Abstract
Inflammatory bowel disease (IBD) is associated with mucosal T cell activation and diarrhea. We found that T cell activation with anti-CD3 mAb induces profound diarrhea in mice. Diarrhea was quantified by intestinal weight-to-length (wt/l) ratios, mucosal Na+/K+-ATPase activity was determined and ion transport changes were measured in Ussing chambers. Anti-CD3 mAb increased jejunal wt/l ratios by more than 50% at 3 hours, returning to base line after 6 hours. Fluid accumulation was significantly reduced in TNF receptor-1 (TNFR-1–/–), but not IFN-γ knockout mice. Anti-CD3 mAb decreased mucosal Na+/K+-ATPase activity, which was blocked by anti-TNF mAb and occurred to a lesser degree in TNFR-1–/– mice. Neither α nor β subunits of Na+/K+-ATPase decreased in abundance at 3 hours. Intestinal tissue from anti-CD3–treated mice exhibited increased permeability to mannitol at 1 hour and decreases in electroneutral Na+ absorption, Na+-dependent glucose absorption, and cAMP-stimulated anion secretion at 3 hours. Furthermore, enteral fluid accumulation was observed in CFTR–/– mice, indicating a minor role of active anion secretion. These data suggest that diarrhea in IBD is due to TNF-mediated malabsorption rather than to secretory processes. T cell activation induces luminal fluid accumulation by increasing mucosal permeability and reducing epithelial Na+/K+-ATPase activity leading to decreased intestinal Na+ and water absorption.
Introduction
Diarrhea is a common clinical feature of immune-mediated bowel dysfunction. Disordered immune cell activation leads to bowel dysfunction via multiple pathways. In disorders associated with cellular inflammation, arachidonic acid metabolites, reactive oxygen radicals, cytokines, and products of enteric nerves are proposed to mediate diarrhea (reviewed in ref. 1). However, despite the abundance of experimental data regarding diarrhea associated with intestinal inflammation, it has been difficult to target a specific pathway for therapeutic intervention until recently.
Results of clinical trials indicate that blockade of the inflammatory mediator TNF effectively reduces disease parameters, including diarrhea, in patients with Crohn disease (2–4). In fact, TNF is detected at high levels in tissues in several disorders characterized by mucosal inflammation and diarrhea, including Crohn disease (3, 5–8), graft-versus-host disease (9), small-bowel allograft rejection (10), and celiac sprue (11). Relatively high levels of TNF have been detected in the stool of patients with diarrheal illnesses due to enteric infections (12, 13). More directly, a phase I study of recombinant TNF infusion in human malignancies found that TNF caused watery diarrhea along with fever, chills, and flu-like symptoms (14). Taken together, these findings suggest that TNF is an important mediator of diarrhea.
A variety of mechanisms are involved in regulating transport of electrolytes and water in the enterocyte. Increased chloride (Cl–) secretion (in crypts) and decreased sodium (Na+) absorption (in villus tips) both lead to net fluid loss from the small intestine. Both Cl– secretion and Na+ absorption are dependent on the function of Na+/K+-ATPase in the basolateral membrane. Sodium absorption is abolished and Cl– secretion reduced when Na+/K+-ATPase is inhibited (15). Not surprisingly, multiple groups have detected decreased activity of epithelial Na+/K+-ATPase in inflamed tissue in inflammatory bowel disease (IBD) (16–18) and infectious enteritis (19). These findings suggest that downregulation of Na+/K+-ATPase is an important factor in the diarrhea associated with intestinal inflammation.
To investigate the mechanism(s) involved in diarrhea associated with immune-mediated bowel disorders, we used a model for activating T cells in vivo with injection of anti-CD3 mAb. Anti-CD3 mAb cross-links T cell receptors and activates T cells when administered systemically or when provided to cells in vitro (20, 21). Infusion of the murine monoclonal anti–human CD3 mAb OKT3 is used in the treatment of human organ rejection, as relatively high-dose stimulation depletes T cells, resulting in its immunosuppressive effects. However, prior to T cell depletion, a self-limited clinical syndrome of fever, hypotension, and diarrhea occurs due to the release of cytokines (TNF, IFN-γ, and IL-2) detected in the serum within hours of the first injection (20). A recent study by Radojevic et al. (22) reported that systemic anti-CD3 mAb administration in mice induced transient diarrhea within 4 hours of injection and increased the base-line jejunal short-circuit current (Isc) measured 20 hours after injection. However, it remains unclear what mediators induced by anti-CD3 mAb were involved in intestinal fluid losses. In the current study, we used anti-CD3 mAb injection to study the mechanism(s) involved in T cell–induced diarrhea. We found that anti-CD3 mAb induced a transient watery diarrhea mediated by TNF. Furthermore, we found that T cell–induced TNF reduced the enzyme activity of Na+/K+-ATPase in epithelial cells without affecting protein levels. Evaluation of mouse intestine mounted in Ussing chambers revealed increased permeability and a profound antiabsorptive effect of T cell activation. Taken together, these data suggest that TNF-mediated downregulation of Na+/K+-ATPase contributes to intestinal fluid losses in patients with immune-mediated bowel disorders by impairing electrolyte absorption.
Methods
Mice.
C57BL/6 mice were obtained from the National Cancer Institute (Frederick, Maryland, USA). Mice deficient in TNF receptor-1 (TNFR-1; p55) and mice deficient in IFN-γ, on C57BL/6 background, were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). CFTR-deficient mice were provided by Laboratory Animal Resources of Dalton Cardiovascular Research Center (University of Missouri–Columbia). B6.129 mice were obtained from The Jackson Laboratory. For all experiments, female mice were primarily used, at 6–8 weeks of age. Similar results were obtained in a limited number of male mice. Mice were maintained under specific pathogen–free conditions in the Veterans Administration Lakeside Medical Center (Medical Science Building, Northwestern University) and Dalton Cardiovascular Research Center (University of Missouri–Columbia). All animal studies were approved by the Center for Experimental Animal Resources of Northwestern University and the University of Missouri Animal Care and Use Committee.
Antibodies and cytokines.
Hamster anti–murine CD3 mAb (2C11) and control hamster mAb (UC8-1B9) were obtained from Pharmingen (San Diego, California, USA). Anti-TNF (XT22), anti–IFN-γ (XMG-1.2), and isotype-matched rat control (GL113) mAb’s were purified from ascites. Antibody was purified over a protein G column (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). Highly purified murine TNF was purchased from PeproTech Inc. (Rocky Hill, New Jersey, USA). Murine IFN-γ was purchased from Life Technologies Inc. (Gaithersburg, Maryland, USA). For all reagents, endotoxin levels were tested using the Limulus amoebocyte lysate assay (BioWhittaker Inc., Walkersville, Maryland, USA) and contained less than 0.1 endotoxin unit/ml.
Enteropooling.
To determine the time course of intestinal fluid accumulation following T cell activation, C57BL/6 mice were sacrificed 1, 3, 6, and 9 hours following intraperitoneal injection of 0.2 mg anti-CD3 (2C11) mAb. For other experiments, mice were sacrificed 3 hours after intraperitoneal injection of 0.2 mg of either anti-CD3 or control hamster mAb (UC8-1B9). Anti-TNF (XT22, 0.5 mg), anti–IFN-γ (XMG-1.2, 2 mg), or control (GL113) mAb’s were administered intraperitoneally 2 hours before T cell activation. TNF was given intraperitoneally in a range of doses as indicated, and IFN-γ was given intraperitoneally in a dose of 10,000 U. TNF- and IFN-γ–treated mice were sacrificed 3 hours after respective treatments.
To quantify the diarrhoegenic activity of in vivo T cell activation as well as to test agents that may block this effect, we measured the weight-to-length (wt/l) ratios of isolated sections of jejunum. The technique was adapted from previously used methods for assessing the accumulation of fluid into the small intestine, called “enteropooling.” Enteropooling is the difference between the fluid being excreted into the lumen and the fluid absorbed (23). The wt/l ratio is a measure of the weight of a section of small bowel in milligrams divided by the length in centimeters. For each measurement, mice were sacrificed at the time interval indicated. Mice were fasted overnight but allowed to drink water ad libitum. The intestine was moved as little as possible so as not to disturb fluid in the lumen. Three jejunal segments (3–6 cm) that could be readily excised were carefully isolated, ligated securely at both ends with equivalent lengths of 4-0 nylon, and removed. Adherent mesentery was cut off, and segments were weighed, their lengths measured, and their wt/l ratios determined.
K+-stimulated phosphatase measurement of Na+/K+-ATPase.
The Na+/K+-ATPase activity was measured as described previously (24). Ten-centimeter segments of jejunum were rinsed in ice-cold saline, and mucosae were scraped off using glass slides. Intestinal scrapings were homogenized in lysis buffer (10 mM Tris [pH 7.4], 3 mM EDTA, 1 mM PMSF, and 10 μg/ml each of pepstatin, leupeptin, aprotinin, and antipain). Nuclei were pelleted (500 g, 2 minutes, 4°C), and then microsomal membranes were pelleted (100,000 g, 20 minutes, 4°C). Membranes were resuspended, and aliquots were added to triplicate tubes of either K+ or Na+ buffer with the Na+/K+-ATPase substrate p-nitrophenol phosphate. Samples were incubated at 37°C for 1 hour, and the reaction was stopped by addition of 10% trichloroacetic acid. After addition of 1N NaOH to neutralize, the samples were centrifuged for 15 minutes. Absorbances were read at 410 nm and compared with standard p-nitrophenol (PNP). Protein in an aliquot was measured by the bicinchoninic acid protein assay.
Western blot of Na+/K+-ATPase subunit.
An aliquot of microsomal membranes isolated above was used for determination of the α and β subunits of Na+/K+-ATPase. Protein was solubilized in Laemmli-SDS stop solution, analyzed on 10% SDS-PAGE, and immediately transferred to a PVDF membrane. Blots were blocked with 5% milk in Blotto buffer (150 mM NaCl, 5 mM KCl, and 10 mM Tris [pH 7.4], with 0.5% vol/vol Tween-20) and incubated overnight at 4°C with mAb’s to either α (clone C464.6) or β (clone 464.8; Upstate Biotechnology Inc., Lake Placid, New York, USA) subunits of rabbit Na+/K+-ATPase (cross-reactive to murine epitopes). Blots were washed, incubated with horseradish peroxidase–conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., Westgrove, Pennsylvania, USA), and developed using an enhanced chemiluminescence system (SuperSignal; Pierce Chemical Co., Rockford, Illinois, USA).
In vitro isotopic flux and bioelectric measurements.
Sections of mid-jejunum were excised from wild-type and Cftr–/– (B6.129-Cftrtm/UNC; C57BL/6J-Cftrtm/UNC) mice and mounted at full thickness in standard Ussing chambers (0.25 cm2 exposed surface area). Intestinal preparations were bathed bilaterally in Krebs bicarbonate Ringers containing (in mM) 115 NaCl, 2.4 K2HPO4, 0.4 KH2PO4, 25 NaHCO3, 1.2 CaCl2, and 1.2 MgCl2 (pH 7.4) and gassed with 95% O2/5% CO2. Glucose (10 mM) was added to the serosal bath; mannitol (10 mM) was substituted for glucose in the mucosal bath to avoid an inward current resulting from Na+-coupled glucose cotransport (25). Transepithelial short-circuit current (Isc, in μEq/cm2/h) was measured using an automatic voltage clamp (VCC-600; Physiologic Instruments, San Diego, California, USA), and total tissue conductance (Gt, mS/cm2 tissue surface area) was determined every 5 minutes by measuring the current deflections resulting from a 5-mV transepithelial pulse and applying Ohm’s law. The serosal bath served as ground in all experiments. For experiments measuring the Isc response to glucose addition or intracellular cAMP stimulation, the jejunal sections were treated by addition of 10 mM glucose to the mucosal bath for 10 minutes or by bilateral addition of 10 μM forskolin for 15 minutes, respectively.
Permeability characteristics of the jejunum were estimated by changes in Gt and in the unidirectional mucosal-to-serosal 3H-mannitol flux (Jmannitol) at 1, 2, and 3 hours after intraperitoneal injection in mice treated with saline or 0.2 mg anti-CD3 mAb. After a 30-minute equilibration, a 30-minute flux period was initiated and flux rates were calculated from aliquots taken at the beginning and end of the flux period. Radioactivity was quantified by liquid scintillation spectroscopy (Packard Instrument Co., Meriden, Connecticut, USA), and flux was calculated as previously described (25). Net Na+ flux was measured as the difference between the unidirectional mucosal-to-serosal (Jms) and serosal-to-mucosal (Jsm) fluxes (Jnet = Jms – Jsm) of 22Na, as previously described (26). Electroneutral Na+ absorption resulting from Na+/H+ exchange activity was estimated by measurement of the unidirectional mucosal-to-serosal flux of 22Na (JmsNa+) across paired jejunal sections (Gt within 10%) in the presence or absence of 100 μM 5-(N-ethyl-N-isopropyl) amiloride (EIPA), as previously described (26). EIPA-sensitive fluxes were calculated using the formula: EIPA-sensitive JmsNa+ = vehicle (DMSO) JmsNa+ – EIPA JmsNa+.
Statistical analysis.
The Student’s t test was used to evaluate differences between the control and experimental groups. When more than two groups were compared, a one-way ANOVA followed by a post hoc Tukey’s t test was used to evaluate differences. Differences were considered significant with P values of less than 0.05.
Results
Anti-CD3–induced enteropooling.
To examine the effects of T cell activation on intestinal fluid and electrolyte losses, mice were given systemic injections of anti-CD3 (2C11) mAb. Within 1–3 hours of in vivo T cell stimulation, mice developed watery stools and rectal prolapse. Examination of the small intestine revealed fluid-filled loops of duodenum, jejunum, and ileum. To quantify the volume of fluid produced (enteropooling), random segments of jejunum were isolated, tied at the ends, and removed for measurement of the wt/l ratio (23). T cell stimulation increased the wt/l ratios of jejunal segments by 33% at 1 hour and more than 50% at 3 hours (Figure 1a). Levels gradually declined to base line by 6 hours after anti-CD3 mAb injection. These results indicate that in vivo T cell activation induces a self-limited accumulation of fluid in the small intestine that is associated with watery diarrhea.
Figure 1.
(a) Time course of effect of anti-CD3 mAb on jejunal wt/l ratio. Mice were injected with 0.2 mg anti-CD3 mAb at time 0. Loop wt/l ratios were measured after varying times. Values are means ± SE for six determinations. (b) Effect of anti-TNF and anti–IFN-γ antibodies on jejunal wt/l ratios at 3 hours. Mice were injected simultaneously with 0.2 mg control or anti-CD3 mAb along with neutralizing mAb to TNF or IFN-γ. (c) Control or anti-CD3 mAb was injected in C57BL/6 or in TNFR-1 or IFN-γ knockout mice. Weight-to-length ratios were determined after 3 hours. Values are means ± SE for six determinations in each group. In all cases, *P < 0.05, **P < 0.01 by comparison using ANOVA. In (a), all comparisons were with 0-time control. In (b) and (c), control mAb-treated, anti–CD3-stimulated mice were initially compared with control mAb-treated, unstimulated controls. In (b), results in anti-TNF mAb and anti–IFN-γ–treated, anti–CD3-stimulated mice were compared with control mAb-treated, anti–CD3-stimulated mice. In (c), results in anti–CD3-stimulated TNFR-1–/– and IFN-γ–/– mice were compared with anti–CD3-stimulated B6 mice. The data indicate that TNF but not IFN-γ inhibition prevented anti–CD3-induced diarrhea.
The role of TNF and IFN-γ in T cell–induced diarrhea.
TNF and IFN-γ can be detected at relatively high levels in the serum within 1 hour of in vivo T cell activation (20–22). To determine the role of IFN-γ and TNF in T cell–induced diarrhea, mice were treated with neutralizing mAb to these cytokines prior to injection of anti-CD3 mAb. Anti-TNF mAb inhibited more than 75% of the increase in intestinal fluid induced by anti-CD3 (Figure 1b). By comparison, IFN-γ blockade reduced the effect of anti-CD3 mAb on intestinal fluid by about 30%. Taken together, these data suggest that TNF largely mediates T cell–induced diarrhea, and that IFN-γ may play a minor role. The TNF- and IFN-γ–neutralizing antibodies by themselves had no effect on enteropooling (data not shown).
To further explore the relationship between T cell–induced cytokines and diarrhea, gene knockout mice were used. Responses to anti-CD3 mAb were reduced by 50% in TNFR-1 knockout (TNFR-1–/–) mice (P < 0.01; Figure 1c). Interestingly, no difference was detected between wild-type and IFN-γ–/– mice in response to anti-CD3 treatment. Although these data suggest that IFN-γ plays little if any role in T cell–induced diarrhea, it is possible that redundant (e.g., TNF-mediated) pathways may have compensated for the absence of IFN-γ in these mice.
Previous studies suggest that TNF and possibly IFN-γ play a role in T cell–induced diarrhea in mice (20–22). To examine the isolated effects of these cytokines on intestinal fluid, we treated mice with injection of exogenous TNF and IFN-γ (Figure 2a). A clear dose effect was detected for TNF-induced intestinal fluid accumulation (Figure 2b). By comparison, only a minor effect was detected for IFN-γ despite administration of 10,000 U per mouse.
Figure 2.
Effect of TNF or IFN-γ on wt/l ratios. (a) Mice were injected with control antibody, anti-CD3 mAb, TNF, or IFN-γ. Jejunal wt/l ratios were measured at 3 hours. Values are means ± SE for six determinations. (b) Dose-dependence of TNF effect. Mice were injected with varying amounts of TNF, and jejunal wt/l ratios were measured at 3 hours. Values are means ± SE for six determinations in each group or each TNF dose. *P < 0.05, **P < 0.01 compared with control antibody in a and 0 TNF in b by ANOVA.
T cell activation results in downregulation of intestinal Na+/K+-ATPase.
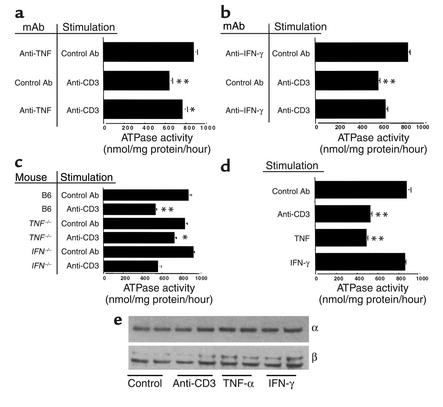
Previous studies (16–18) suggest that diarrhea in immune-mediated bowel disorders is associated with decreased Na+/K+-ATPase activity. To examine the effect of in vivo T cell activation on Na+/K+-ATPase activity, mice were given control or anti-CD3 mAb and jejunal tissues were isolated. Compared with controls, anti-CD3 mAb treatment decreased mucosal Na+/K+-ATPase activity by almost 40% from 863 to 543 nmol PNP/mg protein/hour (P < 0.01; Figure 3a). In contrast, protein levels of α and β subunits of epithelial Na+/K+-ATPase were unchanged (Figure 3e).
Figure 3.
(a–d) Effect of anti-CD3 mAb on Na+/K+-ATPase activity. Mice were injected with control or anti-CD3 mAb and enzyme activity measured in jejunum isolated 3 hours later. Values are means ± SE for four to six determinations in each group. Results in unstimulated mice treated with anti-TNF (a) or anti–IFN-γ (b) mAbs were not different from control mAb-treated, unstimulated mice (data not shown). In (a) and (b), results in control mAb-treated, anti–CD3-stimulated mice were compared with unstimulated mice. Results of anti–cytokine-treated, anti–CD3-stimulated mice were compared with control mAb-treated, anti–CD3-stimulated mice. In (c), data in B6, anti–CD3-stimulated mice were compared with B6, unstimulated mice and results in anti-CD3-stimulated, TNFR-1–/– and IFN-γ–/– mice were compared with B6, anti–CD3-stimulated mice. In (d), results in anti-CD3 mAb-stimulated, TNF-treated and IFN-γ–treated mice were compared with control mAb-treated mice. (e) Effect of anti-CD3 mAb on abundance of Na+/K+-ATPase α and β subunits. Microsomal membranes were isolated and analyzed on SDS-PAGE, and Western blots were performed using mAb to the α and β subunits. *P < 0.05, **P < 0.01 compared with control by ANOVA.
The role of TNF and IFN-γ in T cell–induced downregulation of Na+/K+-ATPase.
To address the role of cytokines in the downregulation of Na+/K+-ATPase activity induced by in vivo T cell activation, anti-CD3 mAb was administered to mice pretreated with control or TNF-neutralizing mAb or to mice deficient in TNFR-1 or IFN-γ. Anti-TNF mAb reduced the effect of T cell activation on Na+/K+-ATPase activity by 45% compared with that in controls (Figure 3a). Similarly, in TNFR-1–deficient mice, the effect of anti-CD3 mAb on Na+/K+-ATPase activity was diminished by 62% (Figure 3c). Taken together, these data suggest that TNF plays a significant role in the downregulation of Na+/K+-ATPase detected in intestinal inflammation.
Compared with TNF, IFN-γ had relatively little effect on T cell–induced downregulation of Na+/K+-ATPase. In mice pretreated with anti–IFN-γ, the effect of T cell activation on Na+/K+-ATPase activity was reduced by only 21% compared with that in control mice pretreated with GL113 (Figure 3b). Likewise, Na+/K+-ATPase activity was decreased to a similar degree in IFN-γ knockout mice compared with C57BL/6 controls (Figure 3c). The difference between control mAb and anti–IFN-γ mAb treatment prior to anti-CD3 was not statistically significant using an ANOVA with a Bonferroni correction.
To address the direct effect of cytokine on Na+/K+-ATPase, mice were injected with exogenous TNF or IFN-γ. Intestinal Na+/K+-ATPase activity was measured in mice treated with a dose of TNF (8 μg) that induced levels of enteropooling similar to those induced by 0.2 mg anti-CD3 mAb. Exogenous TNF reduced intestinal Na+/K+-ATPase activity by 49% from control levels (Figure 3d). In contrast, treatment with relatively high levels of exogenous IFN-γ (10,000 U) failed to affect Na+/K+-ATPase activity (Figure 3d).
To determine whether decreased activity after anti-CD3 mAb treatment was due to decreased abundance of either the α or the β subunit, Western blot analysis of these proteins was determined. The effects of anti-CD3 mAb as well as TNF and IFN-γ on the Na+/K+-ATPase subunits are presented in Figure 3e. At 3 hours, none of the treatments downregulated either the α or β subunit. Therefore, the decreased activity could be due to chemical modification of one or both subunits.
Ion transport alterations in anti-CD3–treated mice.
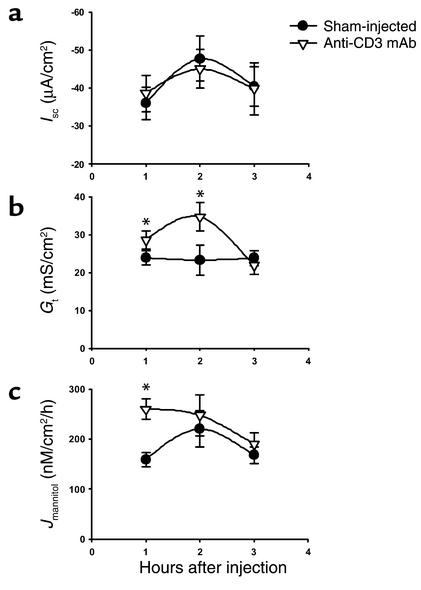
T cell activation produces profound alterations in ion transport of the intestine (22, 27). Downregulation of Na+/K+-ATPase would be anticipated to impinge on nearly every active transport pathway of the cell, similar to the effects of ouabain. The basolateral pump is required to maintain low intracellular Na+ and, thereby, provide the electrochemical driving force for most secondary active transport pathways. Alterations in ion transport pathways induced by T cell activation were investigated using isolated jejunal sections from mice treated with anti-CD3 mAb for 1, 2, or 3 hours. Basal bioelectric parameters of intestinal sections are shown in Figure 4. As compared with the saline-injected control jejuna, the Isc (an index of transcellular electrogenic ion transport in jejunal sections) at all time points was not different in the anti-CD3 mAb–treated jejuna (Figure 4). In contrast, Gt was significantly increased over control at the 1-hour and 2-hour time points, but normalized by 3 hours (Figure 4). It should be emphasized that in a relatively leaky epithelium like the jejunum, the conductance of the paracellular pathway represents approximately 90% of the Gt (28). The increase in paracellular permeability detected by the Gt measurements was mirrored by an increase in passive 3H-mannitol flux (Jmannitol), although the changes were only significant at the 1-hour time point (Figure 4). These findings indicate that T cell activation produces an early (1–2 hours) increase in the paracellular conductance of the jejunum but does not elicit an effect on electrogenic ion transport that persists during in vitro measurements.
Figure 4.
Time course of effect of anti-CD3 mAb on bioelectric parameters and intestinal permeability of murine jejunum. Mice were injected with PBS (Sham-injected) or 0.2 mg anti-CD3 mAb at time 0. Jejunal sections were mounted in Ussing chambers for measurement of Isc, Gt, and Jmannitol. Values are means ± SE for 15 sections (eight mice) at 1 hour, eight sections (five mice) at 2 hours, and eight sections (four mice) at 3 hours. *P < 0.05, **P < 0.1 vs. sham-injected.
An increase in paracellular permeability of the anti-CD3 mAb–treated mice may account for a portion of intestinal fluid accumulation; however, it does not fully explain the maximal enteropooling that occurs at 3 hours after injection. Since the preceding studies indicated that T cell activation does not produce an overt anion secretory response, we investigated the effect of anti-CD3 mAb treatment on Na+ absorption across the jejunum. In the absence of nutrient solutes, sodium is absorbed in the small intestine primarily by the activity of luminal membrane Na+/H+ exchangers, e.g., NHE3 and NHE2 (29, 30). Therefore, changes in the magnitude of this process were investigated by measurement of isotopic Na+ flux across intestine from control and anti-CD3 mAb–injected mice (3 hours after injection). As shown in Table 1, anti-CD3 mAb treatment nearly abolished net Na+ absorption, primarily as a result of a decrease in the mucosal-to-serosal (absorptive) Na+ flux. To verify that the effect of anti-CD3 mAb treatment on net Na+ absorption was due to inhibition of the apical membrane Na+/H+ exchangers, the magnitude of mucosal-to-serosal Na+ flux sensitive to the Na+/H+ exchange inhibitor EIPA was measured in a separate series of experiments (26). Three hours of treatment with anti-CD3 mAb inhibited almost 90% of the EIPA-sensitive Na+ absorption across the jejunum (Figure 5a). Next, to evaluate the effect of T cell activation on Na+-coupled nutrient transport, the Isc response to mucosal addition of 10 mM glucose was measured. The Na-coupled glucose current was reduced by 55% in the anti-CD3 mAb–treated intestine (Figure 5b). The above findings were consistent with inhibition of Na+-absorptive processes, an effect probably secondary to a decrease in Na+/K+-ATPase activity resulting from T cell activation. To further examine the effect of anti-CD3 mAb treatment on secondary active transport, we measured the anion secretory response during acute stimulation of intracellular cAMP. As shown in Figure 5c, the Isc response to forskolin was reduced by nearly 70% in the anti-CD3 mAb–treated intestine, demonstrating that T cell activation inhibits stimulated anion secretion.
Table 1.
Unidirectional and net Na+ flux across the jejunum of sham-injected and anti-CD3 antibody–injected mice 3 hours after injection
Figure 5.
Effect of anti-CD3 mAb on Na+ absorption and anion secretion across murine jejunum. Mice were injected with PBS (Sham-injected) or 0.2 mg anti-CD3 mAb 3 hours before sacrifice. Jejunal sections were mounted in Ussing chambers for measurement of Isc and 22Na flux measurements. (a) Mucosal-to-serosal 22Na flux sensitive to 100 μM EIPA (n = 10 sections from six mice). (b) Isc response to addition of 10 mM glucose in mucosal bath (15 minutes) to stimulate Na+-coupled glucose transport (n = 12 sections from six mice). (c) Isc response to addition of 10 μM forskolin in bathing solutions (10 minutes) to stimulate cAMP-dependent anion secretion (n = 11 sections from six mice). Values are means ± SE. *P < 0.05, **P < 0.1 vs. sham-injected.
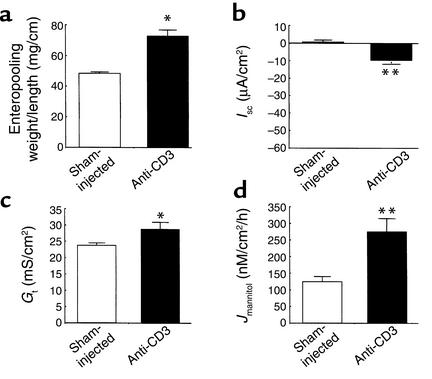
In vitro studies suggested that diarrhea induced by T cell activation does not induce active anion secretion. To test this hypothesis in vivo, effects of anti-CD3 mAb treatment on enteropooling in mice with gene-targeted deletion of CFTR were examined. CFTR is a final common pathway for anion secretion regulated by intracellular cyclic nucleotides or Ca2+ in the murine small intestine (26). However, anti-CD3 mAb treatment increased fluid accumulation in the small intestine of the CFTR-null mice to approximately the same extent as it did in wild-type mice (Figure 6). Note that the base-line fluid accumulation in the saline-injected CFTR-null intestine exceeds that measured in the saline-injected wild-type mice, an effect that may be due to the older age of the CFTR-null mice (8–14 weeks). The in vitro bioelectric parameters of the CFTR-null intestine following anti-CD3 mAb treatment were also investigated and are shown in Figure 6, b–d. Interestingly, anti-CD3 treatment induced a small increase in Isc, suggesting novel activation of a minor conductive pathway (e.g., a separate apical channel for Cl– secretion). Perhaps more importantly, both Gt and Jmannitol were significantly increased in the CFTR-null intestine, as was previously shown in the wild-type intestine. It is interesting to note that increased intestinal permeability was sustained at 3 hours after injection in the CFTR-null intestine, in contrast to the wild-type intestine (compare with Figure 4, Jmannitol), a result suggesting that CFTR activity is involved in the normalization of paracellular permeability in the wild-type intestine following T cell activation.
Figure 6.
Effect of anti-CD3 mAb in CFTR-deficient mice. Mice were injected with PBS (Sham-injected) or 0.2 mg anti-CD3 mAb 3 hours before sacrifice. (a) Effect on jejunal wt/l ratio. Values are means ± SE for three mice in each group. *P < 0.5 vs. sham-injected. (b–d) Effect of anti-CD3 mAb ISC, Gt and Jmannitol of intestine from CFTR-deficient murine jejunum. Values are means ± SE for seven sections (four mice). *P < 0.05, **P < 0.1 vs. sham-injected.
To determine whether increased salivary or gastric secretions may account for the enteropooling, a surgical model was created in which the intestinal contents were diverted at the duodenal-jejunal junction. A heterotopic small bowel transplantation was performed. In these mice the proximal small bowel is made into an ostomy and the efferent limb of the bowel is anastomosed into the host’s distal small bowel. These mice were injected with either control or anti-CD3 mAb, and enteropooling was measured. Fluid accumulation was not different in jejunal loops between the two groups. Weight-to-length ratios at 3 hours were 22 ± 2 and 23 ± 2 in the sham-operated and intestinal-diversion mice, respectively, after injection of control mAb. After anti-CD3 mAb, these values increased to 34 ± 3 and 36 ± 2 in the two groups, respectively. Thus the fluid accumulated in the intestinal lumen was not derived from secretions of proximal sections of the intestine but arose from transmucosal fluid shifts.
Discussion
Using an in vivo model, we examined the mechanism(s) of fluid loss (diarrhea) induced by T cell activation by anti-CD3 mAb. Our results suggest that T cell–induced TNF production induces fluid accumulation in the small bowel by downregulating the activity of epithelial Na+/K+-ATPase. T cell–induced diarrhea has been attributed to several cytokines produced by T cells, including TNF, IFN-γ, IL-1, IL-2, IL-3, IL-6, IL-8, and IL-10 (30–32). The data presented herein suggest that among these, TNF plays a crucial role. In our studies, we observed a dose-dependent fluid accumulation following direct injection of mice with TNF. Clinically, blocking TNF with mAb’s (Infliximab) has been known to prevent diarrhea symptoms in patients with IBD (2, 33). Similarly, we found that in mice, blocking TNF prior to T cell activation prevented fluid accumulation almost completely. Results from experiments were also significant in mice deficient in TNFR-1 (p55). Anti-CD3 mAb–induced fluid accumulation in these mice was lower than in C57BL/6 controls, but not as low as in C57BL/6 mice pretreated with anti-TNF mAb. This slight disparity may reflect the role of another diarrhea-producing cytokine in the knockout mice, perhaps IL-1, as an adaptation to the absence of TNF.
Anti-CD3 mAb–induced fluid accumulation in IFN-γ knockout mice was similar to that in C57BL/6 mice, whereas blocking IFN-γ with mAb prior to anti-CD3 treatment slightly reduced diarrhea. These data suggest that IFN-γ does not play a major role in T cell–induced diarrhea. These results were surprising, as in vitro experiments suggest that this cytokine regulates paracellular permeability (34, 35). It should be noted that the effects of IFN-γ both in vitro and in vivo require many hours to be manifest. The permeability changes observed in the present model, however, occurred rapidly and may indicate a different mechanism. Lastly, it is possible that IFN-γ acts synergistically with TNF, as reported in other biological processes (36, 37), for example by enhancing synthesis of TNF or other diarrhea-inducing cytokines. Thus, further studies may be needed to fully examine the complex role of IFN-γ in immune-mediated diarrhea.
At least three mechanisms might account for the final step in the development of diarrhea in inflammatory intestinal conditions: (a) loss of plasma and fluid through the inflamed and ulcerated mucosa, (b) upregulation of secretory proteins, and (c) downregulation of absorptive proteins. Cytokines are thought to contribute to diarrhea by the latter two processes. Various authors have suggested upregulation of Cl– channels in the apical membrane of crypt epithelial cells during inflammatory conditions, and TNF has been shown to inhibit Cl– absorption and stimulate Cl– secretion in the human colon (31). Absorption of Na+ across the intestine involves the participation of several proteins, including the Na+/H+ exchangers and Na+/nutrient transporters located in the luminal cell membranes. More importantly, absorption requires the active transport of Na+ out of the epithelial cells, mediated by the Na+/K+-ATPase located on the basolateral cell membranes. This transporter consists of two subunits: a 97-kDa α subunit and a 55-kDa β subunit. Its phosphatase activity is assigned to the α subunit, while the β subunit is thought to prevent the proteolytic degradation of newly synthesized α subunits. Using the K+-stimulated phosphatase assay, we demonstrated significant reductions in Na+/K+-ATPase activity in jejunal mucosa with anti-CD3 and TNF treatment. The process responsible for reduced pump activity does not appear to decrease protein abundance in the epithelia, since Western blot analysis of both subunits of Na+/K+-ATPase did not show reduced expression of the protein with either anti-CD3 activation or TNF treatment. This would suggest that a chemical modification of Na+/K+-ATPase that inactivates the enzyme occurs following T cell activation. Previous studies from our laboratory as well as other laboratories have demonstrated that nitric oxide may alter barrier function in intestinal epithelial cells and may do so by inhibiting Na+/K+-ATPase (38, 39). Interestingly, we found that fluid accumulation in iNOS–/– mice was not significantly different from that in control mice (data not shown). Thus, these in vivo data suggest that iNOS-independent signaling pathways decreased Na+/K+-ATPase activity. In vitro in a cultured thyroid epithelial cell line, TNF inhibits activity and expression of Na+/K+-ATPase (40); however, the precise mechanisms of inhibition are not known.
The relationship between pump activity and permeability of the tight junctions is poorly understood. Recent studies using the renal epithelial Madin Darby Canine Kidney cell line suggest that Na+/K+-ATPase activity is required for acute maintenance of tight-junctional permeability (41). The relationship between pump activity and tight-junctional permeability may be influenced by the amount of cell swelling that occurs after pump inhibition and also by how “tight” the occluding junctions are in a particular epithelium. It is also possible that changes in tight-junctional permeability may be mediated by other effects of T cell activation and TNF production. Using an HT29 colonic epithelial cell line subclone, TNF was shown to decrease the number of intercellular strands on electron micrographs (42). These investigators, however, did not determine the mechanism through which TNF acts and whether these junctional-strand changes might involve changes in Na+/K+-ATPase activity, changes in intracellular Na+, or subsequent stress kinase activation. Decreased numbers of junctional strands have been observed in studies of permeability in ulcerative colitis patients (43), but again, the involvement of inhibition of Na+/K+-ATPase and TNF has not been investigated.
The hypothesis that fluid accumulation and diarrhea may arise from decreased absorption rather than stimulated secretion is rather novel. Previous studies have documented the secretory effects of a large variety of hormones and neurotransmitters in tissues from healthy animals (reviewed in ref. 1). In contrast, Sandle et al. reported that short-circuit current decreased in inflamed intestine compared with control (44). These data also indicated that tissue inflammation was associated with decreased Na+ absorption. In animal models of colitis, both hyporesponsiveness to secretagogues (45) and decreased Na+-absorptive processes (Na+-dependent glucose absorption and non–nutrient-dependent Na+ and Cl– transport) have been observed (46, 47). Similar to the present findings, decreased Na+/K+-ATPase activity was measured in inflamed intestine (48). Studies with ouabain indicate that inhibition of Na+/K+-ATPase impairs the ability of enterocytes to maintain an electrochemical gradient, thereby impairing both Na+ absorption and Cl– secretion (49). Taken together, these data and our own suggest that T cell activation reduces Na+ absorption and Cl– secretion in inflamed tissue by TNF-mediated inactivation of Na+/K+-ATPase. It is also possible that other transport proteins may be modified by T cell activation (e.g., Na+-K+-Cl– cotransporter, electrogenic Na+ channels [in colon], CFTR), and these effects might contribute to the antiabsorptive and antisecretory effects of activation of T cells by anti-CD3 mAb. Inhibition of colonic Na+/K+-ATPase has been noted by different groups in two previous studies in human inflammatory bowel diseases (50, 51). In both studies, an inverse relationship of disease activity and Na+/K+-ATPase activity was noted. More recent studies using a rat model demonstrated IL-1β–induced decreases in colonic Na+/K+-ATPase activity (52). All of these authors hypothesize that the decreased ATPase activity relates to the diarrheal fluid losses observed in IBD. Thus, previous studies in IBD states have documented that intense mucosal T cell activation is associated with decreased Na+/K+-ATPase activity. These observations led us to speculate that intestinal transport effects of IBD may be primarily malabsorptive — i.e., decreased electrolyte and water absorption — rather than secretory. Additionally, recent studies suggest that IBD intestinal mucosa has greater permeability. The present studies also suggest that increases in permeability may contribute to fluid losses in diarrhea. Any fluid lost as a result of increased permeability would also not be reabsorbed, because of the impairment in electrolyte (and thus in fluid) absorption.
In summary, our data suggest that intestinal T cell activation induces intestinal fluid accumulation through a cytokine-mediated pathway dominated by TNF. We suspect that these effects contribute to mucosal host defense. Enhanced fluid accumulation in the intestine increases bacterial clearance and may be a mechanism whereby T cell activation impairs tissue invasion and reduces colonization by enteric pathogen. Thus, the results and model described have important implications for the pathophysiology and therapy of diarrhea in infectious enteritis, as well as in aberrant states of intestinal inflammation as seen in IBD.
Acknowledgments
This work was supported by NIH grants DK-54778 and DK-47073 (to T.A. Barrett), DK-38510 (to E.B. Chang), DK-47722 (to E.B. Chang and M.W. Musch), and DK-48816 (to L.L. Clarke), and by research grants from the Crohn’s and Colitis Foundation of America (to T.A. Barrett and E.B. Chang), the Gastrointestinal Research Foundation of Chicago, and the American Heart Association.
Footnotes
Mark W. Musch and Lane L. Clarke contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: inflammatory bowel disease (IBD); TNF receptor-1 (TNFR-1); weight-to-length [ratio] (wt/l).
References
- 1.Ciancio MJ, Chang EB. Epithelial secretory response to inflammation. Ann NY Acad Sci. 1992;664:210–221. doi: 10.1111/j.1749-6632.1992.tb39762.x. [DOI] [PubMed] [Google Scholar]
- 2.Baert FJ, et al. Tumor necrosis factor-alpha antibody (Infliximab) therapy profoundly down-regulates the inflammation in Crohn’s ileocolitis. Gastroenterology. 1999;116:22–28. doi: 10.1016/s0016-5085(99)70224-6. [DOI] [PubMed] [Google Scholar]
- 3.Bell SJ, Kamm MA. Antibodies to tumor necrosis factor alpha as treatment for Crohn’s disease. Lancet. 2000;355:858–860. doi: 10.1016/S0140-6736(99)00442-0. [DOI] [PubMed] [Google Scholar]
- 4.van Dullemen HM, et al. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 5.Casellas F, et al. Intraluminal colonic release of immunoreactive tumour necrosis factor in chronic ulcerative colitis. Clin Sci (Lond) 1994;87:453–458. doi: 10.1042/cs0870453. [DOI] [PubMed] [Google Scholar]
- 6.Reinecker HC, et al. Enhanced secretion of tumor necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanfranchi GA, Tragnone A. Serum and faecal tumour necrosis factor-alpha as marker of intestinal inflammation. Lancet. 1992;33:1053. doi: 10.1016/0140-6736(92)90573-l. [DOI] [PubMed] [Google Scholar]
- 8.Stack WA, et al. Randomised controlled trial of CDP571 antibody to tumour necrosis factor-alpha in Crohn’s disease. Lancet. 1997;349:521–524. doi: 10.1016/s0140-6736(97)80083-9. [DOI] [PubMed] [Google Scholar]
- 9.Brown GR, et al. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116:593–601. doi: 10.1016/s0016-5085(99)70181-2. [DOI] [PubMed] [Google Scholar]
- 10.Bowles MJ, Pockley PG, Wood RF. Effect of anti-LFA-1 monoclonal antibody on rat small bowel allograft survival and circulating leukocyte populations. Transpl Immunol. 2000;8:75–80. doi: 10.1016/s0966-3274(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 11.Nilsen EM, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–563. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 12.Kutukculer N, Caglayan S. Tumor necrosis factor-alpha and interleukin-6 in stools of children with bacterial and viral gastroenteritis. J Pediatr Gastroenterol Nutr. 1997;25:556–557. doi: 10.1097/00005176-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 13.de Silva DG, et al. Concentrations of interleukin 6 and tumour necrosis factor in serum and stools of children with Shigella dysenteriae1 infection. Gut. 1993;34:194–198. doi: 10.1136/gut.34.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinmetz T, et al. Phase I study of 24-hour continuous intravenous infusion of recombinant human tumor necrosis factor. J Biol Response Mod. 1988;7:421–423. [PubMed] [Google Scholar]
- 15.Ewe K. Intestinal transport in constipation and diarrhoea. Pharmacology. 1988;36(Suppl.):73–84. doi: 10.1159/000138424. [DOI] [PubMed] [Google Scholar]
- 16.Rachmilewitz D, Karmeli F, Sharon P. Decreased colonic Na-K-ATPase activity in active ulcerative colitis. Isr J Med Sci. 1984;20:681–684. [PubMed] [Google Scholar]
- 17.Allgayer H, et al. Inverse relationship between colonic (Na+/K+)-ATPase activity and degree of mucosal inflammation in inflammatory bowel disease. Dig Dis Sci. 1988;33:417–422. doi: 10.1007/BF01536025. [DOI] [PubMed] [Google Scholar]
- 18.Ejderhamn J, Finkel Y, Strandvik B. Na, K-ATPase activity in rectal mucosa of children with ulcerative colitis and Crohn’s disease. Scand J Gastroenterol. 1989;24:1121–1125. doi: 10.3109/00365528909089265. [DOI] [PubMed] [Google Scholar]
- 19.Tripp JH, Muller DP, Harries JT. Mucosal (Na+-K+)-ATPase and adenylate cyclase activities in children with toddler diarrhea and the postenteritis syndrome. Pediatr Res. 1980;4:1382–1386. doi: 10.1203/00006450-198012000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Ferran C, et al. Cascade modulation by anti-tumor necrosis factor monoclonal antibody of interferon-gamma, interleukin 3 and interleukin 6 release after triggering of the CD3/T cell receptor activation pathway. Eur J Immunol. 1991;21:2349–2353. doi: 10.1002/eji.1830211009. [DOI] [PubMed] [Google Scholar]
- 21.Ferran C, et al. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990;20:509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- 22.Radojevic N, et al. Characterization of enteric functional changes evoked by in vivo anti-CD3 T cell activation. Am J Physiol. 1999;276:R715–R723. doi: 10.1152/ajpregu.1999.276.3.R715. [DOI] [PubMed] [Google Scholar]
- 23.Robert A, Nezamis JE, Lancaster C, Hanchar AJ, Klepper MS. Enteropooling assay: a test for diarrhea produced by prostaglandins. Prostaglandins. 1976;11:809–828. doi: 10.1016/0090-6980(76)90189-1. [DOI] [PubMed] [Google Scholar]
- 24.Garrahan PJ, Pouchan MI, Rega AF. Potassium activated phosphatase from human red blood cells. The mechanism for potassium activation. J Physiol. 1969;202:305–327. doi: 10.1113/jphysiol.1969.sp008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz SG, Zalusky R. Ion transport in isolated rabbit ileum. I. Short-circuit current and Na fluxes. J Gen Physiol. 1964;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gawenis LR, et al. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol. 2002;282:G776–G784. doi: 10.1152/ajpgi.00297.2001. [DOI] [PubMed] [Google Scholar]
- 27.Barrett TA, et al. Differential function of intestinal intraepithelial lymphocyte subsets. J Immunol. 1992;149:1124–1130. [PubMed] [Google Scholar]
- 28.Frizzell RA, Schultz SG. Ionic conductances of extracellular shunt pathway in rabbit ileum: influence of shunt on transmural sodium transport and electrical potential differences. J Gen Physiol. 1972;59:318–337. doi: 10.1085/jgp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultheis P, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 30.Maher MM, Gontarek JD, Bess RS, Donowitz M, Yeo CJ. The Na/H exchange isoform NHE3 regulates basal canine ileal Na absorption in vivo. Gastroenterology. 1997;112:174–183. doi: 10.1016/s0016-5085(97)70232-4. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz H, et al. Tumor necrosis factor-alpha induces Cl- and K+secretion in human colon driven by prostaglandin E2. Am J Physiol. 1996;271:G669–G674. doi: 10.1152/ajpgi.1996.271.4.G669. [DOI] [PubMed] [Google Scholar]
- 32.Yu LC, Perdue MH. Immunologically mediated transport of ions and macromolecules. Ann NY Acad Sci. 2000;915:247–259. doi: 10.1111/j.1749-6632.2000.tb05248.x. [DOI] [PubMed] [Google Scholar]
- 33.Evans RC, et al. Treatment of ulcerative colitis with an engineered human anti-TNFalpha antibody CDP571. Aliment Pharmacol Ther. 1997;11:1031–1035. doi: 10.1046/j.1365-2036.1997.00251.x. [DOI] [PubMed] [Google Scholar]
- 34.Rocha F, et al. IFN-γ downregulates expression of Na+/H+exchangers NHE2 and NHE3 in rat intestine and human Caco2/bbe cells. Am J Physiol Cell Physiol. 2001;280:C1224–C1232. doi: 10.1152/ajpcell.2001.280.5.C1224. [DOI] [PubMed] [Google Scholar]
- 35.Colgan SP, et al. Interferon-gamma induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am J Physiol. 1994;274:C586–C594. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- 36.Schuerer-Maly CC, Eckmann L, Kagnoff MF, Falco MT, Maly FE. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994;81:85–91. [PMC free article] [PubMed] [Google Scholar]
- 37.Suk K, et al. IFN gamma/TNF alpha synergism as the final effector in autoimmune diabetes: a key role for STAT/IFN regulatory factor-1 in pancreatic beta cell death. J Immunol. 2001;166:4481–4489. doi: 10.4049/jimmunol.166.7.4481. [DOI] [PubMed] [Google Scholar]
- 38.Unno M, Menconi MJ, Smith M, Fink MF. Nitric oxide mediates interferon-gamma-induced hyperpermeability in cultured human intestinal epithelial monolayers. Crit Care Med. 1995;23:1170–1176. doi: 10.1097/00003246-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Sugi K, Musch MW, Field M, Chang EB. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology. 2001;120:1393–1403. doi: 10.1053/gast.2001.24045. [DOI] [PubMed] [Google Scholar]
- 40.Pekary AE, Levin SR, Johnson DG, Berg L, Hershman JM. Tumor necrosis factor-alpha (TNF-alpha) and transforming growth factor-beta 1 (TGF-beta 1) inhibit the expression and activity of Na+/K+-ATPase in FRTL-5 rat thyroid cells. J Interferon Cytokine Res. 1997;17:185–195. doi: 10.1089/jir.1997.17.185. [DOI] [PubMed] [Google Scholar]
- 41.Rajasekaran SA, et al. Na, K, ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell. 2001;12:3717–3732. doi: 10.1091/mbc.12.12.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz H, et al. Tumor necrosis factor alpha reduces tight junction complexity in HT-29/B6 colonic monolayers. Gastroenterology. 1998;114:A414. (Abstr.) [Google Scholar]
- 43.Schmitz H, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 44.Sandle GI, et al. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. doi: 10.1016/0016-5085(90)91235-x. [DOI] [PubMed] [Google Scholar]
- 45.Bell CJ, Gall D, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol. 1995;268:G622–G630. doi: 10.1152/ajpgi.1995.268.4.G622. [DOI] [PubMed] [Google Scholar]
- 46.Sundaram U, Wisel S, Rajendran V, West AB. Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am J Physiol. 1997;273:G913–G919. doi: 10.1152/ajpgi.1997.273.4.G913. [DOI] [PubMed] [Google Scholar]
- 47.Sundaram U, West AB. Effect of chronic inflammation on electrolyte transport in rabbit ileal villus and crypt cells. Am J Physiol. 1997;272:G732–G741. doi: 10.1152/ajpgi.1997.272.4.G732. [DOI] [PubMed] [Google Scholar]
- 48.Wild GE, Thomson AB. Na(+)-K(+)-ATPase alpha 1- and beta 1-mRNA and protein levels in rat small intestine in experimental ileitis. Am J Physiol. 1995;269:G666–G675. doi: 10.1152/ajpgi.1995.269.5.G666. [DOI] [PubMed] [Google Scholar]
- 49.Binder, H.J., and Sandle, G.I. 1987. Electrolyte absorption and secretion in the mammalian colon. In Physiology of the gastrointestinal tract. L.R. Johnson, editor. Raven Press. New York, New York, USA. 1389–1418.
- 50.Ejderhamn J, Finkel Y, Strandvik B. Na,K-ATPase activity in rectal mucosa of children with ulcerative colitis. Scand J Gastroenterol. 1989;24:1121–1125. doi: 10.3109/00365528909089265. [DOI] [PubMed] [Google Scholar]
- 51.Allgayer H, et al. Inverse relationship between colonic (Na+-K+)-ATPase activity and degree of mucosal inflammation in inflammatory bowel disease. Dig Dis Sci. 1988;33:417–422. doi: 10.1007/BF01536025. [DOI] [PubMed] [Google Scholar]
- 52.Kreydiyyeh SI, Al-Sadi R. The mechanism by which interleukin-1 beta reduces net fluid absorption from the rat colon. Eur Cytokine Netw. 2002;13:358–363. [PubMed] [Google Scholar]