Abstract
Physiological hyperglycemia with hyperinsulinemia reduces fat oxidation in skeletal muscle. The mechanism responsible for this decrease in fat oxidation in human muscle is not known and may contribute to the development of insulin resistance. We hypothesized that the transfer of long-chain fatty acids (LCFAs) into the mitochondria via carnitine palmitoyltransferase-1 (CPT-1) is inhibited by increased malonyl coenzyme A (malonyl-CoA) (a known potent inhibitor of CPT-1) in human muscle during hyperglycemia with hyperinsulinemia. We studied six healthy subjects after an overnight fast and during an induced 5-hour period of hyperglycemia with hyperinsulinemia. Muscle fatty acid oxidation was calculated using stable isotope methodology combined with blood sampling from the femoral artery and vein of one leg. Muscle functional CPT-1 activity was assessed by concurrently infusing an LCFA tracer and a CPT-independent medium-chain fatty acid tracer. Muscle biopsies were obtained from the vastus lateralis after the periods of fasting and hyperglycemia with hyperinsulinemia. Hyperglycemia with hyperinsulinemia decreased LCFA oxidation, but had no effect on LCFA uptake or medium-chain fatty acid oxidation across the leg. Malonyl-CoA concentration significantly increased from 0.13 ± 0.01 to 0.35 ± 0.07 nmol/g during hyperglycemia with hyperinsulinemia. We conclude that hyperglycemia with hyperinsulinemia increases malonyl-CoA, inhibits functional CPT-1 activity, and shunts LCFA away from oxidation and toward storage in human muscle.
Introduction
Insulin resistance and type 2 diabetes are characterized by hyperglycemia with hyperinsulinemia, elevated plasma FFA levels, a reduced ability to oxidize fat, and an accumulation of fat within skeletal muscle (1, 2). This increase in muscle fat content is highly associated with insulin resistance (3, 4). Therefore, a better understanding of the mechanisms that lead to these metabolic alterations is important in order to develop interventions to improve insulin sensitivity in type 2 diabetic patients.
We have previously shown that during a hyperinsulinemic (∼250 μU/ml) and hyperglycemic (∼140 mg/dl) clamp, whole-body entry of long-chain fatty acids (LCFAs) into the mitochondria is inhibited (5) in healthy human volunteers. In addition, when the metabolic profile of insulin resistance was simulated by inducing physiological hyperglycemia (∼150 mg/dl) with hyperinsulinemia (∼35 μU/ml), maintaining FFA concentrations resulted in an inhibition of LCFA oxidation across the leg, splanchnic region, and at the whole-body level (6). The mechanism responsible for the decrease in LCFA oxidation in human muscle is not known, but most likely it involves inhibition of carnitine palmitoyltransferase-1 (CPT-1), the enzyme responsible for the transfer of LCFA into the mitochondria.
Malonyl coenzyme A (malonyl-CoA), the product of the acetyl coenzyme A carboxylase (ACC) reaction, is found in a variety of tissues including heart, liver, adipose, and skeletal muscle (7). In lipogenic tissues such as liver and adipose, malonyl-CoA is the first intermediate in the synthesis of LCFA. Malonyl-CoA allosterically binds to CPT-1, inhibiting the enzyme and the transfer of LCFA into the mitochondria (7). An accumulation of malonyl-CoA in liver and adipose tissue stimulates LCFA synthesis while concomitantly inhibiting LCFA oxidation. McGarry et al. (7) have also identified malonyl-CoA in non-lipogenic tissues such as heart and skeletal muscle. Since LCFAs are not significantly synthesized in these tissues, malonyl-CoA appears to play a role in energy balance by affecting CPT-1 and consequently the rates of LCFA oxidation and/or reesterification into muscle triglyceride (8–10). In fact, a recent study (11) reports that mice lacking the ACC enzyme (and thus having no malonyl-CoA) have elevated rates of fat oxidation and reduced fat storage, suggesting that ACC and malonyl-CoA play an important role in overall energy balance. Despite these recent advances, it is still not known whether malonyl-CoA regulates fatty acid oxidation in human skeletal muscle.
This study was designed to determine whether simulating the metabolic profile of insulin resistance by inducing physiological hyperglycemia (156 ± 4 mg/dl) and hyperinsulinemia (43 ± 7 μU/ml) while maintaining constant FFA levels (0.56 ± 0.10 mM) would: (a) increase human muscle malonyl-CoA content; (b) inhibit LCFA entry into the mitochondria of muscle (i.e., inhibit functional CPT-1 activity); and (c) shunt LCFA away from oxidation and toward intramuscular storage.
Methods
Subjects.
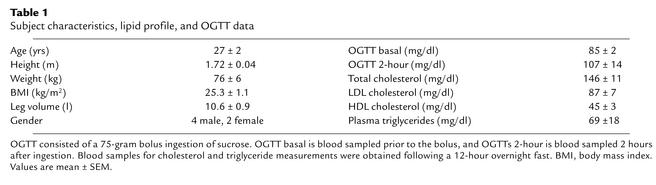
Six volunteers (four males and two females) gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (UTMB) at Galveston. Each subject was screened for determination of health status and given an oral glucose tolerance test (OGTT) at the General Clinical Research Center at UTMB prior to the study. Subjects were not insulin resistant (i.e., had a normal OGTT response) and refrained from physical exercise for at least 24 hours prior to participating in the study. Subject characteristics, lipid profile, and OGTT results are listed in Table 1. Leg volume was calculated using an anthropometric method as previously described (12).
Table 1.
Subject characteristics, lipid profile, and OGTT data
Study protocol.
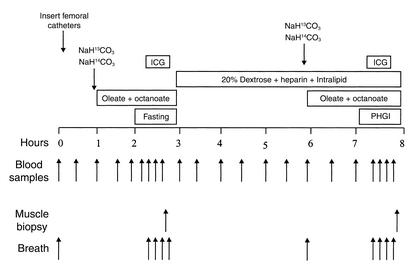
A schematic for the study protocol is shown in Figure 1. Briefly, subjects arrived at the UTMB General Clinical Research Center the evening prior to the study and consumed an evening meal at approximately 9 pm. The study began the next morning at approximately 6 am with the insertion of femoral arterial and venous catheters in one leg and placement of one catheter in each arm. Shortly after insertion of the catheters, background blood and breath samples were obtained, a priming dose of 13C and 14C bicarbonate was given, and a continuous infusion of an LCFA (1-13C-oleate) and a medium-chain fatty acid (1-14C-octanoate) was initiated. Breath was collected throughout the experiment, and blood samples were obtained from the femoral artery and the femoral vein at 75, 90, 105, and 115 minutes. The first sampling period was labeled the “fasting period,” and also included the infusion of indocyanine green to measure blood flow. At the end of the fasting period (120 minutes after infusion), a muscle biopsy was taken from the lateral portion of the vastus lateralis muscle, about 20 cm above the knee, using a 5-mm Bergström biopsy needle (Stille, Stockholm, Sweden). Following the biopsy, the tracer infusions were stopped and a 20% dextrose infusion was initiated and varied to achieve blood glucose values at approximately 150 mg/dl. In addition, heparin and Intralipid (Baxter, Deerfield, Illinois, USA) were started in order to prevent the expected decrease in plasma FFA. Insulin was not infused, and the increase in plasma insulin concentrations during the dextrose infusion was the result of endogenous stimulation. Three hours after the dextrose infusion was initiated, new blood and breath background samples were obtained and the bicarbonate pool was primed with another bolus injection of 13C and 14C bicarbonate. Following this, the oleate and octanoate tracer infusions were restarted as described previously for the fasting period. Blood and breath collection during the period of physiological hyperglycemia with hyperinsulinemia was done identically as during the fasting period, at 375, 390, 405, and 415 minutes after initial tracer infusion. Blood flow was also measured and another muscle biopsy obtained at 420 minutes.
Figure 1.
Study protocol consisting of a fasting period and a period of physiological hyperglycemia with hyperinsulinemia (PHGI). ICG, indocyanine green.
Infusates.
The bicarbonate pools were primed with NaH13CO3 (3 μmol/kg; Cambridge Isotope Laboratories Inc., Andover, Massachusetts, USA) and NaH14CO3 (25 nCi/kg; NEN Life Science Products Inc., Boston, Massachusetts, USA) prior to the fasting period and the period of hyperglycemia with hyperinsulinemia. The infusion rate of 1-13C-oleate (Cambridge Isotope Laboratories Inc.) was 0.04 μmol/kg/min in both periods. Prior to infusion of oleate, the oleate tracer was bound to 5% human albumin to form a solution with an oleate concentration of approximately 2 μmol/ml. A primed (16 nCi/kg), continuous infusion (0.4 nCi/kg/min) of 1-14C-octanoate (NEN Life Science Products Inc.) was also infused along with the oleate tracer. A variable infusion of 20% dextrose (6.7 ± 0.6 mg/kg/min) was performed to clamp blood glucose concentration at approximately 150 mg/dl. A primed (7 U/kg), continuous infusion of heparin (7 U/kg/h) and an infusion of lipids (Intralipid, 0.7 ml/kg/min) was used to prevent the expected insulin-induced decrease in plasma FFA concentration. To measure leg blood flow, a continuous infusion of indocyanine green was given at a rate of 0.5 mg/min.
Isotope analysis.
For determination of plasma arterial and venous oleate enrichments, FFAs were extracted using a solid-phase extraction method and derivatized to their methyl esters (13). Oleate concentration was measured on a gas chromatograph with flame ionization detection using heptadecanoic acid as an internal standard. On average, the arterial oleate concentration was 33% ± 7% and 28% ± 3% of total FFAs during the periods of fasting and physiological hyperglycemia with hyperinsulinemia, respectively. Oleate enrichment (tracer/tracee ratio) was determined by gas chromatography/mass spectrometry (Hewlett-Packard, Palo Alto, California, USA) by selectively monitoring ions mass-to-charge 296 and 297.
Breath samples were collected, and 10 ml of expired air was injected into evacuated tubes. Breath enrichment was analyzed using an isotope ratio mass spectrometer (SIRA; VG Isotech, Cheshire, United Kingdom).
To determine 14C-octanoate specific activity in femoral artery and vein samples, 1 ml of 6 N HCl was added to 1 ml of plasma. After waiting for all CO2 to be eliminated, scintillation fluid was added and octanoate specific activity was determined with a liquid scintillation counter.
The specific activity of 14CO2 in expired breath samples was determined after trapping CO2 by bubbling expired air through 3 ml of a 1:4:9 solution of phenolphthalein, benzethonium hydroxide, and absolute ethanol, titrated to trap 1 mmol of CO2 as previously described (13). Ten milliliters of scintillation fluid was added to the solution and then counted in a liquid scintillation counter.
Femoral arterial and venous 14CO2 specific activity was measured by a method described previously (14). Briefly, a filter paper (1 × 2 cm) was glued to a cap from a 20-ml glass liquid scintillation vial. The filter paper was saturated with 75 μl of ethanolamine immediately before blood sampling. Femoral arterial and venous blood (5 ml) was added to the vials, which contained 1 ml of 4.5 M lactic acid. Vials were immediately capped and incubated for 24 hours to allow the CO2 to be trapped on the filter paper. Following the 24-hour incubation, the filter paper was quickly transferred to another scintillation vial, 10 ml of scintillation fluid was added, and the vials were kept in the dark for 2 days before being analyzed in a liquid scintillation counter.
Kinetic calculations.
Whole-body fat and glucose oxidation rates were calculated from VO2 and VCO2 measurements obtained from indirect calorimetry (Vmax 229; SensorMedics Corp., Yorba Linda, California, USA) and incorporation of these values into established stoichiometric equations (13). Total fatty acid oxidation was determined by converting the rate of total fat oxidation to its molar equivalent and assuming that the average molecular weight of triglyceride is 860 g/mol. The molar rate of triglyceride oxidation was then multiplied by 3 because each triglyceride molecule contains 3 mol of fatty acids.
Whole-body plasma oleate and octanoate oxidation rates were calculated from the equation: oxidation (μmol/kg/min) = (ECO2 × VCO2)/Eo × ar, where ECO2 is the enrichment or specific activity of breath CO2, Eo is the arterial enrichment or specific activity of oleate or octanoate, respectively, and ar is the acetate correction factor. Octanoate oxidation is expressed in pmol/kg/min. The acetate correction factor was determined previously from a nearly identical study design (5). Values of 55% for the fasting period and 50% for the physiological hyperglycemia with hyperinsulinemia period were used. We used the acetate correction factor to fully account for carbon label fixation.
The fractional extraction of labeled oleate and octanoate by the leg was determined by dividing the uptake of label by the arterial concentration of label: percent FFA extracted across the leg = [(EA × CA) – (EFV × CFV)]/(EA × CA), where EA and EFV are the enrichments or specific activity of label in the femoral artery and femoral vein, respectively, and CA and CFV are the concentrations of oleate or octanoate in the femoral artery and femoral vein, respectively.
Plasma FFA (i.e., oleate and octanoate) oxidation across the leg was determined by calculating the absolute rate of uptake of FFA and multiplying this value by the percentage of FFA taken up by the leg that is released as CO2: FFA oxidation = (% FFA extracted across the leg × CA × leg plasma flow) × [(EFVCO2 × CFVCO2) – (EACO2 × CACO2)]/(EA × CA) – (EFV × CFV). The FFA oxidation value was then divided by the acetate correction factor. EFVCO2 and EACO2 are the CO2 enrichment or specific activity in the femoral artery and femoral vein, and CFVCO2 and CACO2 are the concentrations of oleate and octanoate in the femoral artery and vein.
Assays.
Skeletal muscle malonyl-CoA was measured as previously described (7, 15). Briefly, muscle biopsies (>100 mg) were obtained from the vastus lateralis muscle of one leg and were immediately frozen in liquid nitrogen. Muscle samples were placed in a Dewar vessel under liquid nitrogen (liquid nitrogen was added on a daily basis) and stored in a –80°C freezer. The muscle was then ground under liquid nitrogen in a –20°C freezer, weighed, and homogenized in a 6% perchloric acid solution. The neutralized perchloric acid extracts were used to measure malonyl-CoA concentration by assaying malonyl-CoA–dependent incorporation of tritium-labeled acetyl-CoA into fatty acids (catalyzed by fatty acid synthase). The assay was modified for human skeletal muscle by increasing the specific activity and sensitivity of the assay.
Arterial insulin concentrations were measured by radioimmunoassay (Diagnostic Products Corp., Los Angeles, California, USA) and were averaged over the last hour of each period. Blood glucose was determined by a glucose oxidase method immediately after sampling using a YSI 2700 analyzer (Yellow Springs Instruments, Yellow Springs, Ohio, USA). Plasma FFA concentrations were measured using an enzymatic, colorimetric kit (Wako NEFA; Wako Chemicals GmbH, Neuss, Germany) following blood collection in tubes containing a lipase inhibitor (0.4 mM K3-EDTA). This procedure has been previously shown to inhibit in vitro lipolysis of plasma triglycerides in human subjects given heparin (16). Plasma β-hydroxybutyrate was determined enzymatically (Sigma-Aldrich, St. Louis, Missouri, USA). Leg blood and plasma flow was determined from blood samples collected during a continuous infusion of indocyanine green (17). Sera from the blood samples were analyzed in a spectrophotometer with absorbance set at 805 nm. Blood CO2 concentration was measured immediately after sampling by using a 965 Ciba Corning CO2 analyzer (Ciba-Corning Diagnostics Corp., Medfield, Massachusetts, USA).
Blood glucose uptake across the leg was calculated by multiplying the blood flow by the arterial-venous difference of glucose.
Statistical analysis.
The effects of physiological hyperglycemia with hyperinsulinemia on the substrate, hormone, and malonyl-CoA concentrations and the fatty acid kinetic variables were assessed using the two-tailed, paired Student t test. Data are expressed as mean ± SEM. Differences were considered significant at P < 0.05.
Results
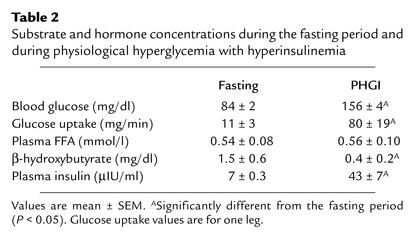
Substrate and insulin data are reported in Table 2. Physiological hyperglycemia with hyperinsulinemia significantly increased arterial glucose concentration, glucose uptake across the leg, and plasma insulin levels (P < 0.05). Plasma FFA levels remained constant because of the simultaneous infusion of lipid and heparin throughout the period of physiological hyperglycemia with hyperinsulinemia, whereas the ketone β-hydroxybutyrate was significantly decreased (P < 0.05).
Table 2.
Substrate and hormone concentrations during the fasting period and during physiological hyperglycemia with hyperinsulinemia
Oleate enrichments in the femoral artery during the fasting period and during the period of physiological hyperglycemia with hyperinsulinemia were in steady state (1.9% ± 0.5% and 1.4% ± 0.2%, respectively). Octanoate specific activity in the femoral artery was 75 ± 9 dpm/ml of blood during the fasting period and 89 ± 7 dpm/ml during the physiological hyperglycemia with hyperinsulinemia period (P > 0.05). Breath 13CO2 enrichments were 0.007% ± 0.001% and 0.0007% ± 0.0003% during the fasting and physiological hyperglycemia with hyperinsulinemia periods, respectively (P > 0.05). Breath 14CO2 specific activity was 2,710 ± 109 dpm/mmol of CO2 during the fasting period and 2,460 ± 146 dpm/mmol during the physiological hyperglycemia with hyperinsulinemia period (P < 0.05).
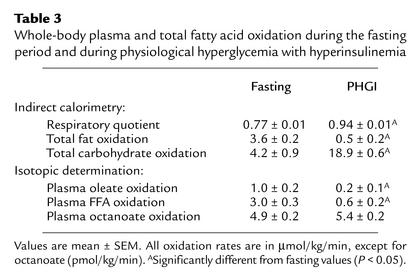
Whole-body total fat oxidation and the respiratory quotient (VCO2/VO2) as measured by indirect calorimetry are presented in Table 3. Physiological hyperglycemia with hyperinsulinemia significantly increased the respiratory quotient and decreased total fat oxidation (P < 0.05). On the other hand, whole-body total carbohydrate oxidation increased significantly during the period of physiological hyperglycemia with hyperinsulinemia (Table 3, P < 0.05).
Table 3.
Whole-body plasma and total fatty acid oxidation during the fasting period and during physiological hyperglycemia with hyperinsulinemia
The tracer-derived calculations of whole-body plasma FFA, oleate, and octanoate oxidation are listed in Table 3. Physiological hyperglycemia with hyperinsulinemia resulted in a significant decrease in plasma FFA and oleate oxidation (P < 0.05), whereas whole-body plasma octanoate oxidation did not change significantly.
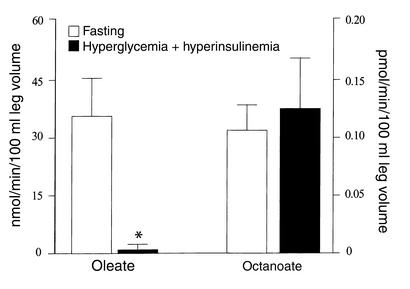
Plasma oleate and octanoate oxidation across the leg are presented in Figure 2. Physiological hyperglycemia with hyperinsulinemia resulted in a significant decrease in oleate oxidation across the leg (P < 0.05), whereas octanoate oxidation remained unchanged. In addition, physiological hyperglycemia with hyperinsulinemia resulted in no change in oleate fractional extraction (41% ± 13% vs. 49% ± 8%) and oleate uptake across the leg (226 ± 123 vs. 264 ± 70 nmol/min/100 ml of leg volume), but the percent of oleate uptake that was oxidized was significantly reduced (15% ± 2% vs. 2% ± 1%; P < 0.05). Thus, LCFAs were shunted away from oxidation and toward reesterification and intramuscular storage. Octanoate fractional extraction across the leg did not change with physiological hyperglycemia with hyperinsulinemia (9% ± 3% vs. 6% ± 2%), and the percent of octanoate uptake that was oxidized remained constant (62% ± 12% vs. 52% ± 18%).
Figure 2.
Graphical representation of in vivo functional CPT-1 activity. Simultaneous measurement of long-chain (oleate) and medium-chain (octanoate) CPT-independent fatty acid oxidation across the leg during conditions of fasting and physiological hyperglycemia with hyperinsulinemia. Values are mean ± SEM. *Significantly different from fasting values (P < 0.05).
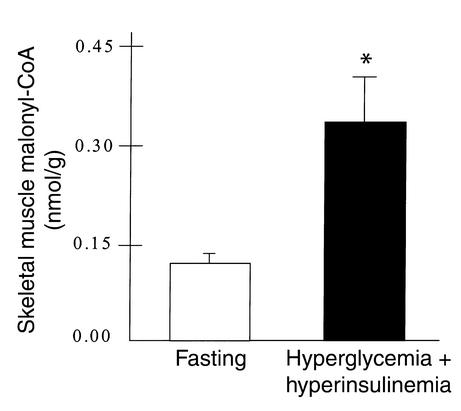
Skeletal muscle malonyl-CoA data is presented in Figure 3. Physiological hyperglycemia with hyperinsulinemia resulted in a 2.7-fold increase (0.13 ± 0.01 vs. 0.35 ± 0.07 nmol/g) in malonyl-CoA concentration (P < 0.05).
Figure 3.
Skeletal muscle malonyl-CoA concentration during conditions of fasting and physiological hyperglycemia with hyperinsulinemia. Values are mean ± SEM. *Significantly different from fasting values (P < 0.05).
Discussion
The primary finding of the present study is that human skeletal muscle malonyl-CoA is increased during physiological hyperglycemia with hyperinsulinemia and that this increase is associated with the inhibition of functional CPT-1 activity and reduced muscle LCFA oxidation. Nonetheless, fatty acid uptake was not inhibited, meaning that intramuscular esterification of LCFA was accelerated. The simultaneous infusion of an LCFA tracer (oleate) and a CPT-independent medium-chain fatty acid tracer (octanoate) in combination with the limb balance technique allowed us to assess, in vivo, functional CPT-1 activity in human skeletal muscle.
Physiological hyperglycemia with hyperinsulinemia, like elevated FFA levels, is common in obese and type 2 diabetic humans. The muscles from these subjects have a decreased capacity to oxidize fat and significantly increased levels of intramuscular triglyceride stores (3). It has been proposed that elevated fat stores within muscle may play a role in the development of insulin resistance (4, 18, 19), although a precise mechanism has yet to be identified. Our data provide the first evidence in humans that physiological hyperglycemia with hyperinsulinemia increases muscle malonyl-CoA concurrently with the decrease in LCFA oxidation. We also found that the fractional extraction of LCFA did not change. This suggests that the increase in malonyl-CoA shunted LCFA away from oxidation and toward storage inside the muscle cell, thus providing a likely explanation for the mechanisms underlying the effects of physiological hyperglycemia with hyperinsulinemia on fat metabolism. In fact, we have estimated that in one leg, the total amount of LCFA shunted away from oxidation and toward reesterification and storage during the 5 hours of physiological hyperglycemia with hyperinsulinemia is significant and amounts to approximately 6.5 mmol/leg. We realize that not all LCFA uptake across the leg can be attributed to skeletal muscle, but it is reasonable to assume that the extent of suppression of oxidation corresponds to the amount of LCFA shunted into the intramuscular triglyceride pool. In agreement with our results, using the proton nuclear magnetic resonance (1H-NMR) spectroscopy technique, it has been shown that a 4-hour hyperinsulinemic, euglycemic clamp with the simultaneous infusion of lipid and heparin increased intramuscular triglyceride concentrations by 9% (20).
We have previously shown that hyperglycemia with hyperinsulinemia decreases fatty acid oxidation to a similar extent at the whole-body level, across the splanchnic region, and across the leg, even when fatty acid availability remained constant (5, 6, 21). Therefore, it was apparent that hyperglycemia with hyperinsulinemia reduces muscle fatty acid oxidation in humans. In addition, at the whole-body level, we have shown that functional CPT-1 activity was reduced during hyperglycemia with hyperinsulinemia and that entry of LCFA into the mitochondria was inhibited (5). However, the precise mechanism for this reduction in functional CPT-1 activity has been elusive, although we demonstrated that long-chain acylcarnitine concentration in human muscle is reduced during these conditions — another indicator that CPT-1 activity had been inhibited (5). The results of the present study, obtained under similar conditions, provide strong evidence for a regulatory role for malonyl-CoA in human muscle, since we found that the increase in malonyl-CoA was associated with decreased fat oxidation.
Our results are consistent with animal studies. For example, muscle malonyl-CoA increases and fat oxidation decreases when rats are exposed to glucose and insulin (22, 23) and during refeeding following fasting (24). It appears that an increase in glucose oxidation results in an increased availability of cytosolic acetyl-CoA (the substrate of ACC) and cytosolic citrate (an allosteric activator of ACC), thus stimulating the production of malonyl-CoA (23). Furthermore, a chronic glucose infusion in rats resulted in significant increases in muscle malonyl-CoA and long-chain acyl-CoA concentrations (25). This suggests that elevations in malonyl-CoA may be shunting the uptake of fatty acids away from CPT-1 and oxidation and toward reesterification (long-chain acyl-CoA) and storage as muscle triglyceride. Interestingly, that study also reported a chronic activation of the protein kinase C-ε isozyme, presumably due to the increase in long-chain acyl-CoA concentration, which may play a role in the development of muscle insulin resistance during conditions of hyperglycemia (25). In support of this view, increasing plasma FFA levels in humans results in a significant increase in muscle triglyceride levels and the development of acute insulin resistance (20). Furthermore, use of a CPT-1 inhibitor for 4 weeks in rats resulted in significant increases in muscle triglyceride stores and muscle insulin resistance (26). Recently, malonyl-CoA in human muscle has been shown to slightly increase (20%) following a hyperinsulinemic (∼120 μU/ml), euglycemic (∼90 mg/dl) clamp, and this increase was associated with a reduction in whole-body fat oxidation measured with indirect calorimetry (27). In our study, muscle malonyl-CoA increased to a greater extent than in that study, most likely due to the fact that we studied the effect of hyperinsulinemia with hyperglycemia, rather than euglycemia, following an overnight fast while maintaining the circulating FFA concentration in order to mimic the insulin-resistant state.
Muscle malonyl-CoA also appears to play a regulatory role during conditions of increased fatty acid oxidation such as fasting and aerobic exercise. In fact, malonyl-CoA concentration in rat skeletal muscle has been shown to decrease with fasting (28, 29), during electrical hind limb stimulation (30, 31), during exercise (15, 32), during the post-exercise recovery state (33), and when exposed to 5-aminoimidazole-4-carboxyamide-1-β-D-ribofuranoside (AICAR), an AMP analogue (14, 34). The decrease in muscle malonyl-CoA during these conditions relieves the inhibition of malonyl-CoA on CPT-1 and allows for increased rates of LCFA oxidation. Muscle contraction and AICAR administration lower malonyl-CoA by activating AMP-activated protein kinase (AMPK), which phosphorylates and inactivates ACC (35). On the other hand, fasting appears to lower malonyl-CoA by allosterically inhibiting ACC (due to an increased concentration of acyl-CoA and a reduction in citrate levels) (24). Recently, three studies have measured human muscle malonyl-CoA following exercise. Two of the studies reported no change in malonyl-CoA concentration (36, 37), whereas a more recent study has reported a significant decrease in malonyl-CoA and ACC activity following exercise (38). The latter study appears to be consistent with studies reporting an increase in human muscle AMPK activity with exercise (39, 40) and supports the notion that a decrease in malonyl-CoA during exercise allows for an increase in the rate of LCFA oxidation.
A well-known paradox regarding malonyl-CoA should be acknowledged. For example, the IC50, (the malonyl-CoA concentration required for 50% inhibition of CPT-1 activity) for malonyl-CoA is much lower than the physiologically reported values, and one would expect CPT-1 to be continually inhibited regardless of any change in muscle malonyl-CoA concentration. One possible explanation is that there are two pools of malonyl-CoA in muscle (i.e., cytoplasmic and mitochondrial), with the cytoplasmic pool being much smaller than the mitochondrial. There is recent evidence suggesting that ACC is embedded in the outer mitochondrial membrane and may be in close proximity to CPT-1 (41). Thus, as suggested recently by McGarry (42), the concentration of malonyl-CoA in the direct vicinity of the CPT-1/ACC complex may be the true regulator of CPT-1 activity, thus explaining this apparent paradox. This observation further stresses the importance of in vivo studies with the measurement of functional enzyme activity, as the anatomic and biochemical conditions in the live organism may be significantly different from the artificial conditions in the in vitro setting.
In summary, we have demonstrated that during physiological hyperglycemia with hyperinsulinemia and maintained FFA concentrations (i.e., a condition that mimics the insulin-resistant state), human skeletal muscle malonyl-CoA concentrations are significantly increased and are directly associated with a reduction in LCFA oxidation and functional CPT-1 activity. Also, LCFA uptake is not altered during these conditions, leading to a shunting of LCFA away from oxidation and toward reesterification and storage as intramuscular triglyceride. We conclude that malonyl-CoA in human skeletal muscle plays a significant role in the regulation of fatty acid oxidation.
Acknowledgments
We would like to thank the nursing staff at the General Clinical Research Center of UTMB for assistance in performing these studies, and William W. Winder, Brigham Young University, for supplying the fatty acid synthase. This study was funded by NIH grant R01 DK-34817 (to R.R. Wolfe) and grant M01RR00073 from the National Center for Research Resources, NIH, (to the General Clinical Research Center, UTMB). U.K. Holmback was a recepient of the STINT (Swedish Foundation for International Cooperation in Research and Higher Education) grant #96/52.
Footnotes
See the related Commentary beginning on page 1607.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: long-chain fatty acid (LCFA); carnitine palmitoyl transferase-1 (CPT-1); malonyl coenzyme A (malonyl-CoA); acetyl coenzyme A carboxylase (ACC); University of Texas Medical Branch (UTMB); oral glucose tolerance test (OGTT); 5-aminoimidazole-4-carboxyamide-1-β-d-ribofuranoside (AICAR); AMP-activated protein kinase (AMPK).
References
- 1.Kelley DE, Mandarin LJ. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990;86:1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandarino LJ, Consoli A, Kelley DE. Effects of obesity and NIDDM on glucose and insulin regulation of substrate oxidation in skeletal muscle. Am J Physiol. 1996;270:E463–E470. doi: 10.1152/ajpendo.1996.270.3.E463. [DOI] [PubMed] [Google Scholar]
- 3.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–941. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 4.Pan DA, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 5.Sidossis LS, Stuart CA, Shulman GI, Lopaschuk GD, Wolfe RR. Glucose plus insulin regulate fat oxidation by controlling the rate of fatty acid entry into the mitochondria. J Clin Invest. 1996;98:2244–2250. doi: 10.1172/JCI119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidossis LS, Mittendorfer B, Chinkes D, Walser E, Wolfe RR. Effect of hyperglycemia-hyperinsulinemia on whole body and regional fatty acid metabolism. Am J Physiol. 1999;276:E427–E434. doi: 10.1152/ajpendo.1999.276.3.E427. [DOI] [PubMed] [Google Scholar]
- 7.McGarry JD, Mills SE, Long CS, Foster DW. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Biochem J. 1983;214:21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol. 1999;276:E1–E18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- 9.Winder WW. Malonyl-CoA—regulator of fatty acid oxidation in muscle during exercise. Exerc Sport Sci Rev. 1998;26:117–132. [PubMed] [Google Scholar]
- 10.Rasmussen BB, Wolfe RR. Regulation of fatty acid oxidation in skeletal muscle. Ann Rev Nutr. 1999;19:463–484. doi: 10.1146/annurev.nutr.19.1.463. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Elheiga L, Matzuk MM, Abo-Hashema KAH, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 12.Katch V, Weltman A. Predictability of body segment volumes in living subjects. Hum Biol. 1975;47:203–218. [PubMed] [Google Scholar]
- 13.Wolfe, R.R. 1992. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. John Wiley & Sons. New York, New York, USA. 471 pp.
- 14.Merrill GF, Kurth EJ, Rasmussen BB, Winder WW. Influence of malonyl-CoA and palmitate concentration on rate of palmitate oxidation in rat muscle. J Appl Physiol. 1998;85:1909–1914. doi: 10.1152/jappl.1998.85.5.1909. [DOI] [PubMed] [Google Scholar]
- 15.Winder WW, Arogyasami J, Barton RJ, Elayan IM, Vehrs PR. Muscle malonyl-CoA decreases during exercise. J Appl Physiol. 1989;67:2230–2233. doi: 10.1152/jappl.1989.67.6.2230. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves M, Kiens B, Richter EA. Effect of increased plasma free fatty acid concentrations on muscle metabolism in exercising men. J Appl Physiol. 1991;70:194–201. doi: 10.1152/jappl.1991.70.1.194. [DOI] [PubMed] [Google Scholar]
- 17.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 18.Storlien LH, et al. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 19.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 20.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 21.Sidossis LS, Wolfe RR. Glucose and insulin-induced inhibition of fatty acid oxidation: the glucose-fatty acid cycle reversed. Am J Physiol. 1996;270:E733–E738. doi: 10.1152/ajpendo.1996.270.4.E733. [DOI] [PubMed] [Google Scholar]
- 22.Duan C, Winder WW. Control of malonyl-CoA by glucose and insulin in perfused skeletal muscle. J Appl Physiol. 1993;74:2543–2547. doi: 10.1152/jappl.1993.74.5.2543. [DOI] [PubMed] [Google Scholar]
- 23.Saha AK, Kurowski TG, Ruderman NB. A malonyl-CoA fuel-sensing mechanism in muscle: effects of insulin, glucose, and denervation. Am J Physiol. 1995;269:E283–E289. doi: 10.1152/ajpendo.1995.269.2.E283. [DOI] [PubMed] [Google Scholar]
- 24.Saha AK, et al. Cytosolic citrate and malonyl-CoA regulation in rat muscle in vivo. Am J Physiol. 1999;276:E1030–E1037. doi: 10.1152/ajpendo.1999.276.6.E1030. [DOI] [PubMed] [Google Scholar]
- 25.Laybutt DR, et al. Muscle lipid accumulation and protein kinase C activation in the insulin-resistant chronically glucose-infused rat. Am J Physiol. 1999;277:E1070–E1076. doi: 10.1152/ajpendo.1999.277.6.E1070. [DOI] [PubMed] [Google Scholar]
- 26.Dobbins RL, et al. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes. 2001;50:123–130. doi: 10.2337/diabetes.50.1.123. [DOI] [PubMed] [Google Scholar]
- 27.Bavenholm PN, Pigon J, Saha AK, Ruderman NB, Efendic S. Fatty acid oxidation and the regulation of malonyl-CoA in human muscle. Diabetes. 2000;49:1078–1083. doi: 10.2337/diabetes.49.7.1078. [DOI] [PubMed] [Google Scholar]
- 28.Winder WW, MacLean PS, Lucas JC, Fernley JE, Trumble GE. Effect of fasting and refeeding on acetyl-CoA carboxylase in rat hindlimb muscle. J Appl Physiol. 1995;78:578–582. doi: 10.1152/jappl.1995.78.2.578. [DOI] [PubMed] [Google Scholar]
- 29.Chien D, Dean D, Saha AK, Flatt JP, Ruderman NB. Malonyl-CoA content and fatty acid oxidation in rat muscle and liver in vivo. Am J Physiol. 2000;279:E259–E265. doi: 10.1152/ajpendo.2000.279.2.E259. [DOI] [PubMed] [Google Scholar]
- 30.Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997;272:E262–E266. doi: 10.1152/ajpendo.1997.272.2.E262. [DOI] [PubMed] [Google Scholar]
- 31.Vavvas D, et al. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol. 1997;83:1104–1109. doi: 10.1152/jappl.1997.83.4.1104. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen BB, Hancock CR, Winder WW. Post-exercise recovery of skeletal muscle malonyl-CoA, acetyl-CoA carboxylase, and AMP-activated protein kinase. J Appl Physiol. 1998;85:1629–1634. doi: 10.1152/jappl.1998.85.5.1629. [DOI] [PubMed] [Google Scholar]
- 34.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 35.Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 36.Odland LM, Heigenhauser GJF, Lopaschuk GD, Spriet LL. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am J Physiol. 1996;270:E541–E544. doi: 10.1152/ajpendo.1996.270.3.E541. [DOI] [PubMed] [Google Scholar]
- 37.Odland LM, Howlett RA, Heigenhauser GJF, Hultman E, Spriet LL. Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am J Physiol. 1998;274:E1080–E1085. doi: 10.1152/ajpendo.1998.274.6.E1080. [DOI] [PubMed] [Google Scholar]
- 38.Dean D, et al. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- 39.Fujii N, et al. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 40.Stephens TJ, et al. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282:E688–E694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Elheiga L, et al. The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci USA. 2000;97:1444–1449. doi: 10.1073/pnas.97.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGarry JD. Banting lecture. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2001;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]