The serine proteinase inhibitors, or serpins, are a superfamily of proteins that are found in a wide range of species, including plants, viruses, and humans. The family includes proteins as diverse as α1-antichymotrypsin, C1 inhibitor, antithrombin, and plasminogen activator inhibitor-1, which have key regulatory functions in the inflammatory, complement, coagulation, and fibrinolytic cascades. Members of the serpin superfamily are characterized by more than 30% sequence homology with α1-antitrypsin and conservation of tertiary structure. The structure is based on three β-sheets (A–C) and an exposed mobile reactive loop that presents a peptide sequence as a pseudosubstrate for the target proteinase. In the case of α1-antitrypsin, the loop presents the P1-P1′ residues methionine-serine as a “bait” for neutrophil elastase. After docking, the proteinase is inactivated by a mousetrap action that swings it from the upper to the lower pole of the protein in association with the insertion of the reactive loop as an extra strand in β-sheet A. This six-stranded protein bound to its target enzyme is then recognized by hepatic receptors and cleared from the circulation.
The reactive loop/β-sheet A interaction of serpins is crucial for their role as effective antiproteinases but also renders them liable to undergo conformational transitions that cause disease. Point mutations can destabilize β-sheet A to allow incorporation of the loop of another serpin molecule (see ref. 1 for review). Sequential reactive loop insertion results in chains of polymers that are retained within the cell of synthesis. This process is best characterized in mutants of α1-antitrypsin that result in liver damage, a consequence of protein retention within hepatocytes (see Perlmutter, this Perspective series, ref. 2; and ref. 3). It is also recognized to underlie plasma deficiency of antithrombin, C1 inhibitor, and α1-antichymotrypsin in association with thrombosis, angio-edema, and emphysema, respectively. Moreover, in the neuron-specific proteinase inhibitor neuroserpin, the same process underlies a novel early-onset inclusion body dementia termed familial encephalopathy with neuroserpin inclusion bodies (FENIB). We review here the role of serpin dysfunction and polymerization in a range of diseases that can be grouped together as the serpinopathies. Our understanding of the structural basis of polymerization provides novel strategies to block polymer formation in vitro. The long-term aim must be to turn these into therapies that are effective in preventing polymerization in vivo.
α1-Antitrypsin deficiency
α1-Antitrypsin deficiency was reported in an Alaskan girl who died 800 years ago, and it may have accounted for the premature death of Frederic Chopin in 1849. It was first described as a clinical entity in 1963 by Laurell and Eriksson, who noted an absence of the α1 band on serum protein electrophoresis (4). Over 70 naturally occurring variants of α1-antitrypsin have been described and characterized by their migration on isoelectric focusing gels. The two most common deficiency variants, S and Z, result from point mutations in the α1-antitrypsin gene that retard the proteins’ migration when compared with normal M α1-antitrypsin. S α1-antitrypsin (Glu264Val) is found in up to 28% of Southern Europeans, and although it results in plasma α1-antitrypsin levels that are 60% of the levels that result from the M allele, it is not associated with any significant clinical disease. The Z variant (Glu342Lys) is present in 4% of Northern Europeans and results in severe plasma deficiency. Individuals who are homozygous for the Z mutation have plasma α1-antitrypsin levels that are 10–15% of the levels of those of the normal M allele, as the aberrant protein accumulates as periodic acid Schiff–positive (PAS-positive), diastase-resistant inclusions in the rough endoplasmic reticulum of the liver (Figure 1). These inclusions can cause juvenile hepatitis, cirrhosis, and hepatocellular carcinoma. The major function of α1-antitrypsin is to protect the lung against the enzyme neutrophil elastase, and severe deficiency of α1-antitrypsin results in an imbalance between proteinases and inhibitors within the lung. This causes tissue destruction and early-onset panacinar emphysema, which accounts for 1–2% of all cases of chronic obstructive pulmonary disease.
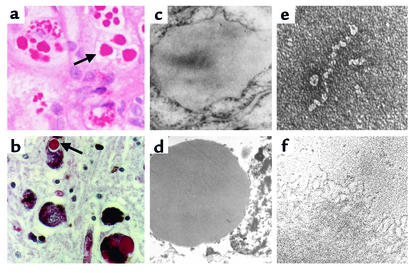
Figure 1.
Inclusion formation in the serpinopathies. Z α1-antitrypsin is retained within hepatocytes as intracellular inclusions, which are PAS-positive and diastase-resistant (a, arrow) and are associated with neonatal hepatitis and hepatocellular carcinoma. (c) Electron micrograph of a hepatocyte from the liver of a patient with Z α1-antitrypsin deficiency shows the accumulation of α1-antitrypsin within the rough endoplasmic reticulum. These inclusions are composed of chains of α1-antitrypsin polymers, shown here from the plasma of a Siiyama α1-antitrypsin homozygote (e) and from the liver of a Z α1-antitrypsin homozygote (f). Similar mutations in α1-antitrypsin deficiency and neuroserpin encephalopathy result in similar intracellular inclusions of α1-antitrypsin and neuroserpin. They are shown here in hepatocytes (a) and neurons (b) with PAS staining (arrow) and as endoplasmic aggregates of the abnormal proteins with electron microscopy (c, hepatocytes; d, neurons). Electron microscopy confirms that the abnormal neuroserpin forms beadlike polymers and entangled polymeric aggregates identical to those shown here with Z α1-antitrypsin (e and f, respectively). Magnification, left to right: ×200, ×20,000, ×220,000. Reproduced with permission from The New England Journal of Medicine (33).
Hepatic accumulation of α1-antitrypsin results from reactive loop into β-sheet polymerization
The Z mutation of α1-antitrypsin is at residue P17 (17 residues proximal to the P1 reactive center) at the head of strand 5 of β-sheet A and the base of the mobile reactive loop. The mutation opens β-sheet A, thereby favoring the insertion of the reactive loop of a second α1-antitrypsin molecule to form a dimer (Figure 2). This process can then extend to form polymers that tangle in the endoplasmic reticulum of the liver and form inclusion bodies (5). Support for this model comes from the demonstration that purified Z α1-antitrypsin forms chains of polymers when incubated under physiological conditions (3). The rate can be accelerated by raising the temperature to 41°C and can be blocked by introducing peptides that compete with the loop for annealing to β-sheet A. The role of polymerization in vivo has been confirmed by the finding of α1-antitrypsin polymers in inclusion bodies from the liver of a Z α1-antitrypsin homozygote with cirrhosis (3) and in cell lines expressing the Z variant (6). Moreover, point mutations that block polymerization increased the secretion of mutants of α1-antitrypsin from a Xenopus oocyte expression system (7). We have now defined the following pathway by which α1-antitrypsin and other members of the serpin superfamily form polymers (equation
 |
1 |
from ref. 8):
Figure 2.
The structure of α1-antitrypsin is centered on β-sheet A (green) and the mobile reactive center loop (red). Polymer formation results from the Z variant of α1-antitrypsin (Glu342Lys at P17; arrow) or mutations in the shutter domain (blue circle) that open β-sheet A to favor partial loop insertion and the formation of an unstable intermediate (M*) (10, 11). The patent β-sheet A can accept either the loop of another molecule, to form a dimer (D), which then extends into polymers (P), or else its own loop, to form a latent conformation (L). The individual molecules of α1-antitrypsin within the polymer are colored red, yellow, and blue.
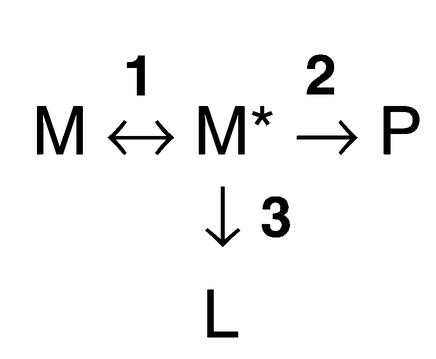
Equation 1
where step 1 represents the conformational change of the serpin to a polymerogenic monomeric form (M*), step 2 represents the formation of polymers (P), and step 3 represents a side pathway that leads to the formation of the stable, monomeric latent conformation (L).
The presence of the unstable, polymerizing intermediate M* was predicted from the biophysical analysis of polymer formation (8), the demonstration of an unfolding intermediate (9), and the solution of the crystal structure of a polymerogenic mutant of α1-antichymotrypsin (10). Indeed, structures can now been assigned to each component in the conformational transition (Figure 2). Our latest data suggest that the Z mutation forces α1-antitrypsin into a conformation that approximates the unstable M* and hence favors polymer formation (11). The quality control mechanisms within the hepatocyte that handle polymers are now being elucidated (12–14). However, despite a more detailed understanding of the disposal pathway, it still remains unclear how the accumulation of Z α1-antitrypsin within hepatocytes causes cell death and liver cirrhosis.
The temperature and concentration dependence of polymerization (3, 8), along with genetic factors (15), may account for the heterogeneity in liver disease among individuals who are homozygous for the Z mutation. The level of α1-antitrypsin synthesis rises during episodes of inflammation as part of the acute phase response. At these times the formation of polymers is likely to overwhelm the degradative pathway, thereby exacerbating the formation of hepatic inclusions and the associated hepatocellular damage. There is anecdotal evidence to support this hypothesis from the prospective study of Sveger and colleagues in Sweden (16). They screened 200,000 newborn babies and identified 120 Z homozygotes, whom they have followed into late adolescence. Two of these patients developed progressive jaundice during the course of the study; in one, this followed appendicitis, and in the other, severe pneumonia. Other asymptomatic infants developed marked derangement of liver function tests in association with coryzal illnesses and eczema. Clearly, further prospective studies are required to assess whether pyrexial episodes are more frequent and cause more intrahepatic polymers in Z α1-antitrypsin homozygotes who develop liver disease than in those individuals who remain asymptomatic.
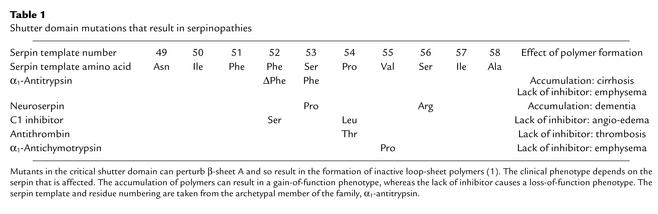
Although many α1-antitrypsin deficiency variants have been described, only two other mutants of α1-antitrypsin have similarly been associated with plasma deficiency and hepatic inclusions: α1-antitrypsin Siiyama (Ser53Phe) and α1-antitrypsin Mmalton (52Phe deleted). The Siiyama mutation is the most common cause of severe α1-antitrypsin deficiency in Japan, and the Mmalton (also known as Mnichinan and Mcagliari) variant is the most common cause of severe α1-antitrypsin deficiency in Sardinia. Both of these mutants lie in the shutter domain that opens to allow the incorporation of the reactive loop during complex formation with the target proteinase (Figure 2 and Table 1). The Siiyama and Mmalton mutations destabilize and open β-sheet A to allow the formation of loop-sheet polymers in vivo (1). Recent investigations have shown that polymerization also underlies the mild plasma deficiency of the S (Glu264Val) and I (Arg39Cys) variants of α1-antitrypsin (17, 18). The point mutations that are responsible for these variants have less effect on β-sheet A than does the Z variant. Thus, the rates of polymer formation are much slower than that of Z α1-antitrypsin, which results in less retention of protein within hepatocytes, milder plasma deficiency, and the lack of a clinical phenotype (8). However, if a mild, slowly polymerizing I or S variant of α1-antitrypsin is inherited with a rapidly polymerizing Z variant, then the two can interact to form heteropolymers within hepatocytes, which give rise to inclusions and cirrhosis (18).
Table 1.
Shutter domain mutations that result in serpinopathies
Polymerization of Z α1-antitrypsin: new insights into the pathogenesis of emphysema
The observation that the intrapulmonary instillation of elastolytic enzymes results in emphysema, and the association between Z α1-antitrypsin deficiency and emphysema, gave rise to the proteinase-antiproteinase hypothesis of tissue damage. α1-Antitrypsin inhibits enzymes such as neutrophil elastase and thereby protects the tissues from proteolytic attack. It enters the lungs by diffusion from the circulation, but α1-antitrypsin is also produced locally by macrophages and bronchial epithelial cells. The most important factor in the development of emphysema is cigarette smoke, and several pathways are now recognized to cause pulmonary damage in the Z α1-antitrypsin homozygote. Firstly, deficiency of α1-antitrypsin within the alveoli allows uncontrolled proteolytic attack. Moreover, the α1-antitrypsin that is available to protect the lungs is approximately fivefold less effective at inhibiting neutrophil elastase than is normal M α1-antitrypsin (19). More recently it has been recognized that the Z mutation also favors the spontaneous formation of α1-antitrypsin loop-sheet polymers within the lung (20). This conformational transition inactivates the protein, thereby further reducing the already depleted levels of α1-antitrypsin that are available to protect the alveoli. The mechanism by which polymers of Z α1-antitrypsin are produced within the lungs is unknown. Their formation may be accelerated by the inflammatory milieu that exists within the lungs of individuals with Z α1-antitrypsin deficiency. In addition, previous studies have shown that polymerization is accelerated at low pH (8) and that cigarette smoke is mildly acidic. Thus, it is possible that cigarette smoke also accelerates polymerization, and hence inactivation of Z α1-antitrypsin, within the lung.
Patients with Z α1-antitrypsin deficiency have an excess number of neutrophils in bronchoalveolar lavage (21) and in tissue sections of lung parenchyma (S. Stewart and R. Mahadeva, unpublished observations) when compared with controls. This may reflect an excess of chemoattractant agents such as LTB4 and IL-8. However, the polymers are themselves chemotactic for human neutrophils in vitro (22). It is plausible that polymers of Z α1-antitrypsin form in vivo and then act as a chronic low-grade chemoattractant that causes an influx of inflammatory cells. The polymers may evade the defense mechanisms of the lung by adhering to the interstitium. Any proinflammatory effect of polymers is likely to be exacerbated by inflammatory cytokines, cleaved or complexed α1-antitrypsin (23), elastin degradation products (24), and cigarette smoke, which themselves cause neutrophil recruitment. Our understanding of the biological properties of α1-antitrypsin thus provides novel pathways for the pathogenesis of emphysema in individuals who are homozygous for the Z mutation (Figure 3). Indeed, the presence of polymers may explain the progression of lung disease in Z α1-antitrypsin homozygotes after smoking cessation and despite adequate intravenous replacement with plasma α1-antitrypsin. The relationship between intrapulmonary Z α1-antitrypsin polymers and smoking, infection, cytokine production, and rate of decline in lung function will require assessment in cell and animal models and prospective studies in Z homozygote individuals.
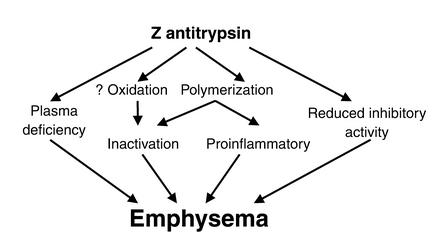
Figure 3.
Proposed model for the pathogenesis of emphysema in patients with Z α1-antitrypsin deficiency. The plasma deficiency and reduced inhibitory activity of Z α1-antitrypsin may be exacerbated by the polymerization of α1-antitrypsin within the lungs. These processes inactivate the inhibitor, thereby further reducing the antiproteinase screen. α1-Antitrypsin polymers may also act as a proinflammatory stimulus to attract and activate neutrophils, thereby increasing tissue damage.
Finally, there is evidence that α1-antitrypsin can be inactivated by oxidation of the P1 methionine residue by cigarette smoke or free radicals from leukocytes. This mechanism has been proposed to explain emphysema in cigarette smokers with normal levels of α1-antitrypsin. However, it is unknown whether this functional deficiency contributes significantly to the inactivation of α1-antitrypsin in vivo, and it is clear that many other factors are likely to be important in the pathogenesis of emphysema in these individuals (25).
The serpinopathies: serpin polymerization in angio-edema, thrombosis, chronic obstructive pulmonary disease, and dementia
The phenomenon of loop-sheet polymerization is not restricted to α1-antitrypsin and has now been reported to underlie the deficiency and inactivation of other serpin variants. This common mechanism allows them to be classified together with α1-antitrypsin deficiency as a new group of disorders, the serpinopathies. Naturally occurring mutations have been described in the shutter (Table 1) and other domains of the plasma proteins C1 inhibitor (Phe52Ser, Pro54Leu, Ala349Thr, Val366Met, Phe370Ser, Pro391Ser), antithrombin (Pro54Thr, Asn158Asp), and α1-antichymotrypsin (Leu55Pro, Pro228Ala). In all cases the residue numbers are based on the serpin template to allow comparison between members of the family (1). These mutations destabilize the serpin architecture to allow the formation of inactive reactive loop into β-sheet polymers. The polymers probably form within the endoplasmic reticulum of the liver but are cleared by the degradative pathway. They are not associated with the formation of inclusions and the liver disease seen in individuals who are homozygous for Z α1-antitrypsin. One can speculate that this is because C1 inhibitor, antithrombin, and α1-antichymotrypsin are synthesized at approximately 10% of the rate of α1-antitrypsin and mutations are usually found in heterozygotes. However, one variant of α1-antichymotrypsin (Pro228Ala) has been shown to form granular inclusions within hepatocytes that are analogous to those formed by Z α1-antitrypsin (26). This mutation also allows the spontaneous formation of polymers in vitro (B. Gooptu and D. Lomas, unpublished observations). The individual with the Pro228Ala α1-antichymotrypsin mutation was infected with the hepatitis C virus, and it seems likely that the viral infection drove the inflammatory response that increased the production of α1-antichymotrypsin polymers to form the inclusions. Clearly, it is impossible to know whether it was the hepatitis C or the inclusions of mutant α1-antichymotrypsin that caused the associated hepatic damage, but the case illustrates that mutants of other serpins can form hepatic inclusions analogous to those of Z α1-antitrypsin. The lack of circulating protein in individuals with C1 inhibitor, antithrombin, and α1-antichymotrypsin deficiency allows uncontrolled activity of proteolytic cascades, and hence angio-edema, thrombosis, and chronic obstructive pulmonary disease, respectively.
The process of polymerization is perhaps most striking in a recently described inclusion body dementia, familial encephalopathy with neuroserpin inclusion bodies (FENIB), that results from polymerization of the neuron-specific serpin neuroserpin (27). The dementia has been described in two Caucasian families in the US. In the first family, 95% of affected individuals presented with dementia between the ages of 45 and 56. The second family had an earlier age of onset of symptoms, with epilepsy and progressive decline in cognitive function occurring in the second and third decades of life. Both were characterized by eosinophilic neuronal inclusion bodies in the deeper layers of the cerebral cortex and in the substantia nigra. The inclusions were PAS-positive and diastase-resistant (Figure 1) but were distinctly different from any previously described entity, including Lewy bodies, Pick bodies, and Lafora bodies. The inclusion bodies had a striking resemblance to those of Z α1-antitrypsin in the hepatocytes of homozygotes with cirrhosis. Biochemical analysis revealed that the inclusions were formed of neuroserpin and that affected individuals carried point mutations in the shutter domain (Ser53Pro, Ser56Arg) that would destabilize the protein to form polymers. Indeed, the Ser53Pro mutation was in the same position as the Siiyama variant, which causes hepatic inclusions and profound plasma deficiency of α1-antitrypsin (Table 1). Structural analysis showed that the mutant neuroserpin had indeed formed intraneuronal polymers that were identical to those of Z α1-antitrypsin (27, 28).
Prevention of polymer formation
Our new understanding of polymer formation may lead to the development of novel therapeutic strategies to prevent disease. We have shown previously that the polymerization of Z α1-antitrypsin can be blocked by annealing of reactive loop peptides to β-sheet A (3). Such peptides were 11–13 residues in length and could anneal to other members of the serpin superfamily, as was most clearly demonstrated by the finding that the reactive loop peptide of antithrombin annealed more readily to β-sheet A of α1-antitrypsin and vice versa. These peptides, although useful in establishing the mechanism of polymerization, are too long and too promiscuous to be used for rational drug design. More recently we have shown that smaller peptides can be designed that can specifically anneal to Z but not to M α1-antitrypsin nor to other native members of the serpin superfamily (11). The value of this observation is that the peptide also completely blocks the polymerization of Z α1-antitrypsin in vitro. The aim is now to convert this small peptide into a mimetic that will enter the endoplasmic reticulum of the cell to block polymerization in vivo.
A second strategy comes from the identification of a hydrophobic pocket in α1-antitrypsin that is bounded by strand 2A and helices C and D. The pocket is patent in the native protein but is filled as β-sheet A accepts an exogenous reactive loop peptide during polymerization (29). Agents that can bind to this pocket will predictably stabilize β-sheet A and so ameliorate polymer formation.
An alternative strategy is to use chemical chaperones (see Kopito, this Perspective series, ref. 30) to stabilize intermediates on the folding pathway. The chaperone trimethylamine oxide has no effect on the secretion of Z α1-antitrypsin in cell culture (31), as it favors the conversion of unfolded Z α1-antitrypsin to polymers (32). In contrast, 4-phenylbutyrate (4-PBA) increases the secretion of Z α1-antitrypsin from cell lines and transgenic mice (31). This agent has been used for several years to treat children with urea-cycle disorders, and, more recently, 4-PBA has been shown to increase the expression of mutant (ΔF508) cystic fibrosis transmembrane conductance regulator protein in vitro and in vivo. These encouraging findings have led to a pilot study to evaluate the potential of 4-PBA to promote the secretion of α1-antitrypsin in patients with α1-antitrypsin deficiency.
Summary
In the last 35 years there have been tremendous advances in our understanding of α1-antitrypsin deficiency. Z α1-antitrypsin polymerizes within the liver to cause cirrhosis. The molecular basis of polymer formation has now been elucidated with biochemical, cellular, and structural studies. The current goals are to determine the cellular response to polymeric α1-antitrypsin and to develop therapeutic strategies to block polymerization in vivo. The recognition of the association between plasma deficiency of α1-antitrypsin and emphysema has led to the proteinase-antiproteinase hypothesis of lung disease. α1-Antitrypsin polymers may have an important role in the progression of emphysema, but this requires further investigation. The evolution of more sophisticated cell and animal models of disease offers a real prospect of dissecting the role of injurious pathways in the pathogenesis of emphysema.
Acknowledgments
We are grateful to B. Gooptu (Department of Medicine, University of Cambridge) and S. Stewart (Department of Pathology, Papworth NHS Trust) for access to unpublished results. This work was supported by the Medical Research Council (United Kingdom), the Wellcome Trust, and the Cystic Fibrosis Trust (United Kingdom). R. Mahadeva is a Wellcome Trust Advanced Clinical Fellow.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: periodic acid Schiff (PAS); 4-phenylbutyrate (4-PBA).
References
- 1.Stein PE, Carrell RW. What do dysfunctional serpins tell us about molecular mobility and disease? Nat Struct Biol. 1995;2:96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- 2.Perlmutter DH. Liver injury in α1-antitrypsin deficiency: an aggregated protein induces mitochondrial injury. J Clin Invest. 2002;110:1579–1583. doi:10.1172/JCI200216787. doi: 10.1172/JCI16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 4.Laurell C-B, Eriksson S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. Scand J Clin Lab Invest. 1963;15:132–140. [Google Scholar]
- 5.Elliott PR, Lomas DA, Carrell RW, Abrahams J-P. Inhibitory conformation of the reactive loop of α1-antitrypsin. Nat Struct Biol. 1996;3:676–681. doi: 10.1038/nsb0896-676. [DOI] [PubMed] [Google Scholar]
- 6.Le A, Ferrell GA, Dishon DS, Quyen-Quyen AL, Sifers RN. Soluble aggregates of the human PiZ α1-antitrypsin variant are degraded within the endoplasmic reticulum by a mechanism sensitive to inhibitors of protein synthesis. J Biol Chem. 1992;267:1072–1080. [PubMed] [Google Scholar]
- 7.Sidhar SK, Lomas DA, Carrell RW, Foreman RC. Mutations which impede loop/sheet polymerisation enhance the secretion of human α1-antitrypsin deficiency variants. J Biol Chem. 1995;270:8393–8396. doi: 10.1074/jbc.270.15.8393. [DOI] [PubMed] [Google Scholar]
- 8.Dafforn TR, Mahadeva R, Elliott PR, Sivasothy P, Lomas DA. A kinetic description of the polymerisation of α1-antitrypsin. J Biol Chem. 1999;274:9548–9555. doi: 10.1074/jbc.274.14.9548. [DOI] [PubMed] [Google Scholar]
- 9.Yu M-H, Lee KN, Kim J. The Z type variation of human α1-antitrypsin causes a protein folding defect. Nat Struct Biol. 1995;2:363–367. doi: 10.1038/nsb0595-363. [DOI] [PubMed] [Google Scholar]
- 10.Gooptu B, et al. Inactive conformation of the serpin α1-antichymotrypsin indicates two stage insertion of the reactive loop: implications for inhibitory function and conformational disease. Proc Natl Acad Sci USA. 2000;97:67–72. doi: 10.1073/pnas.97.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahadeva R, Dafforn TR, Carrell RW, Lomas DA. Six-mer peptide selectively anneals to a pathogenic serpin conformation and blocks polymerisation: implications for the prevention of Z α1-antitrypsin related cirrhosis. J Biol Chem. 2002;277:6771–6774. doi: 10.1074/jbc.C100722200. [DOI] [PubMed] [Google Scholar]
- 12.Cabral CM, Liu Y, Sifers RN. Dissecting the glycoprotein quality control in the secretory pathway. Trends Biochem Sci. 2001;26:619–623. doi: 10.1016/s0968-0004(01)01942-9. [DOI] [PubMed] [Google Scholar]
- 13.Teckman JH, et al. The proteasome participates in degradation of mutant α1-antitrypsin Z in the endoplasmic reticulum of hepatoma-derived hepatocytes. J Biol Chem. 2001;276:44865–44872. doi: 10.1074/jbc.M103703200. [DOI] [PubMed] [Google Scholar]
- 14.Novoradovskaya N, Lee J, Yu Z-X, Ferrans VJ, Brantly M. Inhibition of intracellular degradation increases secretion of a mutant form of α1-antitrypsin associated with profound deficiency. J Clin Invest. 1998;101:2693–2701. doi: 10.1172/JCI549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, et al. A lag in intracellular degradation of mutant α1-antitrypsin correlates with liver disease phenotype in homozygous PiZZ α1-antitrypsin deficiency. Proc Natl Acad Sci USA. 1994;91:9014–9018. doi: 10.1073/pnas.91.19.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sveger T. α1-Antitrypsin deficiency in early childhood. Pediatrics. 1978;62:22–25. [PubMed] [Google Scholar]
- 17.Elliott PR, Stein PE, Bilton D, Carrell RW, Lomas DA. Structural explanation for the dysfunction of S α1-antitrypsin. Nat Struct Biol. 1996;3:910–911. doi: 10.1038/nsb1196-910. [DOI] [PubMed] [Google Scholar]
- 18.Mahadeva R, et al. Heteropolymerisation of S, I and Z α1-antitrypsin and liver cirrhosis. J Clin Invest. 1999;103:999–1006. doi: 10.1172/JCI4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogushi F, Fells GA, Hubbard RC, Straus SD, Crystal RG. Z-type α1-antitrypsin is less competent than M1-type α1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987;80:1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott PR, Bilton D, Lomas DA. Lung polymers in Z α1-antitrypsin related emphysema. Am J Respir Cell Mol Biol. 1998;18:670–674. doi: 10.1165/ajrcmb.18.5.3065. [DOI] [PubMed] [Google Scholar]
- 21.Morrison HM, Kramps JA, Burnett D, Stockley RA. Lung lavage fluid from patients with α1-proteinase inhibitor deficiency or chronic obstructive bronchitis: anti-elastase function and cell profile. Clin Sci. 1987;72:373–381. doi: 10.1042/cs0720373. [DOI] [PubMed] [Google Scholar]
- 22.Parmar JS, et al. Polymers of α1-antitrypsin are chemotactic for human neutrophils: a new paradigm for the pathogenesis of emphysema. Am J Respir Cell Mol Biol. 2002;26:723–730. doi: 10.1165/ajrcmb.26.6.4739. [DOI] [PubMed] [Google Scholar]
- 23.Banda MJ, Rice AG, Griffin GL, Senior RM. The inhibitory complex of human α1-proteinase inhibitor and human leukocyte elastase is a neutrophil chemoattractant. J Exp Med. 1988;167:1608–1615. doi: 10.1084/jem.167.5.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin derived peptides. J Clin Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro SD. Evolving concepts in the pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med. 2000;21:621–632. doi: 10.1016/s0272-5231(05)70172-6. [DOI] [PubMed] [Google Scholar]
- 26.Faber J-P, et al. The molecular basis of α1-antichymotrypsin deficiency in a heterozygote with liver and lung disease. J Hepatol. 1993;18:313–321. doi: 10.1016/s0168-8278(05)80275-2. [DOI] [PubMed] [Google Scholar]
- 27.Davis RL, et al. Familial dementia caused by polymerisation of mutant neuroserpin. Nature. 1999;401:376–379. doi: 10.1038/43894. [DOI] [PubMed] [Google Scholar]
- 28.Belorgey D, Crowther DC, Mahadeva R, Lomas DA. Mutant neuroserpin (Ser49Pro) that causes the familial dementia FENIB is a poor proteinase inhibitor and readily forms polymers in vitro. J Biol Chem. 2002;277:17367–17373. doi: 10.1074/jbc.M200680200. [DOI] [PubMed] [Google Scholar]
- 29.Elliott PR, Pei XY, Dafforn TR, Lomas DA. Topography of a 2.0Å structure of α1-antitrypsin reveals targets for rational drug design to prevent conformational disease. Protein Sci. 2000;9:1274–1281. doi: 10.1110/ps.9.7.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelman MS, Kopito RR. Rescuing protein conformation: prospects for pharmacological therapy in cystic fibrosis. J Clin Invest. 2002;110:1591–1597. doi:10.1172/JCI200216786. doi: 10.1172/JCI16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burrows JAJ, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant α1-antitrypsin (α1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema. Proc Natl Acad Sci USA. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devlin GL, Parfrey H, Tew DJ, Lomas DA, Bottomley SP. Prevention of polymerization of M and Z α1-antitrypsin (α1-AT) with trimethylamine N-oxide. Implications for the treatment of α1-AT deficiency. Am J Respir Cell Mol Biol. 2001;24:727–732. doi: 10.1165/ajrcmb.24.6.4407. [DOI] [PubMed] [Google Scholar]
- 33.Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency: a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]