Abstract
The luteinizing hormone/choriogonadotropin (LH/CG) receptor (R) is a heptahelical R that, upon agonist binding, activates the stimulatory guanine nucleotide-binding protein (Gs) and the downstream effector adenylyl cyclase (AC). Like other G protein-coupled Rs, the LH/CG R subsequently exhibits reduced agonist-dependent effector activity, or desensitization, in response to saturating agonist. Unlike desensitization of many other G protein-coupled Rs, the in vivo desensitization response of LH/CG R-stimulated AC activity of ovarian follicles to the preovulatory surge of LH can be mimicked under cell-free conditions. Based on evidence that porcine ovarian follicular membranes unexpectedly contained β-arrestin-1, the role of arrestins in desensitization of the LH/CG R was investigated. Results showed that neutralizing arrestin antibodies blocked the development of desensitization and that desensitization was rescued with a synthetic peptide corresponding to the antibody-binding epitope on β-arrestin-1. These results suggest that endogenous β-arrestin-1 participates in agonist-dependent desensitization of the LH/CG R. Addition of recombinant purified β-arrestin-1 mimicked human chorionic gonadotrophin to promote desensitization of human chorionic gonadotrophin-stimulated AC activity, in the presence of the ATP phosphorylation antagonist adenylyl-imidodiphosphate, with an ED50 of ≈0.1 nM. Increased levels of an 87-kDa protein reactive with glycoprotein hormone R-reactive antibody, consistent with the LH/CG R, coimmunoprecipitated with follicular membrane β-arrestin-1 in response to LH/CG R activation compared with unactivated R. Taken together, these results show that ovarian follicles contain membrane-associated β-arrestin-1, that β-arrestin-1 participates in agonist-dependent desensitization of the LH/CG R, and that the trigger for β-arrestin-1 binding to the LH/CG R appears to be R activation.
The luteinizing hormone/choriogonadotropin (LH/CG) receptor (R) belongs to the seven-transmembrane β-adrenergic/rhodopsin family of G protein-coupled Rs that mediate their actions through the activation of G proteins and downstream effectors like adenylyl cyclase (AC) (1–4). A characteristic feature of these G protein-coupled Rs is that in the face of continuing stimulation signaling becomes attenuated or desensitized (5). The vertebrate arrestins, which include visual arrestins, β-arrestin-1, and β-arrestin-2, are believed to play an important role in regulation of desensitization of G protein-coupled Rs (6, 7). A role for arrestin to quench R signaling originally was demonstrated for phosphorylated light-activated rhodopsin R (8). For the β-adrenergic R, where agonist-dependent rapid desensitization also has been well studied, R phosphorylation catalyzed by G protein-coupled R kinases (GRKs) triggers but is not sufficient for desensitization (5–7). After GRK-mediated R phosphorylation, β-arrestin recruited from the cytosol binds to the phosphorylated β-adrenergic R with high affinity (6), quenching the coupling of phosphorylated β-adrenergic R to stimulatory guanine nucleotide-binding protein (Gs) (9). High-affinity binding of β-arrestin to ligand-activated, phosphorylated m2 muscarinic cholinergic R has also been shown (10–12).
In preovulatory ovarian follicles, LH/CG R-stimulated AC activity is desensitized by the physiological surge of LH, which induces ovulation and corpus luteum formation (13–16). Desensitization of follicular LH/CG R-stimulated AC activity proceeds relatively slowly in vivo (13–17) and most likely results from a sequential series of events (18). By ovulation, LH/CG R agonist only minimally activates AC (13–15, 17). The initial (60-min) phase of the follicular desensitization response, in which R-stimulated AC activity is reduced ≈50%, is of the homologous type and not mediated by cAMP (18–21) and is not accompanied by a reduction in LH/CG R numbers (17, 22–25). LH/CG R-mediated desensitization has been demonstrated in vitro both in isolated follicles (16–18, 20, 21) and in cell-free follicular membrane preparations (19, 26–32). A hyperdesensitized state, which retains dependence on GTP and agonist for development, can be achieved by preincubating follicular membranes in the presence of 8% ethanol (33). Like cell-free desensitization in the absence of ethanol (refs. 19 and 26; R. M. Ragajopalan-Gupta, S.M., X. Zhu, Y.-K. Ho, H. Hamm, M. Birnbaumer, L. Birnbaumer, and M.H.-D., unpublished data), hyperdesensitization of human chorionic gonadotrophin (hCG)-stimulated AC activity is homologous and not accompanied by a reduction in fluoride-stimulated (19, 33) or forskolin-stimulated (R. M. Ragajopalan-Gupta et al., unpublished data) AC activities. We have shown that desensitization of the LH/CG R in porcine follicular membranes, like that of the angiotensin type I, serotonin type II, cholecystokinin, and secretin Rs (34–37), appears to be independent of R phosphorylation, based on the inability of protein kinase inhibitors to block desensitization (38), the occurrence of desensitization in the presence of the ATP phosphorylation antagonist adenylyl-imidodiphosphate (AMP-PNP) (19, 29, 32), the absence of detectable phosphate incorporation into immunoprecipitated LH/CG R under conditions that support desensitization (32), and the ability of the poorly hydrolyzable GDP analog GDPβS to completely reverse desensitization (31) and ethanol-induced hyperdesensitization (R. M. Rajagopalan-Gupta and M.H.-D., unpublished observation). Based on evidence that arrestins bind not only to phosphorylated Rs but also to agonist-activated unphosphorylated Rs, albeit with lower affinity (9, 11, 39–41), and our preliminary evidence that arrestin immunoreactivity was readily detectable in a plasma membrane preparation of porcine ovarian follicles (R. M. Rajagopalan-Gupta and M.H.-D., unpublished observation), we investigated the role of arrestin in mediating desensitization of the LH/CG R in porcine ovarian follicular membranes. Results show that endogenous β-arrestin-1 participates in desensitization of the LH/CG R and provide evidence for a physiological role for arrestin binding to a G protein-coupled R to mediate desensitization.
MATERIALS AND METHODS
Materials.
Purified hCG (batch CR-127) was obtained through the National Hormone and Pituitary Program, National Institutes of Child Health and Human Development. Neutralizing monoclonal arrestin antibodies to bovine visual arrestin were obtained from Larry Donoso (Wills Eye Hospital Research Division, Philadelphia). mAb A7 (42) was made to amino acid residues (EDIMGY) conserved among glycoprotein hormone Rs (3) located in the extracellular domain proximal to the membrane. Visual arrestin was purified from bovine retinas (43), and recombinant (rec) arrestins were overexpressed in BL-21 cells and purified (44). The sources of materials was as described previously (19, 31, 32) with the following exceptions: Immobilon-P membranes (0.45-μm pore size), Millipore; antibodies to β-arrestin-1 and β-arrestin-2, Transduction Laboratories (Lexington, KY). β-arrestin-1 peptide (VFEDFARQRLKG) was synthesized and purified by the Protein Chemistry Core Facility, Baylor College of Medicine (Houston, TX) and neutralized before use.
Preparation of Follicular Membranes, Desensitization Reaction, and AC Assay.
Walls from pig ovarian follicles larger than 6 mm in diameter were dissected, and a partially purified membrane fraction enriched in AC activity was isolated after sucrose density gradient centrifugation (19) and stored at −70 C at a protein concentration of 6–8 mg/ml in 10 mM Tris⋅HCl, pH 7.2. Final concentrations of reagents in 50 μl reaction volume are indicated throughout. In experiments with arrestin antibody and purified arrestin, follicular membranes (≈30 μg protein) were preincubated in a 20-μl volume in the presence of arrestin antibody, nonimmune serum (NIS), and/or arrestin, as indicated, at room temperature for 15 min then at 4°C for 1 h. Reagents (in 20 μl) for stage 1 desensitization reaction (BSA or hCG at 10 μg/ml in 8% ethanol in 25 mM 1,3 bis-[tris(hydroxymethyl)-methylamino]propane, pH 7.2/10 μM GTP [Sigma G8877, 98% pure]/0.4 mM EDTA/1 mM EGTA/0.2 mg/ml creatine phosphokinase/20 mM phosphocreatine/5 mM MgCl2/1 mM ATP unless otherwise indicated/1 mM [3H]cAMP [≈20,000 cpm]) then were added, and incubation was continued for 40 min at 30°C. Immediately after the desensitization reaction, assay for AC activity (stage 2) was performed at 30°C for 5 min with the addition of a 10-μl volume containing 100 μM GTP, [α-32P]ATP (≈5 μCi, 100–200 cpm/pmol), and 10 μg/ml BSA or hCG. Reaction was stopped (19) and [32P]cAMP was purified and quantified (26, 45). All determinations were run in quadruplicates. The presence of BSA in stages 1 and 2 measured basal AC activity; BSA in stage 1 and hCG in stage 2 measured full hCG-stimulated AC activity; hCG in stages 1 and 2 measured hCG-induced desensitization of AC activity. The percentage of reduction of full hCG-stimulated AC activity above basal AC activity, expressed as percentage desensitization, was used as a measure of the extent of LH/CG R desensitization.
Western Blot Analysis Immunoprecipitation.
Arrestin protein in porcine follicular membranes was detected by enhanced chemiluminescence after separation of follicular membrane proteins by SDS/PAGE, transferring proteins in gel to Immobilon, and incubation with primary and then secondary antibody (46). To immunoprecipitate membrane β-arrestin-1, membrane proteins were solubilized in buffer containing 1% Triton X-100, Triton-soluble extract was precleared with protein A/G agarose, then immunoprecipitating antibody (26 μl anti-β-arrestin-1 antibody) was added (47). Immunocomplexes were collected as protein A/G agarose pellets, proteins were separated by SDS/PAGE and transferred to Immobilon, and the blot was probed with mAb A7, as described above.
Statistics.
Results (means ± SE) were analyzed using Student’s t test (P < 0.05) (48).
RESULTS
Presence of Arrestins in Porcine Ovarian Follicular Membranes.
Western blot analysis was performed on porcine follicular membranes using F4C1 mAb to visual arrestin, which recognizes an epitope (DGVVLVD) conserved among mammalian β-arrestins (7) (Fig. 1 Left), and mAbs (Transduction Laboratories) generated against rat β-arrestin-1 (Fig. 1 Right) and rat β-arrestin-2 (not shown). Results revealed that porcine ovarian follicular membranes contain predominately β-arrestin-1. The β-arrestin-2 antibody did not react with any immunoreactive band shown in Fig. 1A but reacted with rat brain lysate (not shown), suggesting that β-arrestin-2 is absent from porcine follicular membranes.
Figure 1.
Porcine ovarian follicular membranes contain β-arrestin-1. Membrane proteins (100 μg) boiled in 3× SDS sample buffer were separated on a 10.5% SDS-polyacrylamide gel and transferred to Immobilon, and immunoblot analysis was performed on the blot by using F4C1 mAb to visual arrestin (7) (Left) and an mAb (Transduction Laboratories) generated against rat β-arrestin-1 (Right). Molecular weight markers are indicated at the left. Results for each blot are representative of at least duplicate experiments.
Effect of Neutralizing Arrestin Antibody on Desensitization of hCG-Stimulated AC Activity.
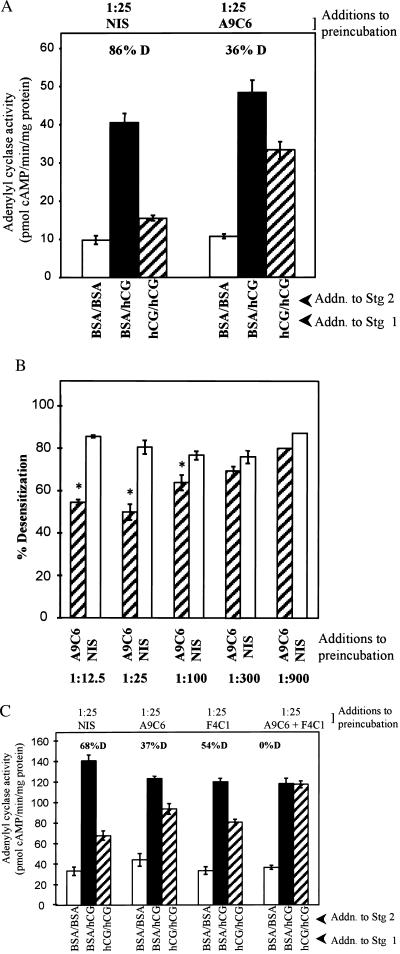
To investigate the role of arrestin in regulating desensitization of the LH/CG R, follicular membranes were preincubated with neutralizing arrestin antibody followed by the two-stage AC incubation, which consists of a 40-min incubation (stage 1 reaction) under conditions that promote development of desensitization of hCG-stimulated AC activity (+hCG) or do not promote desensitization (+BSA) and a subsequent 5-min AC assay (±hCG). The monoclonal arrestin antibody A9C6, made to bovine visual arrestin, recognizes an epitope (VFEEFARHNLK) in the C terminus of visual arrestin (49) homologous among mammalian β-arrestins (7). When preincubation with follicular membranes was conducted with NIS (1:25 dilution in final assay volume), hCG promoted an 86% desensitization of hCG-stimulated AC activity relative to basal AC activity (Fig. 2A, compare BSA/hCG with hCG/hCG), in agreement with previous results (33). Preincubation with A9C6 arrestin antibody (at a final dilution of 1:25) promoted a significant (P < 0.05) increase in hCG-stimulated AC activity when hCG was present in stage 1 (hCG-desensitized AC activity; Fig. 2A, hatched bar). The neutralizing arrestin antibody yielded a concentration-dependent reduction in the extent of desensitization of hCG-stimulated AC activity compared with equivalent concentrations of NIS (Fig. 2B). Up to 50% less desensitization was observed when membranes were preincubated with antibody at a concentration of 1:25 compared with incubation with NIS; however, higher antibody concentrations did not yield less desensitization. Since arrestins may require both C- and N-terminal domains in the process of recognition of an active form of the R (41), we considered the possibility that complete reversal of desensitization could be achieved by combining C-terminal-directed A9C6 antibody with N-terminal-directed antibody to visual arrestin, F4C1. Results in Fig. 2C show that while A9C6 and F4C1 individually led to a partial reversal of desensitization, both antibodies combined resulted in complete reversal of desensitization of hCG-stimulated AC activity. These results suggest that endogenous β-arrestin participates in desensitization of the LH/CG R.
Figure 2.
Effect of pretreatment of porcine follicular membranes with neutralizing arrestin antibodies on desensitization of hCG-stimulated AC activity. (A) Membranes (≈30 μg) were subjected to a preincubation (15 min at room temperature; 1 h at 4°C) with indicated final concentrations (in 50 μl reaction volume) of arrestin antibody A9C6 or NIS, followed by the two-stage desensitization reaction at 30°C, which consisted of a 40-min stage 1 desensitization incubation followed by a 5-min AC assay. Figure shows basal (BSA/BSA), full hCG-stimulated (BSA/hCG), and hormone-desensitized hCG-sensitive AC activity (hCG/hCG). Results are means ± SEM of quadruplicate determinations of a single assay and are representative of four separate experiments. Percentage of desensitization (% D) represents the reduction in hCG-stimulated AC activity when incubations contained hCG in stage 1 compared with activity measured with BSA in stage 1 over basal (BSA/BSA) AC activities. (B) Composite effect of different concentrations of arrestin antibody A9C6 or NIS at indicated final concentrations on percentage of desensitization of hCG-stimulated AC activity. Results are means ± SEM of four separate experiments. Remaining details are as in A. ∗, Differences between percentage desensitization values with arrestin antibody and NIS are significantly differently (P < 0.05). (C) Membranes were preincubated with indicated final concentrations of NIS, A9C6, F4C1, or both A9C6 and F4C1, as in A. Results are means ± SEM of quadruplicate determinations in a single assay.
Effect of Synthetic Peptide Corresponding to Arrestin Antibody-Binding Site on Desensitization of hCG-Stimulated AC Activity.
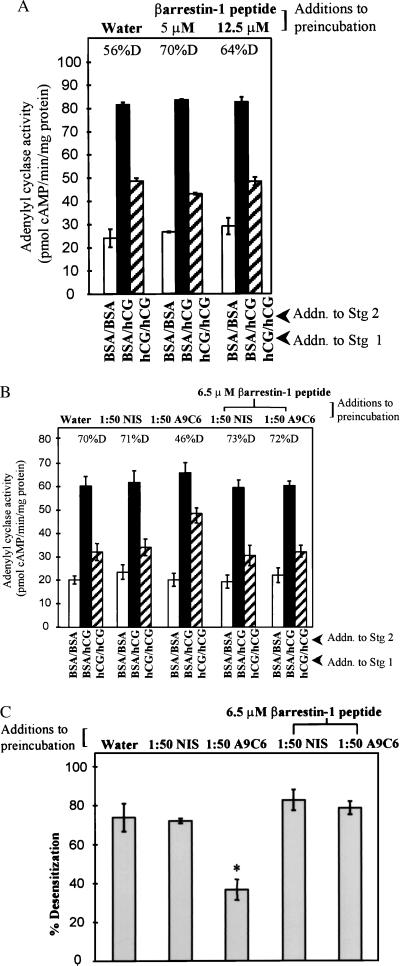
Studies were designed to determine whether the effect of arrestin antibody to block desensitization could be reversed with a synthetic peptide corresponding to the epitope on β-arrestin-1 to which arrestin antibody A9C6 binds (49). Addition of blocking β-arrestin-1 peptide alone in a two-stage AC incubation did not affect any parameter of AC activity measured when compared with water controls (Fig. 3A). Preincubation of the blocking β-arrestin-1 peptide (at 6.5 μM) with arrestin antibody A9C6 (1:50 final dilution) revived desensitization of hCG-stimulated AC activity to levels similar to those for NIS and water controls (Fig. 3 B, compare hatched bars, and C). Percentage of desensitization of hCG-stimulated AC activity was significantly less (P < 0.05) in membranes preincubated with A9C6 antibody, and desensitization was rescued with inclusion of the β-arrestin-1 peptide and A9C6 arrestin antibody (Fig. 3C). The ability of the synthetic peptide corresponding to the A9C6 antibody-binding domain of β-arrestin-1 to reverse the effect of arrestin antibody on LH/CG R desensitization is consistent with a role for an endogenous arrestin in desensitization of the LH/CG R.
Figure 3.
Effect of addition of a synthetic peptide corresponding to arrestin antibody-binding site on hCG-stimulated desensitization of porcine follicular membrane AC activity. (A) Membranes (≈30 μg) were preincubated with indicated amounts of synthetic β-arrestin-1 peptide or water and then subjected to the two-stage AC reaction. Results are mean ± SEM of quadruplicate determinations of a single experiment and are representative of two experiments. Full hCG-stimulated (solid bars) and desensitized hCG-stimulated (hatched bars) AC activities are significantly different (P < 0.05) for all treatments. (B) Membranes were preincubated with NIS or arrestin antibody A9C6 (1:50 dilution in final 50-μl reaction volume), 6.5 μM synthetic β-arrestin-1 peptide, or A9C6 or NIS (1:50 dilution in final 50-μl reaction volume) plus 6.5 μM synthetic arrestin peptide, as indicated, followed by the two-stage desensitization reaction. Results are means ± SEM of quadruplicate determinations of a single experiment and are representative of five experiments. Full hCG-stimulated AC activities (solid bars) for each treatment are not significantly different (P > 0.05). Desensitized hCG-stimulated AC activities (hatched bars) are significantly different (P < 0.05) from full hCG-stimulated AC activities (solid bars) for all treatments except for preincubation with arrestin antibody, where full and desensitized hCG-stimulated AC activities are not significantly different (P > 0.05). (C) Composite effect of synthetic β-arrestin-1 peptide to negate effect of arrestin antibody on desensitization of hCG-stimulated AC activity. Results are means ± SEM of five separate experiments. ∗, Significantly different from other values (P < 0.05).
Effect of Exogenous Arrestin Protein on Desensitization of hCG-Stimulated AC Activity.
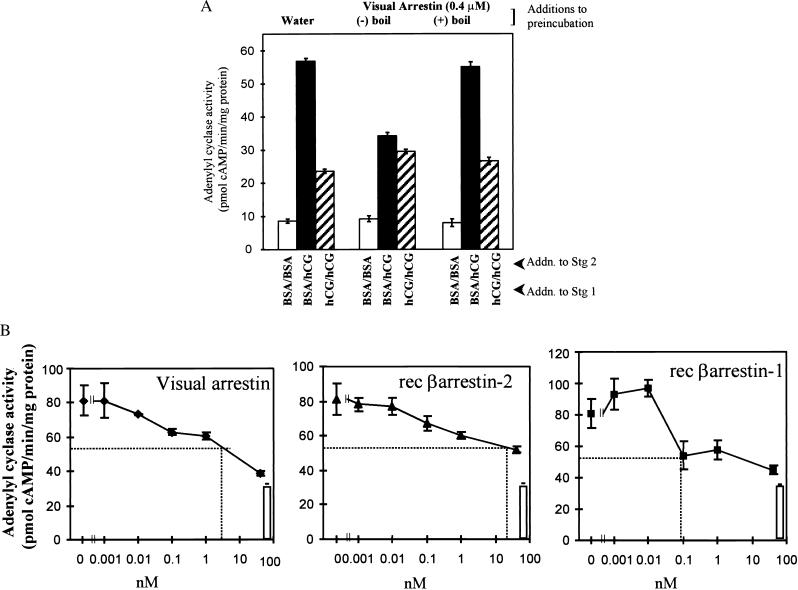
Based on the ability of neutralizing arrestin antibody to limit desensitization, the effect of purified visual arrestin on AC activity was investigated. Visual arrestin was added to membranes in a preincubation step followed by the two-stage AC reaction. Addition of arrestin significantly (P < 0.05) lowered full hCG-responsive AC activity (Fig. 4, compare solid bars) without affecting hormone-desensitized hCG-sensitive AC activity (Fig. 4, compare hatched bars). To evaluate the specificity of the effect of arrestin on follicular membrane desensitization, arrestin was subjected to boiling conditions for 10 min before preincubation with membranes. The effect of purified visual arrestin on full hCG-responsive AC activity was lost with boiling. The potency of arrestins to reduce hCG-stimulated AC activity was evaluated by using recombinant arrestins. These experiments were conducted in the presence of AMP-PNP, a nonhydrolyzable ATP analog that blocks GRK-dependent R phosphorylation (50), to rule out R phosphorylation accomplished by the presence of adequate levels of endogenous ATP or the addition of ATP as a contaminant of assay reagents. Results in Fig. 4B show that rec β-arrestin-1 was the most potent (ED50 ≈ 0.1 nM) and reduced hCG-stimulated AC activity nearly to basal levels (Fig. 4B, open bar). Visual arrestin purified from bovine retina was as effective in reducing hCG-stimulated AC activity as rec β-arrestin-1 but required ≈30-fold-higher concentration. As least 500-fold-higher concentrations of rec β-arrestin-2 were required to promote half-maximal inhibition of hCG-stimulated AC activity. The higher affinity of β-arrestin-1 to reduce hCG-stimulated AC activity is consistent with the presence of β-arrestin-1 in porcine ovarian follicular membranes.
Figure 4.
Effect of addition of arrestins on desensitization of hCG-stimulated AC activity. (A) Membranes (≈30 μg protein) were preincubated (15 min at room temperature; 1 h at 4°C) with water or 0.4 μM visual arrestin that had been boiled (+boil) or not been boiled (−boil), as detailed in the legend to Fig. 2, then subjected to the two-stage AC desensitization incubation. Results are means ± SEM of quadruplicate determinations and are representative of two separate experiments. Desensitized hCG-sensitive AC activity (hatched bars) is significantly different (P < 0.05) from full hCG-stimulated AC activity (solid bars) in membranes incubated with water and boiled arrestin; these values are not significantly different (P > 0.05) for membranes incubated with arrestin that had not been boiled. (B) Membranes (≈30 μg) were preincubated, as in A, with indicated concentrations of purified visual arrestin or rec β-arrestins or water, and then subjected to two-stage desensitization incubation. Solid squares represent BSA in stage 1 and hCG in stage 2. Open bars represent BSA in stages 1 and 2 for water controls (i.e., basal AC activity). Desensitization incubation contained 50 μM AMP-PNP, and AC assay contained 1 mM AMP-PNP. Results are means ± SEM of quadruplicate determinations and are representative of two independent experiments. Dotted lines reflect ED50 values.
Follicular Membrane Arrestin Associates with Glycoprotein Hormone R.
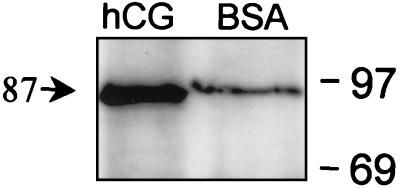
To determine whether LH/CG R activation promotes association of arrestin with the LH/CG R, membranes were subjected to stage 1 desensitization reaction, membrane proteins were then solubilized, and β-arrestin-1 was immunoprecipitated with β-arrestin-1 antibody. Results show that agonist-dependent LH/CG R activation promotes increased association of β-arrestin-1 with an 87-kDa protein reactive with a glycoprotein hormone R-specific antibody (42), consistent with the LH/CG R, upon immunoprecipitation of β-arrestin-1 (Fig. 5).
Figure 5.
Immunoprecipitation of β-arrestin-1. Follicular membranes (800 μg protein) were subjected to stage 1 desensitization incubation in the presence of BSA or hCG, as indicated (in the absence of [3H]cAMP); membrane proteins were solubilized, β-arrestin-1 was immunoprecipitated with β-arrestin-1 antibody, and immunoprecipitated proteins were separated by SDS/PAGE and transferred to Immobilon, then probed with glycoprotein hormone receptor-specific antibody mAb A7. Molecular weight markers are on the right. Molecular weight of indicated band was calculated by linear regression of the migration position of protein standards (30–97 kDa). Results are representative of three separate experiments.
DISCUSSION
Our results support the hypothesis that desensitization of LH/CG R-stimulated AC activity in porcine ovarian follicular membranes is mediated, at least in part, by an endogenous arrestin—likely β-arrestin-1. Neutralizing antibodies that recognize all arrestins disrupt desensitization, and desensitization is rescued by a synthetic peptide corresponding to the antibody-binding epitope, consistent with previous evidence that this synthetic peptide disrupts antibody binding to arrestins (49). These results are consistent with a role for β-arrestin-1 to bind to the ligand-activated LH/CG R and to participate in LH/CG R desensitization.
Neutralizing arrestin antibody promoted a concentration-dependent decrease in the extent of desensitization by preventing the reduction in hCG-stimulated AC activity when hCG was present during the desensitization reaction (hCG/hCG in stage 1/stage 2). These results suggest that β-arrestin-1 preferentially binds to the ligand-activated R. Consistent with this conclusion, increased amounts of an 87-kDa protein consistent with the LH/CG R coimmunoprecipitated with β-arrestin-1 after LH/CG R activation, suggesting that there is a physical association between arrestin and the LH/CG R (58).
There is now abundant evidence for a number of G protein-coupled Rs (6, 7), including a recent report for the LH/CG R, that an arrestin is required for R uncoupling from downstream effectors. For each of these Rs, the trigger for arrestin binding to the R and apparently for arrestin recruitment from the cytosol is R phosphorylation (6, 7). One to three moles of Pi/mole R are required for arrestins to recognize the phosphorylated forms of the β-adrenergic R and rhodopsin (10, 41). Arrestin is believed to bind through distinct binding regions in a constrained conformation with low affinity to intracellular regions of the activated R and in an unconstrained conformation with high affinity to GRK-catalyzed phosphorylated serine and threonine residues on the R (6). Single amino acid mutations in the phosphorylation recognition domain of arrestin result in a “constitutively active” arrestin mutant, which binds with high affinity to phosphorylated and unphosphorylated ligand-activated Rs (51–53). Consistent with evidence that arrestins bind albeit with a lower affinity to unphosphorylated, ligand-activated Rs (9, 11, 39–41) is the report that overexpression of β-arrestin rescued sequestration of β-adrenergic R mutants lacking putative GRK- and cAMP-dependent protein kinase phosphorylation sites or C-terminal tails (54).
The LH/CG R in ovarian follicular membranes therefore presents a unique model system in which the primary trigger for arrestin binding to the LH/CG R appears to be R activation. It also appears to be unique that an adequate concentration of β-arrestin-1 to promote R desensitization is associated with AC-rich ovarian follicular membranes, as arrestins are localized to soluble fraction in other cells (6, 7, 50, 55). Only visual arrestin lacking 30–40 C-terminal residues (generated by proteolysis, or the naturally occurring 44-kDa splice variant) binds tightly to membranes (56). We do not know whether the follicular β-arrestin-1 represents a unique variant of β-arrestin-1, two of which have been described (57), but, based on the size of the follicular β-arrestin-1, it does not appear to be lacking a significant number of amino acid residues. In summary, these results constitute a report for a physiological role for an arrestin to promote desensitization of a G protein-coupled R to mediate desensitization.
Acknowledgments
This work was supported by National Institutes of Health Grants P01 HD 21921 (M.H.-D.), EY 09339 (K.P.), GM 47417 (J.L.B.), and EY 11500 (V.G.). J.B.L. is an established Investigator of the American Heart Association.
ABBREVIATIONS
- LH/CG
luteinizing hormone/choriogonadotropin
- NIS
nonimmune serum
- AMP-PNP
adenylyl-imidodiphosphate
- GRKs
G protein-coupled receptor kinases
- AC
adenylyl cyclase
- rec
recombinant
- R
receptor
- hCG
human chorionic gonadotrophin
Note
While this manuscript was in preparation, Lazari et al. (58) reported a role for an arrestin in ligand-stimulated LH/CG R internalization but not in uncoupling of ligand-activated R from cAMP synthesis in a heterologous expression system.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Dohlman H G, Thorner J, Caron M G, Lefkowitz R J. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 2.Guderman T, Birnbaumer M, Birnbaumer L. J Biol Chem. 1992;267:4479–4488. [PubMed] [Google Scholar]
- 3.Loosfelt H, Misrahi M, Atger M, Salesse R, Vuhai-Luuthi M T, Jolivet A, Guiochon-Mantel A, Sar S, Jallal B, Garnier J, Milgrom E. Science. 1989;245:525–528. doi: 10.1126/science.2502844. [DOI] [PubMed] [Google Scholar]
- 4.McFarland K C, Sprengel R, Phillips H S, Kohler M, Rosembilt N, Nikolics K, Segaloff D L, Seeburg P H. Science. 1989;245:494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 6.Ferguson S S G, Barak L S, Zhang J, Caron M G. Can J Physiol Pharmacol. 1996;74:1094–1110. doi: 10.1139/cjpp-74-10-1095. [DOI] [PubMed] [Google Scholar]
- 7.Palczewski K. Protein Sci. 1994;3:1355–1361. doi: 10.1002/pro.5560030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilden U, Hall S W, Kuhn H. Proc Natl Acad Sci USA. 1986;83:1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohse M J, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure J-P, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 10.Gurevich V V, Benovic J L. J Biol Chem. 1993;268:11628–11638. [PubMed] [Google Scholar]
- 11.Gurevich V V, Richardson R M, Kim C M, Hosey M M, Benovic J L. J Biol Chem. 1993;268:16879–16882. [PubMed] [Google Scholar]
- 12.Gurevich V V, Benovic J L. J Biol Chem. 1992;267:21919–21923. [PubMed] [Google Scholar]
- 13.Marsh J M, Mills T M, Lemaire W J. Biochem Biophys Acta. 1973;304:197–202. doi: 10.1016/0304-4165(73)90128-1. [DOI] [PubMed] [Google Scholar]
- 14.Hunzicker-Dunn M, Birnbaumer L. Endocrinology. 1976;99:185–197. doi: 10.1210/endo-99-1-185. [DOI] [PubMed] [Google Scholar]
- 15.Hunzicker-Dunn M, Birnbaumer L. Endocrinology. 1976;99:198–210. doi: 10.1210/endo-99-1-198. [DOI] [PubMed] [Google Scholar]
- 16.Hunzicker-Dunn M. Biol Reprod. 1981;24:279–286. doi: 10.1095/biolreprod24.2.279. [DOI] [PubMed] [Google Scholar]
- 17.Lamprecht S A, Zor U, Koch Y, Ahren K, Lindner H R. J Cyclic Nucleotide Res. 1977;3:69–83. [PubMed] [Google Scholar]
- 18.Hunzicker-Dunn M, Birnbaumer L. Endocrinology. 1981;109:345–351. doi: 10.1210/endo-109-2-345. [DOI] [PubMed] [Google Scholar]
- 19.Ekstrom R C, Hunzicker-Dunn M. Endocrinology. 1989;124:956–963. doi: 10.1210/endo-124-2-956. [DOI] [PubMed] [Google Scholar]
- 20.Lamprecht S A, Zor U, Tsafriri A, Lindner H R. J Endocrinol. 1973;57:217–233. doi: 10.1677/joe.0.0570217. [DOI] [PubMed] [Google Scholar]
- 21.Zor U, Lamprecht S A, Misulovin Z, Koch Y, Lindner H R. Biochem Biophys Acta. 1976;428:761–765. doi: 10.1016/0304-4165(76)90206-3. [DOI] [PubMed] [Google Scholar]
- 22.Rao M C, Richards J S, Midgley A R, Reichert L E. Endocrinology. 1977;101:512–523. doi: 10.1210/endo-101-2-512. [DOI] [PubMed] [Google Scholar]
- 23.Amsterdam A, Berkowitz A, Nimrod A, Kohen F. Proc Natl Acad Sci USA. 1980;77:3440–3444. doi: 10.1073/pnas.77.6.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwall R H, Erickson G F. Endocrinology. 1984;114:1114–1123. doi: 10.1210/endo-114-4-1114. [DOI] [PubMed] [Google Scholar]
- 25.Meduri G, Vu Hai M T, Takemori S, Kominami S, Draincourt M A, Milgrom E. J Endocrinol. 1996;148:435–446. doi: 10.1677/joe.0.1480435. [DOI] [PubMed] [Google Scholar]
- 26.Bockaert J, Hunzicker-Dunn M, Birnbaumer L. J Biol Chem. 1976;251:2653–2663. [PubMed] [Google Scholar]
- 27.Ezra E, Salomon Y. J Biol Chem. 1980;255:653–658. [PubMed] [Google Scholar]
- 28.Ezra E, Salomon Y. J Biol Chem. 1981;256:5377–5382. [PubMed] [Google Scholar]
- 29.Ekstrom R C, Hunzicker-Dunn M. Endocrinology. 1989;125:2470–2474. doi: 10.1210/endo-125-5-2470. [DOI] [PubMed] [Google Scholar]
- 30.Ekstrom R C, Hunzicker-Dunn M. Endocrinology. 1990;126:1191–1198. doi: 10.1210/endo-126-2-1191. [DOI] [PubMed] [Google Scholar]
- 31.Ekstrom R C, Carney E M, Lamm M L G, Hunzicker-Dunn M. J Biol Chem. 1992;267:22183–22189. [PubMed] [Google Scholar]
- 32.Lamm M L G, Hunzicker-Dunn M. Mol Endocrinol. 1994;8:1537–1546. doi: 10.1210/mend.8.11.7877622. [DOI] [PubMed] [Google Scholar]
- 33.Ekstrom R C, Hunzicker-Dunn M. Endocrinology. 1990;127:2578–2586. doi: 10.1210/endo-127-5-2578. [DOI] [PubMed] [Google Scholar]
- 34.Vouret-Craviari V, Auberger P, Pouyssegur J, Van Obberghen-Schilling E. J Biol Chem. 1995;270:4813–4821. doi: 10.1074/jbc.270.9.4813. [DOI] [PubMed] [Google Scholar]
- 35.Roettger B F, Rentsch R U, Hadac E M, Hellen E H, Burghardt T P, Miller L J. J Cell Biol. 1995;130:579–590. doi: 10.1083/jcb.130.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtmann M H, Roettger B F, Pinon D I, Miller L J. J Biol Chem. 1996;271:23566–23571. doi: 10.1074/jbc.271.38.23566. [DOI] [PubMed] [Google Scholar]
- 37.Allgeier A, Laugwitz K L, Van Sande J, Schultz G, Dumont J E. Mol Cell Endocrinol. 1997;127:81–90. doi: 10.1016/s0303-7207(96)03996-2. [DOI] [PubMed] [Google Scholar]
- 38.Lamm M L G, Ekstrom R C, Maizels E T, Rajagopalan R M, Hunzicker-Dunn M. Endocrinology. 1994;134:1745–1754. doi: 10.1210/endo.134.4.8137739. [DOI] [PubMed] [Google Scholar]
- 39.Pulvermuller A, Maretzki D, Rudnicka-Nawrot M, Smith W C, Palczewski K, Hofmann K P. Biochemistry. 1997;36:9253–9260. doi: 10.1021/bi970772g. [DOI] [PubMed] [Google Scholar]
- 40.Gurevich V V, Chen C, Kim C M, Benovic J L. J Biol Chem. 1994;269:8721–8727. [PubMed] [Google Scholar]
- 41.Gurevich V V, Dion S B, Onorato J J, Ptasienski J, Kim C M, Sterne-Marr M, Hosey M M, Benovic J L. J Biol Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson L B, Vlase H, Graves P, Nilsson M, Hyang G C, Morgenthaler N G, Davies T F, MacGregor A M, Banga J P. J Mol Endocrinol. 1996;16:159–170. doi: 10.1677/jme.0.0160159. [DOI] [PubMed] [Google Scholar]
- 43.Palczewski K, Hargrave P A. J Biol Chem. 1991;266:4201–4206. [PubMed] [Google Scholar]
- 44.Gurevich V V, Pals-Rylaarsdam R, Benovic J L, Hosey M M, Onorato J J. J Biol Chem. 1997;272:28849–289852. doi: 10.1074/jbc.272.46.28849. [DOI] [PubMed] [Google Scholar]
- 45.Salomon Y, Londos C, Rodbell M. Anal Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 46.Rajagopalan-Gupta R M, Lamm M L, Mukherjee S, Rasenick M M, Hunzicker-Dunn M. Endocrinology. 1998;139:4547–4555. doi: 10.1210/endo.139.11.6302. [DOI] [PubMed] [Google Scholar]
- 47.Rajagopalan-Gupta R M, Rasenick M M, Hunzicker-Dunn M. Mol Endocrinol. 1997;11:538–549. doi: 10.1210/mend.11.5.9929. [DOI] [PubMed] [Google Scholar]
- 48.Bender F E, Douglass L W, Kramer A. Statistical Methods for Food and Agriculture. Westport, CT: AVI Publishing; 1982. pp. 87–107. [Google Scholar]
- 49.Knospe V, Donoso L A, Banga J P, Yue S, Kasp E, Gregerson D S. Eye Res. 1988;7:1137–1147. doi: 10.3109/02713688809001885. [DOI] [PubMed] [Google Scholar]
- 50.Benovic J L, Kuhn H, Weyand I, Codina J, Caron M G, Lefkowitz R J. Proc Natl Acad Sci USA. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurevich V V, Benovic J L. Mol Pharmacol. 1997;51:161–169. doi: 10.1124/mol.51.1.161. [DOI] [PubMed] [Google Scholar]
- 52.Gray-Keller M P, Detwiler P B, Benovic J L, Gurevich V V. Biochemistry. 1997;36:7058–7063. doi: 10.1021/bi963110k. [DOI] [PubMed] [Google Scholar]
- 53.Gurevich V V, Benovic J L. J Biol Chem. 1995;270:6010–6016. doi: 10.1074/jbc.270.11.6010. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson S S G, Downey W E, III, Colapietro A-M, Barak L S, Menard L, Caron M G. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 55.Barak L S, Ferguson S S, Zhang J, Caron M G. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- 56.Smith W C, Milar A H, Dugger D, Arendt A, Hargrave P A, Palczewski K. J Biol Chem. 1994;269:15407–15410. [PubMed] [Google Scholar]
- 57.Parruti G, Peracchia F, Sallese M, Ambrosini G, Masini M, Rotilio D, DeBlasi A. J Biol Chem. 1993;268:9753–9761. [PubMed] [Google Scholar]
- 58.Lazari M D F M, Bertrand J E, Nakamura K, Liu X, Krupnick J G, Benovic J L, Ascoli M. J Biol Chem. 1998;273:18316–18324. doi: 10.1074/jbc.273.29.18316. [DOI] [PubMed] [Google Scholar]