Abstract
Central to inflammatory responses are the integrin-mediated adhesive interactions of cells with their ECM-rich environment. We investigated the role of the collagen-binding integrin α1β1 in intestinal inflammation using the mouse model of colitis induced by dextran sodium sulfate (DSS). mAb’s directed against murine α1 were found to significantly attenuate inflammation and injury in DSS-treated wild-type mice; similar protection was seen in mice deficient for α1β1 integrin. Blockade or loss of α1β1 was also associated with decreased mucosal inflammatory cell infiltrate and cytokine production. Importantly, we demonstrated that development and α1-mediated inhibition of DSS-induced colitis occurred independently of lymphocytes (Rag-2–/– mice), and identified the monocyte as a key α1β1-expressing cell type involved in the development of colitis in this model. In response to DSS, both α1 deficiency and anti-α1 mAb treatment significantly reduced monocyte accumulation and activation within the lamina propria. In summary, the data demonstrate that engagement of leukocyte-associated α1β1 receptors with ECM plays a pivotal role in mediating intestinal inflammation via promotion of monocyte movement and/or activation within the inflamed interstitium. Therapeutic strategies designed to disrupt such interactions may prove beneficial in treating intestinal inflammation.
Introduction
Human inflammatory bowel diseases (e.g., Crohn disease, ulcerative colitis) are associated with chronic, relapsing inflammation of the intestinal tract of unknown origin (1). Tissue obtained from patients with inflammatory bowel disease is characterized by a dense leukocyte infiltrate that may play an important role in the pathophysiology of inflammatory tissue injury (2). This leukocyte accumulation is a cardinal histopathologic feature of acute and chronic inflammatory diseases of the gastrointestinal tract. Leukocyte–endothelial cell interactions that result in recruitment of circulating cells into areas of inflammation are now recognized to represent an early and rate-limiting step in leukocyte-mediated tissue injury (3). However, this initial adhesive event is followed by ano-ther critical step that occurs within the perivascular compartment and has yet to be fully explored. Emigrated and residing cells must migrate along a chemotactic signal toward the site of infection/injury, identify the offending antigen, and undergo activation to perform their respective cell-specific functions.
Members of the integrin family of molecules mediate cell adhesion to ECM proteins such as collagen, fibronectin, and laminin (4). Recent studies suggest that interactions between leukocyte-associated integrins and the interstitial matrix may promote the migration and/or activation of extravasated leukocytes (e.g., T cells and monocytes) within the perivascular compartment (5, 6). The ECM-rich environment of perivascular tissue is thought to be an important site where certain human leukocytes undergo differentiation (e.g., monocytes) and activation (neutrophils, monocytes, lymphocytes) upon extravascular migration (7). The α1β1 integrin is a major cell surface receptor for collagens with a preference for type IV collagen (8). Expression of α1β1 in the adult is largely confined to mesenchymal cells, notably smooth muscle cells, fibroblasts, stellate cells, hepatocytes, and microvascular endothelium (9–12). While little detectable α1β1 is expressed on resting leukocytes, α1β1 expression is induced on the surface of T lymphocytes and monocytes upon activation (4, 13). Expression of α1β1 has also been demonstrated on tissue-infiltrating T cells from a variety of chronic inflammatory settings, including the synovium of rheumatoid arthritis patients, lung tissue of sarcoidosis patients, and atherosclerotic plaques (4, 14). The importance of α1β1 interaction with the ECM-rich environment of peripheral tissue was recently demonstrated in several in vivo models of inflammation outside the gut (e.g., hypersensitivity and arthritis) (5, 15).
The presumed role of the ECM environment in the inflammatory process, and the fact that chronically activated immune cells can express this integrin, led us to the hypothesis that α1β1 integrin may contribute to the pathophysiology of intestinal inflammation. Therefore we wanted to assess whether α1β1-mediated interactions, which occur in the perivascular compartment independently of the adhesive interactions responsible for leukocyte recruitment at the endothelium, contribute to the pathophysiology of mucosal injury and inflammation in the dextran sodium sulfate (DSS) mouse model of colitis.
In this report, we found that treatment of DSS-exposed wild-type (WT) mice with a blocking anti-α1 mAb resulted in significant attenuation of colitis, and that similar protection was conferred to DSS-exposed mice lacking α1β1 integrin. Blockade of α1β1 was also associated with decreased mucosal inflammatory cell infiltrate and cytokine production. Importantly, we demonstrated that both induction of DSS-induced colitis and α1-mediated inhibition of colitis occurred independently of lymphocytes, and identified the monocyte as a key α1β1-expressing cell type involved in the development of colitis in this model. Given that α1β1 represents a main collagen-binding integrin expressed on leukocytes, our study highlights the important role ECM-leukocyte interactions play in the pathogenesis of colitis, with particular relevance to monocytes.
Methods
Mice.
Six- to eight-week-old male BALB/c and recombinase-activating gene-2 knockout mice [Rag2–/– mice; C.129(B6)-Rag2tmlN12] were purchased from Taconic Farms (Germantown, New York, USA). α1 integrin knockout mice (α1–/–) on a BALB/c background were obtained as previously described (16).
Induction of colitis.
Mice were fed 5% (wt/vol) DSS (molecular weight, 40 kDa; ICN Biomedicals Inc., Aurora, Ohio, USA) dissolved in water that was filtered using water purification systems from Millipore Corp. (Bedford, Massachusetts, USA) for 7 days (17). No mortality was observed during the 7 days of DSS administration.
mAb’s.
Function-blocking mAb against murine α1 integrin chain Ha31/8 (hamster anti-α1) (18) and the hamster isotope control mAb Ha4/8 (hamster anti-KLH) (18) were prepared in an azide-free and low-endotoxin format. Two hundred micrograms of mAb was administered intraperitoneally 12 hours prior to induction of DSS-colitis; this dose was repeated every 48 hours for the duration of the experiment. For immunohistochemistry, Ha31/8 and Ha4/8 mAb’s were fluorescently labeled using the Alexa 488 protein labeling kit as recommended (Molecular Probes Inc., Eugene, Oregon, USA). FITC- and phycoerythrin-conjugated (PE-conjugated) mAb’s 145-2C11 (hamster anti-CD3e), M1/70 (rat anti-CD11b), rmC5-3 (rat anti-CD14), and RB6-8C5 (rat anti–Ly-6G/Gr-1) were from Pharmingen (San Diego, California, USA); PE-conjugated CI:A3-1 (rat anti-F4/80) was from Serotec Ltd. (Kidlington, United Kingdom).
Assessment of inflammation in DSS-treated mice.
Daily clinical assessment of DSS-treated animals included measurement of drinking volume and body weight, evaluation of stool consistency, and the presence of blood in the stools by a guaiac paper test (ColoScreen; Helena Laboratories Corp., Beaumont, Texas, USA). A validated clinical disease activity index (19) ranging from 0 to 4 was calculated using the following parameters: stool consistency, presence or absence of fecal blood, and weight loss. Mice were sacrificed at day 7 and the colons were removed; length and weight were measured after exclusion of the cecum and prior to division for histology, myeloperoxidase activity, and RNA extraction.
Histology.
Histology was performed on three samples of distal colon for each animal. Samples were fixed in Zamboni solution prior to embedding in JB-4 (Polysciences Inc., Warrington, Pennsylvania, USA) and staining with hematoxylin and eosin. All histologic quantitation was performed in a blinded fashion using a validated scoring system (20). Three independent parameters were measured: severity of inflammation (0–3: none, slight, moderate, severe); depth of injury (0–3: none, mucosal, mucosal and submucosal, transmural); and crypt damage (0–4: none, basal one-third damaged, basal two-thirds damaged, only surface epithelium intact, entire crypt and epithelium lost). The score of each parameter was multiplied by a fac-tor reflecting the percentage of tissue involvement (×1: 0–25%, ×2: 26–50%, ×3: 51–75%, ×4: 76–100%) and these values were summed to obtain the histology score (maximum possible score, 40). In addition, the percentage of ulcerated mucosal surface was measured by computer-aided morphometric analysis (MetaMorph; Universal Imaging Corp., West Chester, Pennsylvania, USA). Images of three cross sections of the distal colonic wall were captured via an ECLIPSE E600 upright light microscope (Nikon Inc., Melville, New York, USA) coupled to a SenSys CCD camera system (Roper Scientific Inc., Trenton, New Jersey, USA). The ulcerated mucosal surface was defined by loss of mucosal epithelium and expressed as percentage of the total luminal perimeter for each section.
For immunohistochemical staining, freshly isolated colonic tissue from the distal portion of the colon was frozen in dry ice using OCT compound (Sakura Finetek, Torrance, California, USA). Acetone-fixed frozen sections (10-μm thick) were blocked in a 3% BSA/PBS solution for 30 minutes at room temperature. Slides were washed and sections were incubated with 5 μg/ml of Alexa 488–labeled anti-α1 mAb in 3% BSA/PBS for 1 hour at room temperature. Slides were then washed in PBS and mounted in Citifluor (Ted Pella Inc., Redding, California, USA). Dual-color immunohistochemistry was performed with the inclusion of PE-conjugated mAb during the primary staining step. The stained sections were examined by dual immunofluorescent microscopy (Leica Microsystems AG, Wetzlar, Germany).
The number and activation state of monocytes/macrophages infiltrating the lamina propria was quantitated by dual-color immunohistochemical analysis using FITC-conjugated anti-CD14 and PE-conjugated anti-F4/80 mAb’s. The numbers of F4/80+ and F4/80+CD14+ cells per high-power field (hpf) observed following different treatment regimens were counted by two independent observers. A total of eight randomly selected hpf’s per animal were counted, with five animals analyzed per group.
Tissue myeloperoxidase activity.
Samples of mid-to-distal colon (adjacent to tissue used for histology) were obtained from either control or DSS-treated animals, rinsed with cold PBS, blotted dry, and immediately frozen in liquid nitrogen. The samples were stored at –80°C until being thawed for myeloperoxid-ase activity determination using the O-dianisidine method previously described (21). Myeloperoxidase activity was expressed as the amount of enzyme necessary to produce a change in absorbance of 1.0 per minute per gram wet weight of tissue.
RNA extraction.
Segments from the distal colon (five per group) were snap frozen and stored at –80°C. Total cellular RNA was isolated by homogenizing tissue in TRIzol (Gibco BRL, Life Technologies, Paisley, United Kingdom). RNA was then chloroform-extracted and isopropanol was precipitated. The yield and purity of RNA was determined by spectroscopic analysis; RNA was stored at –80°C until use.
RNase protection assay.
Cytokine mRNA levels were analyzed by RNase protection assay using the RiboQuant multiprobe set (Pharmingen) as described (22). In brief, RNA obtained from distal colons was hybridized overnight to the 32P-labeled RNA probe from a customized mouse template set (Pharmingen). Protected RNA was analyzed on a 6% denaturing polyacrylamide gel and quantitated using a PhosphorImager (Storm 860) and ImageQuant 5.0 software (both from Molecular Dynamics, Sunnyvale, California, USA). Cytokine values were expressed as fold increase over the mean values for healthy control colon tissue corrected for the housekeeping gene GAPDH for each condition/gel lane.
Data analysis.
Statistical analyses were performed using one-way ANOVA with Scheffé’s post hoc test or the Kruskal-Wallis test when appropriate. Two-way ANOVA for repeated measures was used to test for group and time effects on the clinical data (e.g., disease activity index) over the 7 successive days of clinical observation. A P value below 0.05 was considered significant.
Results
Anti-α1 integrin mAb protects from colitis associated with DSS administration.
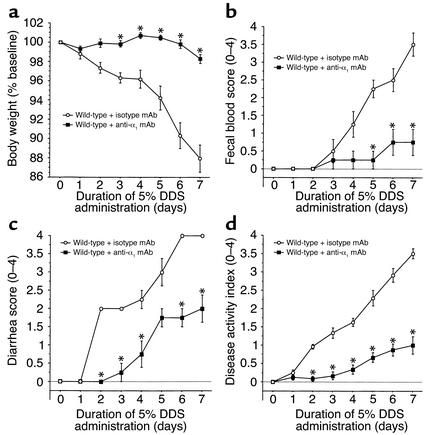
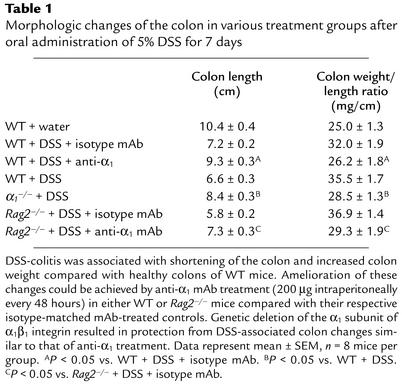
To evaluate the role of α1β1 in DSS-mediated colitis (17), anti-α1 or control mAb administration was initiated 12 hours prior to DSS exposure in the drinking water (for 7 days) of WT mice; mAb administration was repeated every 48 hours thereafter for the duration of the study. DSS administration in isotype control mAb–treated WT mice was associated with significant clinical changes, including weight loss (starting on day 2), the appearance of occult fecal blood (on day 3), and diarrhea (on day 4) (Figure 1). In contrast, anti-α1 mAb administration resulted in a significant amelioration in the severity of DSS-colitis by day 7, as shown by a 71% reduction in the clinical disease activity index (Figure 1). The improvement in disease parameters characteristically associated with DSS-induced inflammation such as colon shortening and colon weight gain confirmed the observed protection (Table 1).
Figure 1.
Treatment with anti-α1 mAb prevents DSS-mediated colitis. Mice were fed DSS over 7 days and treated with mAb (control or anti-α1) every second day starting at day 0. Disease severity was measured daily and is expressed in terms of (a) body weight, (b) fecal blood, (c) diarrhea, and (d) disease activity index (combined index of a + b + c). The isotype mAb-treated group exhibited no significant differences from WT mice receiving DSS alone (see Figure 2). *P < 0.05; anti-α1 mAb vs. isotype mAb. Data represent mean ± SEM.
Table 1.
Morphologic changes of the colon in various treatment groups after oral administration of 5% DSS for 7 days
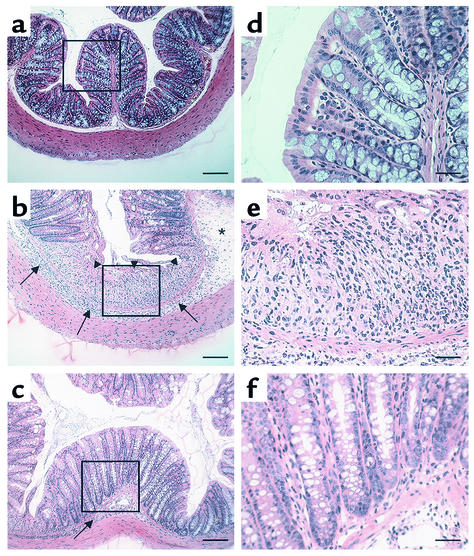
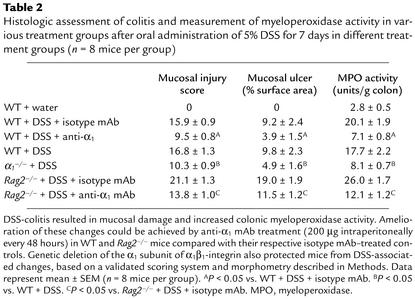
Histologic analysis of the inflamed colons confirmed the ability of anti-α1 mAb to dramatically improve disease (Figure 2). Compared with colons from mice given regular water (Figure 2, a and d), mice receiving DSS in their drinking water for 7 days showed obvious signs of colonic inflammation and tissue destruction (Figure 2, b and e). Colons from DSS-treated mice showed extensive cellular infiltrate, submucosal edema, and large areas of epithelial erosion. In contrast, histologic analysis of colons from DSS-treated mice receiving anti-α1 mAb showed greatly reduced numbers of infiltrating cells; these mice were largely protected from DSS-associated mucosal injury and edema (Figure 2, c and f). Computerized morphometry quantified and confirmed that the degree of mucosal injury and ulceration caused by DSS was significantly reduced in the anti-α1 mAb–treated mice (Table 2). Consistent with the dramatic effects anti-α1 mAb treatment had on reducing the cellular infiltrate associated with DSS-induced colitis, neutrophil accumulation, as measured by colonic myeloperoxidase activity, was reduced by more than 75% in DSS-treated mice receiving anti-α1 mAb (Table 2).
Figure 2.
Treatment with anti-α1 mAb results in histologic improvement in DSS-induced colitis in WT mice. The experiment was carried out as described in the Figure 1 legend. Colons were excised after 7 days of DSS treatment and stained with hematoxylin and eosin. Tissue sections are from colons of WT mice given regular water (a and d); treated with DSS + isotype control mAb (b and e); and treated with DSS + anti-α1 mAb (c and f). Boxed regions in a–c are shown at higher magnification in d–f. DSS administration to WT mice (b and e) resulted in an extensive cellular infiltrate (arrows, also in c), submucosal edema (asterisk), and epithelial erosion (arrowheads). Treatment with anti-α1 mAb (c and f) almost completely inhibited leukocyte infiltration and protected from DSS-associated mucosal injury and edema. In a–c, magnification is ×100 and bar represents 100 μm. In d–f, magnification is ×400 and bar represents 25 μm.
Table 2.
Histologic assessment of colitis and measurement of myeloperoxidase activity in various treatment groups after oral administration of 5% DSS for 7 days in different treatment groups (n = 8 mice per group)
Mice lacking α1β1 integrin are similarly protected against DSS-induced colitis.
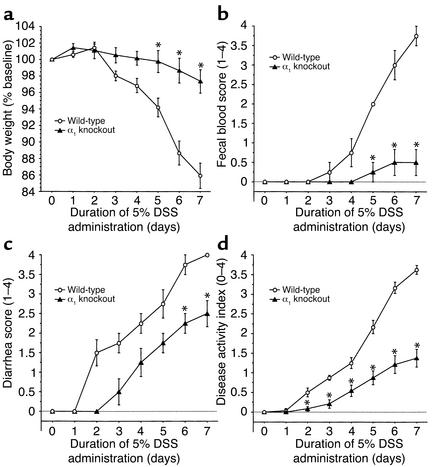
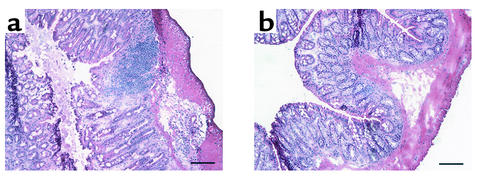
While the anti-α1 mAb used has been shown to be a highly specific function-blocking inhibitor of murine α1 (5), we wanted to confirm in an mAb-independent manner the importance of α1β1 in the DSS colitis model. The DSS colitis model was examined in mice that were deficient in the α1 subunit of α1β1 integrin. Consistent with the degree of inhibition seen with anti-α1 mAb, the severity of DSS-induced colitis was significantly reduced (62% inhibition) in α1 integrin–deficient mice (Figure 3). Other disease-associated parameters such as colon shortening and colon weight gain were reduced in the α1-deficient mice compared with WT mice; the degree of reduction in the α1-deficient mice was comparable to that seen in WT mice treated with anti-α1 mAb (Table 1). The improvement achieved by genetic disruption of α1 was confirmed histologically. Colons from DSS-treated α1-deficient mice showed decreased leukocyte infiltration and were protected from mucosal injury (Figure 4). The decreased ulceration, mucosal injury, and neutrophil infiltration seen in the DSS-treated α1-deficient mice was comparable to that seen with anti-α1 mAb treatment (Table 2).
Figure 3.
Genetic deletion of the α1 subunit of the murine α1β1 integrin protects mice against DSS-induced colitis. Colitis was induced in either BALB/c WT or α1-deficient BALB/c mice as described in Figure 1 legend. Disease severity was measured daily and is noted in terms of (a) body weight, (b) fecal blood, (c) diarrhea, and (d) disease activity index (combined index of a + b + c). α1–/– mice exhibited similar protection to that observed in mice treated with the function-blocking anti-α1 mAb (compare with Figure 1). *P < 0.05, α1–/– vs. WT. Data represent mean ± SEM.
Figure 4.
α1-deficient mice showed decreased leukocyte infiltration and were protected from DSS-associated mucosal injury. Shown are representative photomicrographs at ×100 magnification of colonic cross sections after hematoxylin and eosin staining from WT (a) or α1-deficient mice (b) treated for 7 days with DSS. Bar represents 100 μm.
Blockade of α1β1 results in decreased inflammatory cytokine mRNA expression in WT mice.
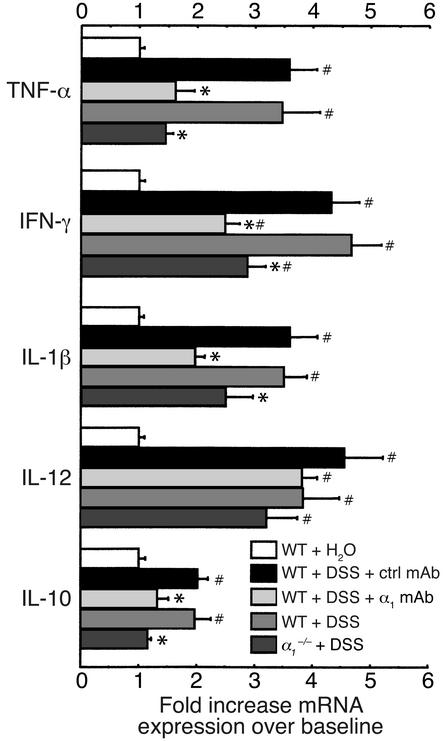
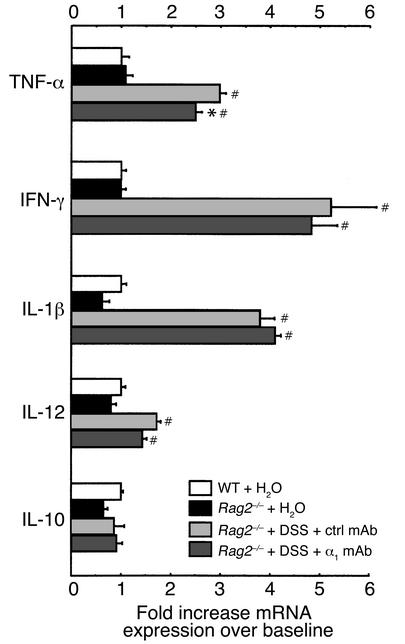
Since certain cytokines (such as TNF-α) also contribute to disease progression and tissue damage in human inflammatory bowel disease, we were interested in whether the α1-mediated protection observed in murine DSS colitis was reflected by changes in mRNA expression of Th1 and Th2 cytokines thought to be important in ulcerative colitis. Administration of DSS to WT mice led to significant upregulation of TNF-α, IFN-γ, IL-1β, IL-12, and IL-10 mRNA expression in the colon (Figure 5). Anti-α1 treatment or α1 deficiency was associated with a significant reduction in TNF-α, IFN-γ, IL-1β, and IL-10 mRNA expression (Figure 5).
Figure 5.
Blockade of α1 inhibits cytokine mRNA expression in DSS-treated murine colons. Levels of TNF-α, IFN-γ, IL-1β, IL-12, and IL-10 mRNA expression were measured in colons from WT or α1-deficient mice treated for 7 days with DSS. DSS-treated WT mice received either no mAb, isotype control mAb, or anti-α1 mAb. For quantitation, cytokine values are expressed as fold increase over the mean values obtained for healthy control colon tissue. *P < 0.05, anti-α1 mAb vs. isotype mAb and α1–/– vs. WT, respectively. #P < 0.05 vs. WT + H2O. Data represent mean ± SEM. ctrl, control.
Mediation of the protective effect by cells of the innate immune system.
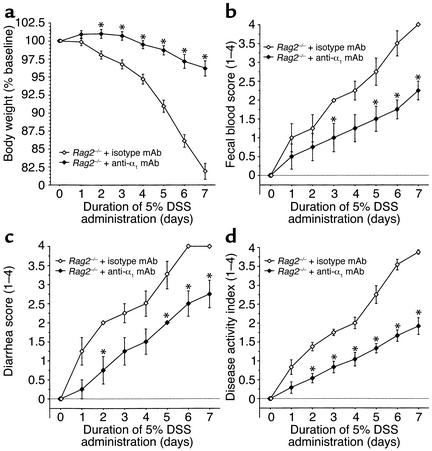
To address whether lymphocytes are important in DSS-colitis and to directly examine whether these cells are important in mediating the beneficial effects of α1 immunoneutralization, the effect of anti-α1 mAb treatment was tested in DSS-treated Rag2–/– mice (Figure 6, Figure 7, Table 1, and Table 2). Isotype control Ig–treated Rag2–/– mice responded to DSS administration with even more severe colitis than was seen in WT mice with DSS-colitis. Interestingly, when T cells and B cells were absent, the protective effect of anti-α1 mAb was maintained, suggesting that the inhibitory effect of α1 blockade is independent of lymphocytes and is most likely mediated by cells of the innate immune system. Colons of Rag2–/– mice exposed to DSS and treated with either control or anti-α1 mAb were also analyzed for induction of inflammatory cytokines (Figure 7). Exposure of Rag2–/– mice to DSS resulted in similar fold induction of TNF-α, IFN-γ, and IL-1β to that seen in WT mice. In contrast to DSS-exposed WT mice and consistent with their immunodeficient status, Rag2–/– mice showed decreased induction of IL-12 and no induction of IL-10 in response to DSS. Unlike what was seen in WT mice, anti-α1 mAb treatment of DSS-exposed Rag2–/– mice resulted in little change in inflammatory cytokine production, with the exception of TNF-α, for which a small but significant reduction was seen (Figure 7).
Figure 6.
Development and α1-mediated inhibition of DSS-induced colitis is independent of lymphocytes. Immunodeficient Rag2–/– mice were fed DSS over 7 days and treated with mAb (control or anti-α1) every second day starting at day 0. Disease severity was measured daily and is expressed in terms of (a) body weight, (b) fecal blood, (c) diarrhea, and (d) disease activity index (combined index of a + b + c). The isotype mAb-treated group exhibited an exaggerated course and severity of colitis compared with WT mice receiving either DSS alone or with the isotype-matched mAb (compare with Figure 1 and Figure 2). *P < 0.05, anti-α1 mAb vs. isotype mAb. Data represent mean ± SEM.
Figure 7.
Inflammatory cytokine mRNA expression in response to DSS is altered in Rag2–/–-immunodeficient mice compared with WT mice and is largely uninhibited by anti-α1 mAb treatment. Levels of TNF-α, IFN-γ, IL-1β, IL-12, and IL-10 mRNA expression were measured in colons from Rag2–/– mice exposed for 7 days to DSS and treated with either isotype control mAb or anti-α1 mAb. For quantitation and to allow comparison with Figure 5, cytokine values are expressed as fold increase over the mean values obtained for healthy control colon tissue from WT mice. *P < 0.05, anti-α1 mAb vs. isotype mAb; #P < 0.05 vs. Rag2–/– + H2O. Data represent mean ± SEM. ctrl, control.
Expression of α1β1 on lamina propria–infiltrating leukocytes and its effect on monocyte recruitment and activation.
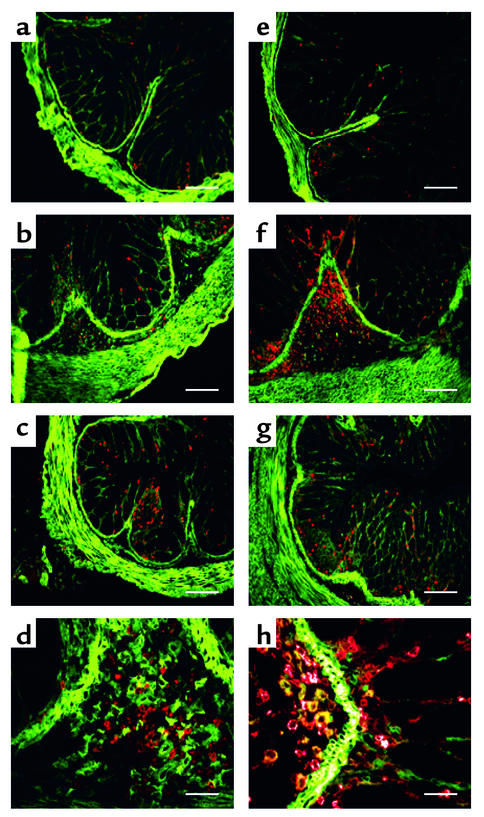
Given the fact that α1β1 is a prominent leukocyte-associated collagen-binding integrin, α1β1 may enhance intestinal inflammation by promoting migration and/or activation of extravasated leukocytes within the tissue interstitium. Since α1β1 integrin can be expressed on both activated T lymphocytes and mono-cytes (4, 5, 13), dual-color immunohistochemistry was performed on colons from DSS-treated mice to determine the nature of the infiltrating cells and whether they express α1β1 integrin (Figure 8). Colons were examined for α1β1 expression and cell lineage markers. Consistent with previous reports (9), α1β1 was highly expressed on smooth muscle cells within the colon. Colons from mice given normal drinking water showed very few infiltrating T cells (CD3+) or granulocytes/monocytes (CD11b+) (Figure 8, a and e, respectively). In contrast, mice with DSS-induced colitis showed extensive infiltration of granulocytes/monocytes, and a slight increase in the number of infiltrating T cells (Figure 8, b and f). Anti-α1 mAb treatment of mice with DSS-colitis resulted in an almost complete reduction in the number of infiltrating granulocytes/monocytes and T cells in the submucosa (Figure 8, c and g). Examination of α1β1 expression on the infiltrating cells revealed little expression on T cells, while a subset of CD11b+ granulocytes/monocytes expressed high levels of the α1β1 integrin (Figure 8, d and h).
Figure 8.
DSS-induced colitis results in accumulation of granulocytes/monocytes that is significantly inhibited by treatment with anti-α1 mAb. Immunohistochemical staining of colonic cross sections from WT mice receiving either regular water (a and e) or mice that were treated for 7 days with DSS in the absence (b, d, f, and h) or presence (c and g) of anti-α1 mAb. Dual immunofluorescent staining of colon tissue was performed with Alexa 488–conjugated anti-α1 mAb and either PE-conjugated anti-CD3 mAb (a–d) or anti-CD11b mAb (e–h). PE-conjugated mAb’s were specific for granulocytes/monocytes (anti-CD11b) and T lymphocytes (anti-CD3). No staining was seen with Alexa 488– and PE-conjugated isotype control mAb’s (not shown). In a–c and e–g, magnification is ×100 and bar represents 100 μm. In d and h, magnification is ×400 and bar represents 25 μm.
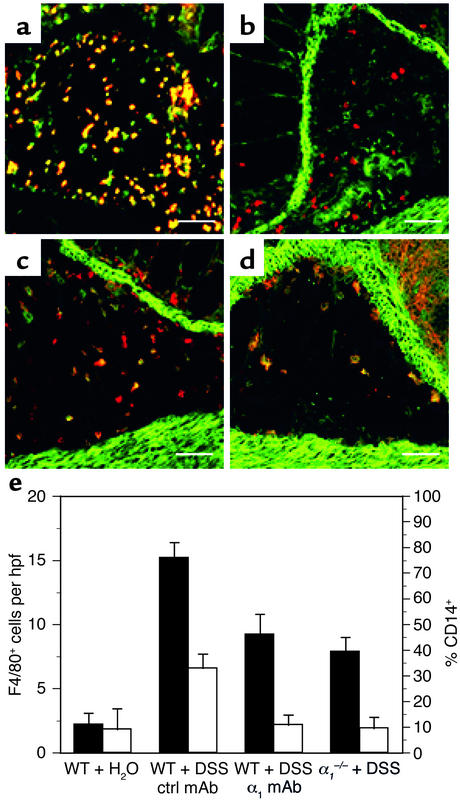
Further delineation of the CD11b+ population was possible using the granulocyte marker Gr1 (23) and the monocyte/macrophage marker F4/80 (24). Immunohistochemical analysis revealed expression of α1β1 on CD11b+ cells to be restricted to monocytes and macrophages as judged by its colocalization on F4/80+ and CD11b+Gr1– cells (Figure 9, a–d). Furthermore, immunohistochemical analysis using F4/80 and CD14 mAb’s was carried out to quantitate the effect of in vivo α1β1 blockade on the number and activation state of monocytes/macrophages infiltrating the lamina propria (Figure 9e). Treatment with DSS resulted in a fivefold increase over normal in the number of infiltrating F4/80+ cells found in the lamina propria, which was reduced 40–50% by α1β1 blockade by mAb or knockout. Likewise, blockade of α1β1 also affected the activation state of the infiltrating monocytes/macrophages. Upon exposure to DSS, roughly one-third of all F4/80+ cells in WT mice expressed the CD14 activation marker. The percentage of activated F4/80+ cells in the α1-deficient and anti-α1 mAb–treated mice receiving DSS was significantly reduced (to 10% of F4/80+ cells) and was comparable to the percentage of activated monocytes/macrophages seen under resting conditions (Figure 9e).
Figure 9.
Expression of α1β1 on CD11b+ cells is restricted to monocytes/macrophages, and in vivo blockade of α1β1 results in decreased number and activation state of monocytes/macrophages infiltrating the lamina propria. Colonic cross sections from WT mice that were treated for 7 days with DSS were stained with a combination of directly labeled mAb’s: (a) FITC anti-CD11b and PE anti-Gr1 mAb’s; (b) Alexa 488 anti-α1 and PE anti-Gr1 mAb’s; (c) Alexa 488 anti-α1 and PE anti-CD11b mAb’s; (d) Alexa 488 anti-α1 and PE anti-F4/80 mAb’s. Expression of α1β1 on CD11b+ cells was restricted to monocytes and macrophages as judged by its colocalization on F4/80+ and CD11b+Gr1– cells. Gr1 and F4/80 are granulocyte and monocyte/macrophage markers, respectively. The effect of in vivo α1β1 blockade on the number and activation state of the monocytes/macrophages infiltrating the lamina propria was quantitated by dual-color immunohistochemical analysis using F4/80 and CD14 mAb’s (e). The number of F4/80+ (black bars) cells per hpf and the percentage of CD14+ (white bars) F4/80 cells infiltrating the lamina propria following various treatment regimens (outlined in legends for Figure 1 and Figure 3) was quantitated. Data are expressed as number of F4/80+ cells per hpf and percentage of F4/80+ cells expressing the CD14 activation marker, mean ± SEM. In a–d, magnification is ×200 and bar represents 50 μm.
Discussion
In this report, we used a well-established model of colitis associated with administration of DSS to demonstrate the functional importance of α1β1 integrin in the pathogenesis of this disease. Furthermore, we have identified the monocyte as a key α1β1-expressing cell type involved in the development of colitis in this model. Given that α1β1 represents a main collagen-binding integrin expressed on leukocytes, our study highlights the important role ECM-leukocyte interactions play in the pathogenesis of colitis, with particular relevance to monocytes.
DSS is a sulfated polymer that is thought to induce mucosal injury and inflammation initially through a direct toxic effect on epithelial cells with subsequent recruitment and activation of macrophages and T cells, resulting in upregulation of both Th1 and Th2 cytokines and other inflammatory mediators, and thus leading to the development of severe colitis (20). DSS-induced colitis was originally viewed as a T cell–independent model because it is observed in severe combined immunodeficient (SCID) mice lacking both T and B lymphocytes (25). However, recent reports demonstrating that DSS-colitis is more severe in T cell– and B cell–deficient mice suggest that regulatory T cells may be critical in later phases of disease (26). These findings are consistent with our data showing that DSS-colitis is more severe in Rag2–/– mice and support the hypothesis that subsets of T cells are important for limiting the severity of DSS-colitis (2).
We found that DSS-exposed WT mice receiving anti-α1 mAb had significant attenuation of colitis, suggesting that α1β1 integrin is involved in mechanisms that regulate the initiation and/or progression of inflammation in this model of colitis. Genetic deletion of α1 resulted in a similar degree of protection from DSS-induced inflammation compared with anti-α1 mAb treatment in WT mice, confirming the importance of α1β1 integrin in gut inflammation. Protection from DSS-associated disease can also be achieved by blockade with anti-α1 mAb in Rag2–/– mice despite the lack of mature T and B cells, suggesting that α1 antagonism can modulate inflammation by acting on other cell populations. The DSS colitis model is thought to have a strong neutrophil/monocyte-based component in the acute phase of inflammation (17). Our immunohistologic analysis of the infiltrating cells in DSS-colitis revealed that while both neutrophils and monocytes were present, α1β1 expression was restricted to the monocyte population. These findings agree with previous reports demonstrating that monocytes rapidly upregulate expression of α1β1 upon activation (13), and that α1β1 is expressed on monocytes at sites of inflammation (5).
Although the pathogenesis of DSS-mediated colitis is incompletely understood, the recruitment and activation of macrophages within the intestinal mucosa seems to have a critical role (27). The importance of monocytes and ECM in the inflammatory cascade is underscored by recently published gene expression data demonstrating that integrin-mediated attachment of monocytes to ECM resulted in increased expression of numerous inflammatory and immune response genes important in promoting cell recruitment and activation (6). Antagonism of α1β1-mediated interaction with collagen may serve to inhibit monocyte movement and/or activation within the inflamed interstitium, resulting in an attenuated inflammatory response. Consistent with both hypotheses, blockade of α1β1 by mAb or knockout resulted in a sharp reduction in the number and activation status of lamina propria–infiltrating monocytes/macrophages seen in response to DSS.
In general, disruption of adhesive interactions can affect leukocyte recruitment by affecting entry into tissues (endothelial/leukocyte interactions) or by affecting migration of cells within the inflamed tissue. In the case of α1β1, disruption of endothelial/leukocyte adhesion as a possible mechanism of action can be excluded because α1β1 ligands (collagen, laminin) are not expressed on the surface of endothelial cells. Direct involvement of α1β1 in endothelium-dependent leukocyte adhesion was also ruled out experimentally in a pilot intravital microscopy study that showed no effect of either α1 blockade or deletion on endothelium-dependent leukocyte recruitment (data not shown). In vitro studies clearly indicate that α1β1 is important in regulating cell movement as this integrin has been shown to be critically involved in controlling cell migration on collagen matrices (16, 28). The potential involvement of α1β1 in regulating in vivo cell migration within ECM-rich tissues represents a possible mechanism of action requiring further study.
While engagement of α1β1 may be important in allowing migration of leukocytes to inflammatory sites, it is also capable of promoting cellular activation. For instance, integrin-mediated binding to collagen provides a costimulatory signal for T cell activation, resulting in increased proliferation and secretion of proinflammatory cytokines such as TNF-α and IFN-γ (29–31). The proinflammatory roles of IFN-γ and TNF-α in the activation of macrophages in animal models of colitis and in human inflammatory bowel disease have been well established (32, 33). Both these cytokines have also been shown to be important in the pathology of DSS-induced colitis. For instance, the severity of DSS-colitis was significantly attenuated in mice given anti–IFN-γ and/or anti–TNF-α mAb’s (34). Antagonism of α1β1 in WT mice resulted in dramatic reductions in the levels of proinflammatory cytokines induced by DSS, including both TNF-α and IFN-γ. The decreased cytokine expression caused by α1β1 blockade was not skewed toward Th1- or Th2-type cytokines, as levels of both Th1 (IFN-γ, IL-1α, TNF-α) and Th2 (IL-10) cytokines were similarly reduced. Consistent with a role for α1β1 in regulating monocyte/macrophage activation is the fact that both α1 deficiency and anti-α1 mAb treatment significantly reduced the activation status of lamina propria–infiltrating monocytes/macrophages as judged by coexpression of the CD14 activation marker. Whether the impaired monocyte/macro-phage activation that is seen in WT mice upon α1β1 blockade is due solely to blockade of proinflammatory cytokines is unknown. Inhibition of α1β1 can potentially also have other anti-inflammatory effects, as evidenced by the ability of anti-α1 mAb treatment to improve disease in Rag2–/– mice while having minimal impact on expression of the proinflammatory cytokines studied. Clearly, the mechanistic and cellular pathways responsible for cytokine production in Rag2–/– mice in response to DSS are different from what is seen in the presence of an intact immune system. This is perhaps not surprising given their immunocompromised status, and is also borne out by the altered pattern of cytokines induced by DSS in WT and Rag2–/– mice.
It is not clear whether the inhibition of leukocyte infiltration in the inflamed colons that is observed with anti-α1 mAb treatment or α1 deficiency represents a direct inhibitory effect on cell migration or is a consequence of disruption of a cellular activation event that then leads to decreased leukocyte recruitment. While α1 blockade may directly affect both cell migration and activation, α1 antagonism can clearly also act indirectly to decrease inflammation. For example, in response to DSS, colonic recruitment of both monocytes and neutrophils is significantly reduced by α1 blockade, yet only monocytes were found to express α1β1. Thus, while the monocyte represents a key cell type that α1 antagonism is acting upon in the DSS-colitis model, the reduction in neutrophil recruitment is likely a secondary effect of inhibiting monocyte recruitment and activation.
Anti-adhesive therapies for inflammatory bowel diseases have traditionally focused on preventing leukocyte recruitment to inflammatory sites by interfering with leukocyte-endothelial interactions. Development of DSS-colitis can be reduced through blockade of either α4β1/VCAM-1 (35) or αLβ2/ICAM-1 interactions (36, 37), and in humans, an anti-α4 mAb shows much promise in treating Crohn disease (38). Our study expands the role of adhesion molecules in inflammatory bowel disease beyond recruitment of circulating cells at the endothelial surface, and supports an emerging paradigm shift highlighting the importance of adhesion molecules within the ECM-rich environment of peripheral tissue (29).
The finding that emigrated leukocytes at sites of inflammation can express α1β1, a main collagen receptor, on their surface suggests that expression of this integrin is physically and temporally situated to play an important role in the inflammatory cascade. The importance of α1β1 in the inflammatory process is underscored by the strong protective effects that both mAb-based inhibition and genetic deletion of this integrin have in the DSS model of colitis. A similar protective effect of anti-α1 mAb and α1 deficiency was previously seen in animal models of hypersensitivity and arthritis (5, 15). Our study not only reinforces the importance of peripheral tissues to the inflammatory disease process by extending it to include inflammatory bowel disease, but also specifically highlights the monocyte as an important α1β1-expressing cell type in the pathogenesis of colitis. We demonstrated that development and α1-mediated inhibition of DSS-induced colitis was independent of lymphocytes, and that in this model, activated monocytes represent the main α1β1-expressing cell type seen within the inflammatory infiltrate. The ability of α1 blockade to prevent monocyte accumulation, activation, and proinflammatory cytokine production within the inflamed colon is particularly important, given that activated monocytes and their production of proinflammatory cytokines has been linked to the pathogenesis of inflammatory bowel disease in humans (33). In summary, we have demonstrated the importance of the ECM-rich peripheral tissue environment in modulating immune responses, and propose that therapies targeting monocyte interaction with ECM may represent a powerful new approach in the treatment of patients with inflammatory bowel disease.
Acknowledgments
This study was supported by NIH grants P01 DK-43785 (to D.N. Granger) and R01 DK-47663 (to M.B. Grisham), and grants from the Broad Medical Research Program of the Eli and Edythe L. Broad Foundation, and the Center of Excellence in Arthritis and Rheumatism at Louisiana State University Health Sciences Center at Shreveport. We would also like to thank T. Crowell and H. Gardner (Biogen Inc.) for assistance with tissue sectioning and helpful discussions, respectively.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: dextran sodium sulfate (DSS); wild type (WT); recombinase-activating gene-2 (Rag2); phycoerythrin (PE); high-power field (hpf).
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 3.Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- 4.Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu. Rev. Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 5.de Fougerolles AR, et al. Regulation of inflammation by collagen-binding integrins α1β1 and α2β1 in models of hypersensitivity and arthritis. J. Clin. Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Fougerolles AR, et al. Global expression analysis of extracellular matrix-integrin interactions in monocytes. Immunity. 2000;13:749–758. doi: 10.1016/s1074-7613(00)00073-x. [DOI] [PubMed] [Google Scholar]
- 7.Werb, Z. 1987. Phagocytic cells: chemotaxis and effector functions of macrophages and granulocytes. In Basic and clinical immunology. D.P. Sites, J.P. Stobe, and J.V. Wells, editors. Appleton-Century-Crofts. Norwalk, Connecticut, USA. 96.
- 8.Gullberg D, et al. Analysis of α1β1, α2β1, and α3β1 integrins in cell-collagen interactions: identification of conformation dependent α1β1 binding sites in collagen type I. EMBO J. 1992;11:3865–3873. doi: 10.1002/j.1460-2075.1992.tb05479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkin VM, Belkin AM, Koteliansky VE. Human smooth muscle VLA-1 integrin: purification, substrate specificity, localization in aorta, and expression during development. J. Cell Biol. 1990;111:2159–2170. doi: 10.1083/jcb.111.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duband JL, Belkin AM, Syfrig J, Thiery JP, Koteliansky VE. Expression of α1 integrin, a laminin-collagen receptor, during myogenesis and neurogenesis in the avian embryo. Development. 1992;116:585–600. doi: 10.1242/dev.116.3.585. [DOI] [PubMed] [Google Scholar]
- 11.Racine-Samson L, Rockey DC, Bissell DM. The role of α1β1 integrin in wound contraction: a quantitative analysis of liver myofibroblasts in vivo and in primary culture. J. Biol. Chem. 1997;272:30911–30917. doi: 10.1074/jbc.272.49.30911. [DOI] [PubMed] [Google Scholar]
- 12.Kocken JM, et al. Blocking of α1β1 integrin strongly improves survival of hepatocytes in allogeneic transplantation. Lab. Invest. 1997;77:19–28. [PubMed] [Google Scholar]
- 13.Rubio MA, Sotillos M, Jochems G, Alvarez V, Corbi AL. Monocyte activation: rapid induction of α1β1 (VLA-1) integrin expression by lipopolysaccharide and inteferon-γ. Eur. J. Immunol. 1995;25:2701–2705. doi: 10.1002/eji.1830250945. [DOI] [PubMed] [Google Scholar]
- 14.Stemme S, Holm J, Hansson GK. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler. Thromb. 1992;12:206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- 15.Ianaro A, et al. Anti-very late antigen-1 monoclonal antibody modulates the development of secondary lesion and T-cell response in experimental arthritis. Lab. Invest. 2000;80:73–80. doi: 10.1038/labinvest.3780010. [DOI] [PubMed] [Google Scholar]
- 16.Gardner H, Kreidberg J, Koteliansky VE, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev. Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 17.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 18.Mendrick DL, Kelly DM, duMont SS, Sandstrom DJ. Glomerular epithelial cells differentially modulate the binding specificities of VLA-1 and VLA-2. Lab. Invest. 1995;72:367–375. [PubMed] [Google Scholar]
- 19.Cooper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sodium sulfate experimental murine colitis. Lab. Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 20.Dieleman LA, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer P, Russel JM, Granger DN. Role of endotoxin in intestinal reperfusion-induced expression of E-selectin. Am. J. Physiol. 1999;276:G479–G484. doi: 10.1152/ajpgi.1999.276.2.G479. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Weber CB, Alexander SI, Henault LE, James L, Lichtman AH. IL-4 enhances IL-10 gene expression in murine Th2 cells in the absence of TCR engagement. J. Immunol. 1999;162:238–244. [PubMed] [Google Scholar]
- 23.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 24.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 25.Dieleman LA, et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 26.Beck PL, et al. The role of T cell subsets and chemokines in the regulation of the inflammatory response in dextran sodium sulfate-induced colitis. Gastroenterology. 1999;116:A797. (Abstr.) [Google Scholar]
- 27.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 28.Gotwals PJ, et al. The α1β1 integrin is expressed during neointima formation in rat arteries and mediates collagen matrix reorganization. J. Clin. Invest. 1996;97:2469–2477. doi: 10.1172/JCI118693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dustin ML, de Fougerolles AR. Reprogramming T cells: the role of extracellular matrix in coordination of T cell activation and migration. Curr. Opin. Immunol. 2001;13:286–290. doi: 10.1016/s0952-7915(00)00217-x. [DOI] [PubMed] [Google Scholar]
- 30.Miyake S, Sakurai T, Okumura K, Yagita H. Identification of collagen and laminin receptor integrins on murine T lymphocytes. Eur. J. Immunol. 1994;24:2000–2005. doi: 10.1002/eji.1830240910. [DOI] [PubMed] [Google Scholar]
- 31.Rao WH, Hales JM, Camp RD. Potent costimulation of effector T lymphocytes by human collagen type I. J. Immunol. 2000;165:4935–4940. doi: 10.4049/jimmunol.165.9.4935. [DOI] [PubMed] [Google Scholar]
- 32.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 33.Podolsky DK. Inflammatory bowel disease. N. Engl. J. Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 34.Obermeier F, et al. Interferon-γ (IFN-γ)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin. Exp. Immunol. 1999;116:238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soriano A, et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab. Invest. 2000;80:1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 36.Bendjelloul F, et al. Intercellular adhesion molecule-1 (ICAM-1) deficiency protects mice against severe forms of experimentally induced colitis. Clin. Exp. Immunol. 2000;119:57–63. doi: 10.1046/j.1365-2249.2000.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taniguchi T, et al. Effects of the anti-ICAM-1 monoclonal antibody on dextran sodium sulphate-induced colitis in rats. J. Gastroenterol. Hepatol. 1998;13:945–949. doi: 10.1111/j.1440-1746.1998.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 38.Gordon FH, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to α4 integrin in active Crohn’s disease. Gastroenterology. 2001;121:268–274. doi: 10.1053/gast.2001.26260. [DOI] [PubMed] [Google Scholar]