Abstract
IgG Fc receptors (FcγRs, especially FcγRIII) and complement (in particular, C5a anaphylatoxin) are critical effectors of the acute inflammatory response to immune complexes (ICs). However, it is unknown whether and how these two key components can interact with each other in vivo. We use here a mouse model of the acute pulmonary IC hypersensitivity reaction to analyze their potential interaction. FcγRIII and C5aR are coexpressed on alveolar macrophages (AMs), and both FcγRIII and C5aR mutant mice display impaired immune responses. We find that recombinant human C5a (rhC5a) can control inverse expression of various FcγRs, and costimulation of ICs with rhC5a results in strong enhancement of FcγRIII-triggered cellular activation in vitro and in vivo. Moreover, we show here that early IC-induced bioactive C5a, and its interaction with C5aR, causes induction of activating FcγRIII and suppression of inhibitory FcγRII on AMs that appears crucial for efficient cytokine production and neutrophil recruitment in lung pathology. Therefore, C5a, which is a potent chemoattractant, has a broader critical function in regulating the inhibitory/activating FcγRII/III receptor pair to connect complement and FcγR effector pathways in immune inflammation.
Introduction
Enhanced effector cell activation to deposited IgG immune complexes (ICs) is a significant factor causing fatal inflammatory responses in many im-munologic diseases like systemic lupus erythema-todes, rheumatoid arthritis, Goodpasture syndrome, nephritis, and hypersensitivity pneumonitis/alveolitis (1–6). Despite the identification of several putative effector activities (in particular, the complement system and IgG Fc receptors [FcγRs]) associated with IC disease in animal models, the mechanisms through which ICs initiate inflammation are still not fully resolved. In particular, the issue of potential interaction between FcγRs and complement in the pathogenesis of IC disease remains controversial (7, 8).
Complement is an important regulator of IC-dependent tissue injury and contributes to IC clearance by CR1- and CR3-dependent phagocytosis, tissue destruction by the terminal C5b/C9 complex, and mobilization of inflammatory immune cells through the anaphylatoxins C3a, C4a, and C5a. C3 is the central protein in complement activation, and C3 mutant mice (9) display diminished or partial activation responses in several disease models, including Ab-induced arthritis and IC alveolitis (10–12). The genetic deletion of C5aR is very effective in lowering IC inflammation or T-cell–mediated contact hypersensitivity, and preventing acute arthritis (12–15). In addition, pharmacological inhibition of C5aR has beneficial effects in tissue damage, ischemia/reperfusion injury, and sepsis (16, 17). These data suggest that the interaction of C5a with C5aR may be essential for the majority of complement-mediated inflammatory reactions.
FcγRs are the other key players in inflammatory autoimmune disease, modulating cellular effector responses through activating FcγRIII and inhibitory FcγRII receptors (18). FcγRII-deficient mice show increases in the humoral immune response and enhanced susceptibility in various models of IC inflammation and antibody-dependent autoimmunity (19–23). FcγRIII mutant mice display protection in autoimmune hemolytic anemia, arthritis, alveolitis, and nephritis (11, 24–30). FcγRI-deficient mice also indicate that the high-affinity FcγRI can contribute to some of the activating FcγR-dependent pathologies (31, 32). However, the stronger phenotype of FcγRIII–/– mice as compared with FcγRI–/– mice, as well as the similarity of FcγRIII–/– mice and FcRγ–/– mice (defective in FcγRI and FcγRIII) (33) may indicate that the critical role of FcγRs is mediated largely, but not entirely, through FcγRIII.
The strict requirement of FcγRs defined for the majority of inflammatory disease models may support the view that the participation of complement is independent of or only secondary to FcγRs (7, 34). However, complement and FcγRs, specifically C5aR and FcγRIII, have been reported to play codominant roles in cutaneous and pulmonary Arthus reaction, which implies that FcγR-mediated responses can be integrated through C5aR activation (35). In this study, we show that C5a/C5aR is directly involved in the regulation of FcγRs (through induction of FcγRIII and suppression of FcγRII) on macrophages. Moreover, we describe initial production of C5a and C5a/C5aR-dependent modulation of FcγRs in an acute model of IC-induced lung pathology. These data establish the critical link between complement and FcγRs in immune inflammation and show that C5a/C5aR is an important regulator of the activating FcγRIII and inhibitory FcγRII receptor pair in vivo.
Methods
Mice.
FcγRIII-deficient mice were generated as previously described (24). They were bred for eight generations onto C57BL/6 mice under pathogen-free conditions in the animal facility of Hannover Medical School. The homozygous FcγRIII–/– were selected, and wild-type (WT) FcγRIII-positive C57BL/6 littermates were used for all comparisons. C57BL/6 mice homozygous for FcγRII–/– and C5aR–/– were kindly provided by T. Takai and C. Gerard (14, 19). All these mice were used at 8–14 weeks of age. Experiments were conducted in accordance to the regulations of the local authorities.
mAbs and FACS analysis.
The following antibodies were used: anti-FcγRII/III (clone 2.4G2, rat anti-mouse IgG) (PharMingen, BD Biosciences, Heidelberg, Germany), anti-FcγRII (Ly17.2; clone K9.361, mouse anti-mouse IgG) (20), and anti-C5aR (clone 20/70, rat anti-mouse IgG; generated by J. Zwirner, Goettingen, Germany). Isotype control mAbs with irrelevant specificities were obtained from Immunotech (Hamburg, Germany). Expression of C5aR and FcγR was measured by flow cytometry, using a FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany). Direct binding of FITC- and phycoerythrin-conjugated (PE-conjugated) 20/70 and 2.4G2 mAbs to the respective antigens was analyzed on peripheral blood cells (PBCs), alveolar macrophages (AMs) obtained by bronchoalveolar lavage (BAL), or cultured MH-S AM cells (36).
Experimental pulmonary IC inflammation.
Mice were anesthetized with ketamine and xylazine, the trachea was cannulated, and 150 μg of protein G chromatography–purified rabbit anti-OVA IgG Ab (Sigma-Aldrich, Munich, Germany) was applied. In some experiments, recombinant human C5a (rhC5a, 200 ng per mouse) were applied intratracheally along with anti-OVA IgG. Immediately thereafter, 20 mg/kg OVA antigen (Ag) was given intravenously. Ab control animals received PBS instead of OVA Ag. Mice were killed at various time points (2, 4, 8, and 24 hours) after initiation of pulmonary IC inflammation. BAL was performed five times with 1 ml PBS at 4°C. The total cell count of the BAL fluid (BALF) was assessed with a hemocytometer (Neubauer Zählkammer, Gehrden, Germany). The amount of erythrocytes represented the degree of hemorrhage. For quantitation of alveolar polymorphonuclear leukocyte (PMN) accumulation, differential cell counts were performed on cytospins (10 min at 55 g) stained with May-Grünwald-Giemsa using 300 μl BALF. The concentrations of TNF-α and macrophage inflammatory protein–2 (MIP-2) in BALF were assayed in duplicate in appropriately diluted samples with TNF-α– and MIP-2–specific ELISA kits (R&D Systems, Wiesbaden, Germany). The detection limits of the assays were 5.1 pg/ml (TNF-α) and 1.5 pg/ml (MIP-2). Myeloperoxidase (MPO) activity of lavaged lung tissue was assayed as previously described (11). In brief, homogenized tissue was suspended in 50 mM potassium phosphate buffer (pH 6) and 0.5% hexadecyltrimethyl ammonium bromide, subsequently exposed to three freeze-thaw cycles, and finally sonicated. A total of 0.167 mg/ml o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide was added to the supernatant. The change in OD at λ = 450 nm was recorded. A serial dilution of MPO from human PMNs (Calbiochem-Novabiochem, Bad Soden, Germany) served as a standard. Samples were run in duplicate.
Detection of C5a-dependent chemotactic activity in vivo.
Bone marrow cells (containing 64–68% PMNs) from C57BL/6 and C5aR–/– mice were suspended at 7.5 × 106 cells per ml RPMI 1640 medium and 0.5% BSA. One hundred microliters of the bone marrow cell suspension was placed into the insert of a Transwell chemotaxis chamber, and the bottom well was filled with 600 μl RPMI/0.5% BSA (negative control) or the same medium supplemented with 50 ng/ml rhC5a (internal positive control) or BALF diluted 1:2 in RPMI/1% BSA. BALFs were obtained from C57BL/6 mice at 2 and 4 hours after OVA:anti-OVA IC inflammation. BALFs from Ab-treated mice served as controls. Inserts were transferred to the lower chambers and incubated at 37°C and 6% CO2 for 2 hours. Where indicated, bone marrow cells were preincubated with the anti-C5aR mAb 20/70. After the incubation, 50 μl of 70 mM EDTA solution was added into the lower chambers to release adherent cells from the lower surface of the membrane and from the bottom of the well. Plates were further incubated for 30 min at 4°C, inserts were removed, and the transmigrated neutrophils were vigorously suspended and counted with a FACScalibur for 1 min at 60 μl/min with gating on forward and side scatter. Migration of PMNs from the insert to the bottom well was quantitated as the percentage of total PMNs loaded into the upper chamber.
Expression analysis in vivo.
Total RNA was prepared from BAL-AM cells of indicated mice at 2 hours after IC/rhC5a treatments using RNAzol reagent (WAK-Chemie Medical GmbH, Steinbach, Germany). FcγR/C5aR mRNA expression levels normalized to tubulin were quantitated by TaqMan real-time RT-PCR using published FcγRII, FcγRIII, and β-tubulin primers or the following FcRγ/C5aR-specific primers and probes: FcRγ, sense 5′-ATCTTGTTCTTGCTCCTTTTGGTG-3′ and antisense 5′-GCATCCAGGATATAGCAGAGCTG-3′; probe, 6-FAM-AGCA-GCCGCCCTGGGAGAGC-TAMRA; C5aR, sense 5′- TGT-GGGTGACAGCCTTCGA-3′ and antisense 5′-CCGCCAGA-TTCAGAAACCAG-3′; and probe, 6-FAM-CCAGACGGG-CCGTCAAACGC-TAMRA (21, 37, 38).
Functional analysis of alveolar macrophages in vitro.
Mouse alveolar macrophage MH-S cells (36) expressing both C5aR and FcγRs in RT-PCR and FACS analysis were maintained in 10% FCS/RPMI 1640 medium containing supplements. In functional experiments, 106 adherent MH-S cells were incubated for 24 hours in six tissue-culture wells containing 1% FCS/RPMI 1640 medium and activated with either 100 μg/ml heat aggregated IgG (mouse IgG1) as previously described (21) or 50 ng/ml recombinant human C5a (Sigma-Aldrich), or a combination of both stimuli. After various time points (0, 2, 4, 6, 8, and 16 hours), appropriate dilutions of culture supernatants from untreated and stimulated MH-S cells were examined for production of TNF-α and MIP-2 by ELISA. Total RNA was prepared and analyzed for C5aR, FcγRs and TNF-α, and MIP-2 mRNA expression by TaqMan real time RT-PCR (TNF-α, sense 5′-GTGACCA-GGCTGTCGCTACA-3′ and antisense 5′-AGGGCAATTAC-AGTCACGGC-3′; probe, 6-FAM-ACTGAACCTCTGCTCCCCACGGG-TAMRA; MIP-2, sense 5′-TGTGACGCCCCC-AGGA-3′ and antisense 5′-TTTGACCGCCCTTGAGAGTG-3′; probe, 6-FAM-CCCACTGCGCCCAGACAGAAGTCA-TA-TAMRA) (39, 40).
Statistical analysis.
Statistical analysis was performed using the SPSS V. 9.0 statistical package (SPSS, Chicago, Illinois, USA). To analyze differences in mean values between groups, a two-sided unpaired Student’s t test was used.
Results
Impaired pulmonary hypersensitivity reaction in C5aR and FcγRIII mutant mice.
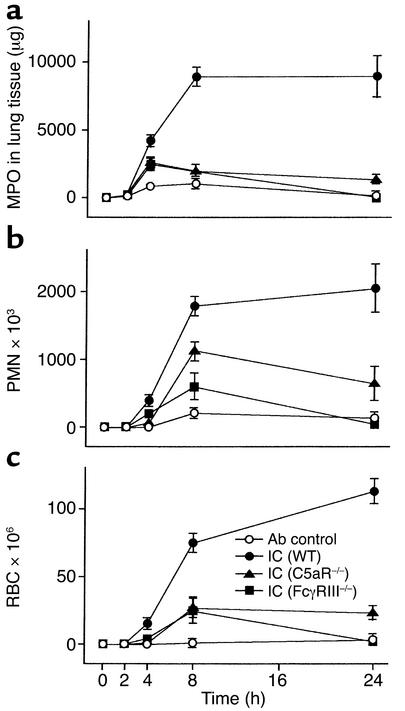
The hypersensitivity reaction was induced by OVA:anti-OVA IgG IC challenge in the lungs of C57BL/6 mice lacking C5aR (C5aR–/–) and FcγRIII (FcγRIII–/–) and followed in kinetic studies by MPO measurement of lung tissue as a marker of interstitial PMN influx (Figure 1a), by analysis of BALFs for accumulation of PMNs in alveoli (Figure 1b), and by quantitation of red blood cells in BALF indicating the degree of pulmonary hemorrhage (Figure 1c). Substantial IC-induced signs of inflammation were first revealed after 4 hours, reaching maximal levels at 8 to 24 hours in WT C57BL/6 control mice for all these parameters. In FcγRIII–/– mice, recruitment of interstitial PMNs and hemorrhage were markedly decreased and comparable to that seen in C5aR–/– animals at 4 and 8 hours, with a decline to background levels at 24 hours in both strains of mice (Figure 1, a and c). Alveolar PMN migration differed in that the attenuation in FcγRIII mutant mice was substantially more reduced in C5aR null mice at 4 hours (FcγRIII–/– vs. C5aR–/–: 1.61 ± 0.32 × 105 PMNs vs. 0.92 ± 0.18 × 105 PMNs; n = 7–19, P < 0.05), but less profound at 8 hours (FcγRIII–/– vs. C5aR–/–: 6.25 ± 2.54 × 105 PMNs vs. 11.54 ± 1.79 × 105 PMNs; n = 7–10, P = 0.10) and 24 hours (FcγRIII–/– vs. C5aR–/–: 2.17 ± 1.41 × 105 PMN vs. 7.41 ± 1.65 × 105 PMNs; n = 7–9, P < 0.05) (Figure 1b). These results suggest that C5aR and FcγRIII are critical effectors in pulmonary IC inflammation and, consistent with published data (14, 35), may indicate that C5aR contributes more to the initial events of neutrophil infiltration in IC-induced lung pathology.
Figure 1.
Attenuation of IC-induced lung injury by C5aR and FcγRIII deficiency. C57BL/6 WT (filled circles), FcγRIII–/– (filled squares), and C5aR–/– (filled triangles) mice received 150 μg anti-OVA Ab intratracheally and 20 mg/kg OVA Ag intravenously, and the inflammatory response in the lung was allowed to proceed for 2 to 24 hours (IC). Mice not receiving OVA Ag served as Ab controls (open circles). At the indicated times, mice were killed, and PMN accumulation in lung tissue (a), PMN influx in the alveolar space (b), and hemorrhage (c), were evaluated. The results are expressed as means ± SEM (n = 7–18 mice for each group). Differences for hemorrhage and alveolar and interstitial PMN infiltration were significant (P < 0.05) for the IC treatment groups of WT mice as compared with FcγRIII–/– and C5aR–/– mice at 4, 8, and 24 hours. FcγRIII–/– and C5aR–/– mice only differed significantly for alveolar PMN accumulation (see text). Ab control values do not differ between WT, FcγRIII–/–, and C5aR–/– mice (data not shown).
In vivo effects of rhC5a stimulation and C5aR inhibition in lung IC inflammation.
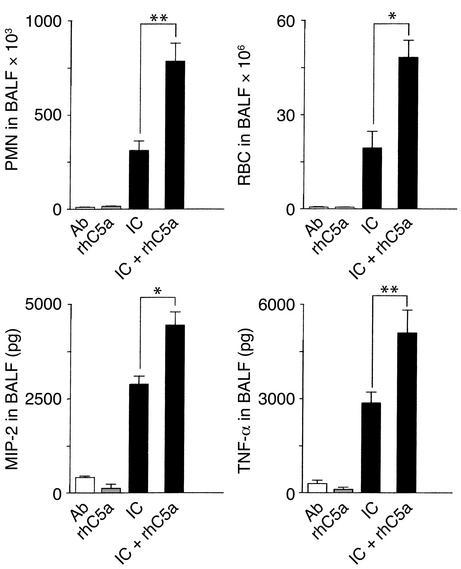
Given the diminished phenotype of C5aR mutant mice early rather than late in alveolar PMN migration, we determined the stimulatory effects of rhC5a and C5aR inhibition in the pulmonary Arthus reaction at early time points. Assessment of lung inflammation at 4 hours revealed that local intratracheal application of rhC5a, which was associated with no signs of inflammation when given alone, synergistically enhances alveolar PMN influx, hemorrhage, and mediator production of MIP-2 and TNF-α in IC-challenged C57BL/6 mice (Figure 2). IC inflammation has been described to be markedly suppressed by inhibition of C5aR, resulting in a 70–90 % reduction in PMN and red blood cell levels (35). IC-induced contents of MIP-2 and TNF-α in BALF of C57BL/6 mice (2854 ± 243 pg MIP-2 and 2843 ± 336 pg TNF-α, n = 13) were found to be significantly decreased after blockade of C5aR (1369 ± 171 pg MIP-2 and 1972 ± 174 pg TNF-α/BALF; n = 6, P < 0.05) as well as in C5aR mutant mice (1540 ± 535 pg MIP-2 and 1478 ± 269 pg TNF-α; n = 10, P < 0.05) or FcγRIII mutant mice (1660 ± 223 pg MIP-2 and 1463 ± 269 pg TNF-α; n = 10, P < 0.05). It has previously been shown that AMs but not mast cells are the main cellular source of FcγRIII-triggered MIP-2/TNF-α production in vivo (11, 28). Thus, the results of rhC5a-dependent enhancement and C5aR-specific inhibition of cytokine production and neutrophil infiltration suggest a link between C5a/C5aR and FcγRIII in the activation of AMs in pulmonary IC inflammation.
Figure 2.
Pulmonary IC inflammation in mice receiving rhC5a. The induction of the inflammatory response in the lung was performed by intratracheal application of 150 μg of purified anti-OVA Ab, followed by systemic 20 mg/kg OVA Ag in C57BL/6 wild-type mice (IC) or WT mice treated with rhC5a (IC + rhC5a). Mice receiving only Ab or rhC5a served as controls (Ab, rhC5a). After 4 hours, lungs were lavaged and BALFs were assayed for PMN infiltration (upper left), pulmonary hemorrhage (upper right), and production of MIP-2 and TNF-α (lower panels). Data are expressed as means ± SEM (n = 6–13 mice for each group). Differences in IC as compared with ICs + rhC5a treatment groups were significant for all parameters (*P < 0.05, **P < 0.001).
Coexpression of C5aR and FcγRIII on alveolar macrophages in vitro and in vivo.
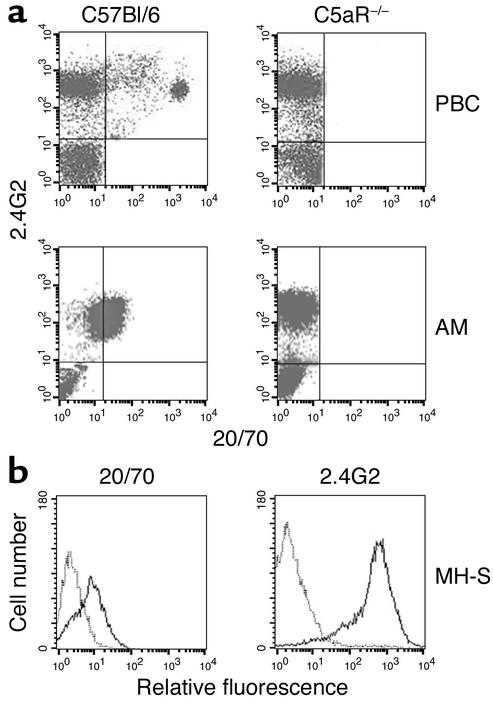
In vivo studies of AMs have shown the important role of these effector cells in various models of lung pathology (41–43). Thus, we examined expression levels of C5aR and FcγR in freshly isolated AMs and in vitro–cultured MH-S AMs (36). To measure C5aR protein, we used the rat anti-mouse C5aR mAb 20/70. The C5aR specificity of 20/70 mAb was validated by flow cytometric analysis demonstrating positive staining of PBCs and AMs from C57BL/6 WT but not C5aR mutant mice (Figure 3a). The combined incubation of 20/70 and 2.4G2 (which recognizes a common epitope on FcγRII and FcγRIII) mAbs showed double-positive staining of AMs from normal mice (Figure 3a) or MH-S AMs (Figure 3b), which suggested that alveolar macrophages coexpress FcγRII/III and C5aR on their surfaces.
Figure 3.
Flow cytometric detection of C5aR and FcγRII/III on AMs. (a) Control cells from peripheral blood (PBC) and resident AMs isolated from BAL fluid of C57BL/6 WT and C5aR–/– mice were stained with the newly developed anti-C5aR mAb 20/70 conjugated to FITC in combination with PE anti-FcγRII/III 2.4G2 mAb and analyzed on a FACScan. (b) MH-S AM cells were cultured under 10% FCS medium conditions. Simultaneous expression of FcγRII/III and C5aR was detected by FACS analysis using PE-2.4G2 and FITC-20/70 mAbs.
rhC5a synergistically enhances FcγRIII-dependent ICs activation of AMs in vitro.
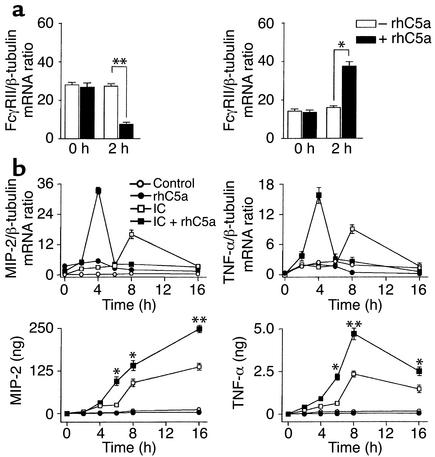
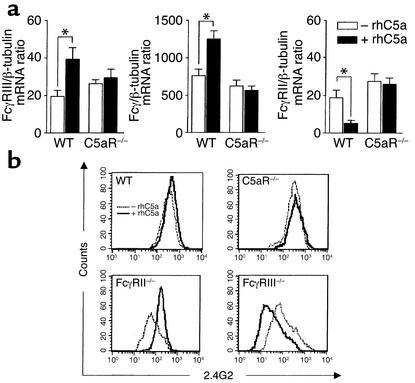
Studies in rats investigating the role of complement in lung injury have shown that C5a synergistically enhances IC-induced chemokine production of AMs in vitro and in vivo (44). To examine the molecular basis of C5a-increased IC activation, we first tested whether rhC5a has a direct effect on the expression of FcγRs on macrophages. In MH-S AM cells, 2 hour incubation with 50 ng/ml of rhC5a was associated with a significant reduction in FcγRII mRNA and strongly enhanced FcγRIII expression (Figure 4a) but did not alter C5aR levels (data not shown). We next determined whether a C5a-dependent increase of the FcγRIII/FcγRII mRNA ratio contributes to enhanced MH-S cell activation. Stimulations with rhC5a alone were not sufficient to mediate induced synthesis of TNF-α and MIP-2. However, IC-activated mediator production was found to be significantly increased by additional rhC5a (Figure 4b). Moreover, RNA analysis showed a strong kinetic contribution that resulted in rapidly pronounced transcriptional induction of TNF-α/MIP-2 (Figure 4b). These findings suggest that binding of rhC5a to C5aR amplifies IC-mediated activation of AMs through modulation of FcγRs in vitro.
Figure 4.
rhC5a enhances FcγRIII-dependent IC activation of AMs in vitro. (a) MH-S AM cells were cultured in medium containing 1% FCS, stimulated (black bars, +rhC5a) or not (white bars, –rhC5a) with 50 ng/ml recombinant human C5a for 2 hours, and assayed for rhC5a-dependent changes in FcγRII/III mRNA normalized to β-tubulin by TaqMan RT-PCR. (b) FCS-cultured MH-S cells (medium control, open circles) were incubated for the indicated time points with rhC5a (filled circles), heat-aggregated IgG (IC, open squares), or the combination of both stimuli (filled squares) and analyzed for production of MIP-2/TNF-α mRNA by TaqMan RT-PCR (upper panels) and MIP-2/TNF-α protein by ELISA (lower panels). Results are expressed as means ± SEM from three independent experiments performed in duplicate. Significant differences were determined by Student’s t test (*P < 0.05; **P < 0.001). Note the more rapid induction of MIP-2 and TNF-α mRNA correlating with significantly increased MIP-2/TNF-α protein concentrations in culture supernatants of ICs + rhC5a as compared with IC treatment groups.
rhC5a regulates expression of activating FcγRIII and inhibitory FcγRII on AMs in vivo.
Because rhC5a is a critical regulator of FcγRII/III mRNA expression in cultured MH-S cells enhancing IC-triggered cytokine production, we speculated that rhC5a can also regulate the activating FcγRIII and inhibitory FcγRII receptors on AMs in vivo. We therefore analyzed rhC5a-induced changes in FcγR expression by TaqMan RT-PCR and flow cytometry (Figure 5). In AMs of all B6 WT mice analyzed, mRNA expression of FcγRIII and the FcRγ chain was markedly upregulated, whereas FcγRII mRNA was suppressed after intratracheal application of rhC5a (Figure 5a). C5aR mutant mice did not display such alterations (Figure 5a). In line with the regulated mRNA expression, AM surface expression of FcγRII/III proteins was found to be inversely regulated by rhC5a, which was absent in C5aR–/– mice (Figure 5b). Since 2.4G2 mAb cross-reacts with both FcγRII and FcγRIII, simultaneous FcγRII/III staining obscured the regulatory effects of rhC5a in WT mice. However, flow cytometric analysis of FcγR mutant mice revealed the expected rhC5a-dependent changes of increased FcγRIII staining in AMs from FcγRII–/– mice and reduced FcγRII staining in AMs from FcγRIII–/– mice (Figure 5b). Thus, rhC5a does indeed have a regulatory role in modulating the balance of surface expression of activating FcγRIII and inhibitory FcγRII in vivo.
Figure 5.
rhC5a modulates FcγR expression on AMs in vivo. (a) BAL-AM cells were isolated from C57BL/6 WT and C5aR–/– mice 4 hours after intratracheal application of 200 ng of rhC5a in 40 μl of PBS (black bars, +rhC5a) or PBS alone (white bars, –rhC5a). TaqMan RT-PCR analysis reveals significantly increased FcγRIII and reduced FcγRII mRNA levels in BAL-AMs from WT mice but not C5aR–/– mice on rhC5a treatment. Data are represented as means ± SEM (n = 6 mice for each group, *P < 0.05). (b) BAL-AM cells (2 × 104) of the indicated mice were stained with PE anti-FcγRII/III 2.4G2 mAb and analyzed on a FACScan (representative results from individual mice are shown). Different FcγRII/III staining patterns are specifically observed in FcγRII–/– and FcγRIII–/– mice after intratracheal injection of rhC5a (solid line, +rhC5a) as compared with PBS (dashed line, –rhC5a), demonstrating inverse regulation of AM surface expression of inhibitory FcγRII (reduced) and activating FcγRIII (increased) by rhC5a.
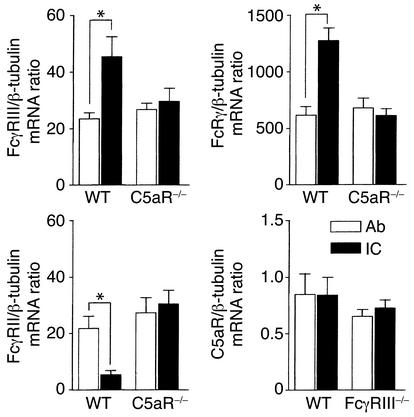
Local generation of C5a and C5aR-dependent inverse modulation of FcγRII versus FcγRIII mRNA/protein in IC inflammation.
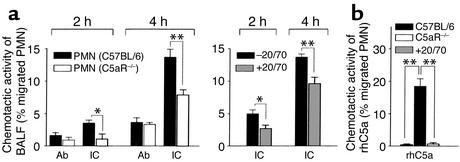
To test the hypothesis that C5a is induced early in acute inflammation, we analyzed the presence of IC-induced C5a bioactivity in vivo. BALFs of IC-treated mice recovered at 2 and 4 hours demonstrated chemotactic activity on PMNs from C57BL/6 mice that was strongly reduced on PMNs from C5aR–/– mice (left panel in Figure 6a) and neutralized in part in vitro by anti-C5aR mAb 20/70 (right panel in Figure 6a), which suggested that local generation of C5a (in addition to MIP-2 and KC CXC chemokines) (28) is an early event in the initiation of IC inflammation. Moreover, we found that BAL-AM cells of B6 mice display changed mRNA expression of FcγRs as short as 2 hours after IC challenge (Figure 7). IC-mediated modulation of FcγRs, which was not observed in Ab controls, is dependent on the presence of C5aR, as verified in C5aR mutant mice showing neither transcriptional induction of FcγRIII α/γ chains nor suppression of FcγRII (Figure 7). In contrast to FcγRs, expression of C5aR mRNA remained unchanged in AMs of IC-treated C57BL/6 and FcγRIII–/– mice (Figure 7).
Figure 6.
Functional detection of bioactive C5a in BALF from IC-challenged mice. Pulmonary IC inflammation was induced in C57BL/6 mice and assayed for IC-induced C5a (IC). Controls received anti-OVA Ab without OVA antigen (Ab). (a) Chemotactic activity was determined at the indicated times by Transwell migration assays of neutrophils (PMNs isolated from bone marrow of C57BL/6 and C5aR–/– mice or C57BL/6 PMNs preincubated with or without anti-C5aR mAb 20/70) elicited with 300 μl of BALF pools obtained from five mice of the IC and Ab treatment groups. (b) Assays using an optimal concentration of 50 ng/ml rhC5a instead of BALF served as positive controls for the indicated PMN preparations. Results are expressed as the percentage of PMNs loaded into the upper chamber that had migrated to the bottom well (means ± SEM for five individual experiments). Differences in PMN migration of C57BL/6 and C5aR–/– mice, or after anti-C5aR 20/70 mAb treatment, were significant (*P < 0.05, **P < 0.001).
Figure 7.
IC-induced modulation of FcγR mRNAs is impaired in C5aR–/– mice. mRNA expression of C5aR and FcγRs was assessed in BAL-AM cells from the indicated mice obtained 2 hours after OVA/anti-OVA challenge (IC). Mice not receiving the OVA antigen served as Ab controls (Ab). mRNA analysis by TaqMan RT-PCR showed significantly increased FcγRIII (upper left panel) and FcRγ (upper right panel) versus reduced FcγRII (lower left panel) mRNA levels in C57BL/6 (WT) mice, but not C5aR–/– mice, in response to IC treatment. C5aR mRNA expression do not differ between Ab and IC treatment groups of C57BL/6 WT and FcγRIII–/– mice. Data are represented as means ± SEM (n = 5–6 mice in each group, *P < 0.05).
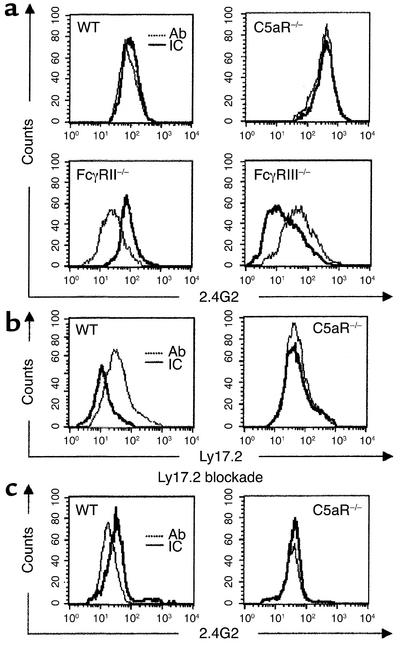
FACS analysis of FcγR mutant mice revealed similar IC-induced changes of increased 2.4G2 mAb staining of FcγRIII in FcγRII–/– mice (Ab vs. IC groups: mean fluorescence ± SEM, 40.13 ± 6.10 vs. 75.40 ± 9.83; n = 4, P = 0.0018) and reduced 2.4G2 mAb staining of FcγRII in FcγRIII–/– mice (Ab vs. IC groups: mean fluorescence ± SEM, 66.94 ± 9.92 vs. 33.82 ± 2.86; n = 4, P = 0.0014) (Figure 8a), indicating that the observed changes of FcγR transcription correlate with modulated FcγRII/III surface membrane expression. We recently showed that mAbs detecting the mouse Ly17.1/2 alloantigen system are specific for FcγRII with no cross-reactivity to FcγRIII (20). Here, we used the Ly17.2 mAb to examine the significance of C5aR for IC-induced suppression of FcγRII protein. As shown in Figure 8b, BAL-AM cells revealed surface FcγRII that was downregulated in IC-challenged WT (Ab vs. IC groups: mean fluorescence ± SEM, 37.99 ± 7.63 vs. 14.43 ± 3.82; n = 4, P = 0.0031) but not C5aR mutant mice (Ab vs. IC groups: mean fluorescence ± SEM, 72.83 ± 8.46 vs. 69.51 ± 7.70; n = 4, P = not significant). In a second approach, surface expression of FcγRIII was specifically determined by FACS analysis using prior blockade of FcγRII by unlabeled Ly17.2 mAb followed by 2.4G2 mAb staining. The expected IC-dependent increase of FcγRIII staining was found in WT mice (Ab vs. IC groups: mean fluorescence ± SEM, 23.95 ± 2.86 vs. 41.29 ± 4.95; n = 4, P = 0.0019) but was absent in C5aR–/– mice (Ab vs. IC groups: mean fluorescence ± SEM, 39.81 ± 3.46 vs. 35.73 ± 4.68; n = 4, P = not significant) (Figure 8c). Taken together, these results strongly suggest that inverse regulation of FcγRII/III mRNA/protein is determined by initial C5a production and C5aR activation in IC inflammation in the lung.
Figure 8.
FACS analysis of FcγRII/III surface expression in IC-challenged mice. Protein expression of the inhibitory/activating FcγRII/III receptor pair was assessed in BAL-AM cells from indicated mice obtained 2 hours after OVA/anti-OVA challenge (ICs). Mice not receiving the OVA antigen served as Ab control (Ab). (a–c) Representative results of individual mice are shown. (a) AM cells were stained with PE anti-FcγRII/III 2.4G2 mAb. Different FcγRII/III staining patterns are specifically observed in FcγRII–/– and FcγRIII–/– mice after IC challenge (solid line, IC) as compared with Ab control (dashed line, Ab), demonstrating inverse regulation of surface expression of inhibitory FcγRII (reduced) and activating FcγRIII (increased) by ICs. (b) AM cells from WT or C5aR–/– mice were stained with the anti-FcγRII mAb Ly17.2 conjugated to FITC. Note the IC-induced suppression of surface FcγRII in WT but not C5aR–/– mice. (c) In order to achieve FcγRIII specificity, AM cells were first treated with unlabeled anti-FcγRII Ly17.2 mAb (Ly17.2 blockade) followed by PE-2.4G2 mAb staining. Substantial IC-induced upregulation of surface FcγRIII was detected in WT mice but is impaired in C5aR–/– mice.
Discussion
C5a anaphylatoxin has several tissue effects — such as vasodilation, increased vascular permeability, and edema formation — in the inflammatory response, especially in the lung (45). Our findings here establish the pivotal role played by C5a and C5aR in the inverse regulation of the inhibitory/activating FcγRII/III receptor pair on alveolar macrophages in vivo. Both C5aR and FcγRs are expressed on AMs, and upregulation of FcγRIII and suppression of FcγRII occur in response to rhC5a. Genetic ablation of C5aR expression in C5aR mutant mice completely abolishes this regulation of FcγRs, and C5aR inhibition results in decreased TNF-α and MIP-2 production and neutrophil infiltration in IC alveolitis. Costimulation with rhC5a intensifies the IC-mediated inflammatory reaction with increases of hemorrhage, cytokine production, and neutrophil infiltration. In vitro studies on IC activation indicate that C5aR-dependent modulation of FcγRs on AMs contributes to the synergistic enhancement by rhC5a. Thus, our results provide strong evidence for a direct regulatory link of C5a/C5aR and FcγRs in cellular activation of macrophages and during the initiation of pulmonary IC disease.
Currently, it is thought that hypersensitivity type III reactions in lung pathology and other immunologic diseases are mediated by the activating FcγRIII (28) controlled by the inhibitory FcγRII (46). FcγRIII is expressed in association with dimers of the signal-transducing FcRγ chain, which contains an ITAM sequence in its cytoplasmic tail. Coligation of FcγRIII and FcγRII, which has an ITIM motif in the cytoplasmic domain, results in tyrosine phosphorylation of FcγRII ITIM and subsequent inhibition of the FcγRIII ITAM-triggered activation signal (18). Recently, the regulatory importance of FcγRII has been validated in animal models of nephritis, collagen-induced arthritis, and Goodpasture syndrome (21–23). Downregulation of FcγRII on kidney cells is a significant factor contributing to renal inflammation in anti–glomerular basement membrane nephritis (21). Our results in lung inflammation show that formation of soluble ICs also results in suppression of FcγRII and upregulation of FcγRIII. However, absence of FcγR regulation in C5aR mutant mice indicates that C5a/C5aR interaction has a causative role in IC-induced changes of FcγR expression. C5a-dependent bioactivity is detectable in BAL samples early in the onset of lung injury, and both C5aR and FcγRIII mutant mice display diminished IC responses. Thus, our data suggest that increased generation of local C5a and its binding to C5aR mediate FcγR regulation that is essential for the observed lung phenotype. It will be interesting to assess the role of C5a in other inflammatory models by genetic or pharmacological inhibition of C5aR, and to determine whether C5a-dependent modulation of activating FcγRIII and inhibitory FcγRII is a common mechanism operative in many forms of IC disease.
Our work significantly extends previous reports that describe both dependent and alternative contributions of complement and FcγR effector pathways, C5a/C5aR and FcγRIII being the most prominent, in mouse models of inflammatory autoimmune diseases (11, 12, 14, 24, 34, 35, 45). The results presented here provide evidence that the C5a/C5aR interaction determines macrophage FcγR expression levels, thus serving as the initial amplification step of FcγRIII activation in immune inflammation. In line with this, genetic inactivation of C5aR is effective in preventing early events of mediator release (TNF-α, MIP-2) and neutrophil migration into alveoli. We also note that loss of FcγRIII and not C5aR correlates with profound dysfunction of late neutrophil influx, as well as generation of IL-1β (28) (N. Shushakova, unpublished data), indicating that the condition of C5aR deficiency results in a delay but not complete abrogation of cellular infiltration. The possibility of C5aR-independent but FcγRIII-dependent compensatory changes in upregulation of certain cytokines needs to be determined.
Together, the results demonstrate that C5a anaphylatoxin, which is a potent chemoattractant, has a broader critical function acting as an early regulator of induction of the activating FcγRIII and suppression of the inhibitory FcγRII in inflammatory immune reactions in vivo. Its generation and the subsequent C5aR stimulation provide initial activity, setting the threshold for IC FcγRIII activation. Thus, complement activation at the site of inflammation can control FcγRs to bridge humoral and cellular immunity. The regulatory role that C5a plays may enable the immune system to respond efficiently to immune complexes that are potentially harmful for the host.
Acknowledgments
We are grateful to T. Takai for kindly providing FcγRII–/– mice, U. Höpken and C. Gerard for C5aR–/– mice, A. Fiebeler and H. Haller for their support with TaqMan RT-PCR analysis, and O. Felda for excellent technical assistance. J. Skokowa received Medical School Hannover Graduate Program funding. This research was supported, in part, by a grant from the Deutsche Forschungsgemeinschaft to J.E. Gessner (Ge892/7-1:2).
Footnotes
See the related Commentary beginning on page 1759.
Nelli Shushakova and Julia Skokowa contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: IgG Fc receptors (FcγRs); immune complexes (ICs); alveolar macrophages (AMs); recombinant human C5a (rhC5a); wild type (WT); peripheral blood cells (PBCs); phycoerythrin (PE); bronchoalveolar lavage (BAL); antigen (Ag); BAL fluid (BALF); polymorphonuclear leukocyte (PMN); macrophage inflammatory protein–2 (MIP-2); myeloperoxidase (MPO).
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 3.Korganow A-S, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 4.Madaio MP. The role of autoantibodies in the pathogenesis of lupus nephritis. Semin. Nephrol. 1999;19:48–56. [PubMed] [Google Scholar]
- 5.Bolton WK. Goodpasture’s syndrome. Kidney Int. 1996;50:1753–1766. doi: 10.1038/ki.1996.495. [DOI] [PubMed] [Google Scholar]
- 6.Ando M, Suga M. Hypersensitivity pneumonitis. Curr. Opin. Pulm. Med. 1997;3:391–395. doi: 10.1097/00063198-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu. Rev. Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- 8.Köhl J, Gessner JE. On the role of complement and Fcγ-receptors in the Arthus reaction. Mol. Immunol. 1999;36:893–903. doi: 10.1016/s0161-5890(99)00111-x. [DOI] [PubMed] [Google Scholar]
- 9.Wessels MR, et al. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 11.Baumann U, et al. Distinct tissue site-specific requirements of mast cells and complement components C3/C5a receptor in IgG immune complex-induced injury of skin and lung. J. Immunol. 2001;167:1022–1027. doi: 10.4049/jimmunol.167.2.1022. [DOI] [PubMed] [Google Scholar]
- 12.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 13.Bozic CR. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 14.Höpken UE, Lu B, Gerard NP, Gerard C. Impaired inflammatory responses in the reverse Arthus reaction through genetic deletion of the C5a receptor. J. Exp. Med. 1997;186:749–756. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuji RF, et al. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J. Immunol. 2000;165:1588–1598. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- 16.Heller T, et al. Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J. Immunol. 1999;163:985–994. [PubMed] [Google Scholar]
- 17.Riedemann NC, et al. Increased C5a receptor expression in sepsis. J. Clin. Invest. 2002;110:101–108. doi:10.1172/JCI200215409. doi: 10.1172/JCI15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravetch JV, Bolland S. IgG Fc receptors. Annu. Rev. Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 19.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 20.Schiller C, et al. Mouse FcγRII is a negative regulator of FcγRIII in IgG immune complex triggered inflammation but not in autoantibody induced hemolysis. Eur. J. Immunol. 2000;30:481–490. doi: 10.1002/1521-4141(200002)30:2<481::AID-IMMU481>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Radeke HH, et al. Opposite regulation of type II and III receptors for immunoglobulin G in mouse glomerular mesangial cells and in the induction of anti-glomerular basement membrane (GBM) nephritis. J. Biol. Chem. 2002;277:27535–27544. doi: 10.1074/jbc.M200419200. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura A, et al. FcγRIIb-deficient mice develop Goodpasture’s syndrome upon immunization with type IV collagen. J. Exp. Med. 2000;191:899–905. doi: 10.1084/jem.191.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuasa T, et al. Deletion of Fcγ receptor IIB renders H-2b mice susceptible to collagen-induced arthritis. J. Exp. Med. 1999;189:187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazenbos WLW, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in FcγRIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 25.Meyer D, et al. FcγRIII (CD16) deficient mice show IgG-isotype dependent protection to experimental autoimmune hemolytic anemia. Blood. 1998;92:3997–4002. [PubMed] [Google Scholar]
- 26.Fossati-Jimack L, et al. Markedly different pathogenicity of four IgG isotype-switch variants of an anti-erythrocyte autoantibody is based on their capacity to interact in vivo with the low-affinity FcγRIII. J. Exp. Med. 2000;191:1293–1302. doi: 10.1084/jem.191.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieswandt B, et al. Acute systemic reaction and lung alterations induced by an anti-platelet integrin gpIIb/IIIa antibody in mice. Blood. 1999;94:684–693. [PubMed] [Google Scholar]
- 28.Chouchakova N, et al. FcγRIII-mediated production of TNF-α induces immune complex alveolitis independently of CXC chemokine generation. J. Immunol. 2001;166:5193–5200. doi: 10.4049/jimmunol.166.8.5193. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe N, et al. Mast cells induce autoantibody-mediated vasculitis syndrome through tumor necrosis factor production upon triggering Fcγ receptors. Blood. 1999;94:3855–3863. [PubMed] [Google Scholar]
- 30.Coxon A, et al. FcγRIII mediates neutrophil recruitment to immune complexes: a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 31.Barnes N, et al. FcγRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 32.Ioan-Facsinay A, et al. FcγRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 33.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 34.Trcka J, et al. Redundant and alternative roles for activating Fc receptors and complement in an antibody-dependent model of autoimmune vitiligo. Immunity. 2002;16:861–868. doi: 10.1016/s1074-7613(02)00327-8. [DOI] [PubMed] [Google Scholar]
- 35.Baumann U, et al. A codominant role of FcγRI/III and C5aR in the reverse Arthus reaction. J. Immunol. 2000;164:1065–1070. doi: 10.4049/jimmunol.164.2.1065. [DOI] [PubMed] [Google Scholar]
- 36.Mbawuike IN, Herscowitz HB. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J. Leuk. Biol. 1989;46:119–126. doi: 10.1002/jlb.46.2.119. [DOI] [PubMed] [Google Scholar]
- 37.Ra C, Jouvin MH, Kinet JP. Complete structure of the mouse mast cell receptor for IgE (Fc epsilon RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J. Biol. Chem. 1989;264:15323–15327. [PubMed] [Google Scholar]
- 38.Gerard C, et al. Structural diversity in the extracellular faces of peptidergic G-protein-coupled receptors. Molecular cloning of the mouse C5a anaphylatoxin receptor. J. Immunol. 1992;149:2600–2606. [PubMed] [Google Scholar]
- 39.Semon D, Kawashima E, Jongeneel CV, Shakhov AN, Nedospasov SA. Nucleotide sequence of the murine TNF- locus, including the TNF-alpha (tumor necrosis factor) and TNF-beta (lymphotoxin) genes. Nucleic Acids Res. 1987;15:9083–9084. doi: 10.1093/nar/15.21.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tekamp-Olson P, et al. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J. Exp. Med. 1990;172:911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henson PM, et al. Complement fragments, alveolar macrophages, and alveolitis. Am. J. Pathol. 1979;97:93–110. [PMC free article] [PubMed] [Google Scholar]
- 42.Lentsch AB, Czermak BJ, Bless NM, van Rooijen N, Ward PA. Essential role of alveolar macrophages in intrapulmonary activation of NF-kB. Am. J. Respir. Cell Mol. Biol. 1999;20:692–698. doi: 10.1165/ajrcmb.20.4.3414. [DOI] [PubMed] [Google Scholar]
- 43.Broug-Holub E, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czermak BJ, et al. Synergistic enhancement of chemokine generation and lung injury by C5a or the membrane attack complex of complement. Am. J. Pathol. 1999;154:1513–1524. doi: 10.1016/S0002-9440(10)65405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Köhl J. Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol. Immunol. 2001;38:175–187. doi: 10.1016/s0161-5890(01)00041-4. [DOI] [PubMed] [Google Scholar]
- 46.Clynes R, et al. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J. Exp. Med. 1999;189:179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]