Abstract
For many years it has been recognized that sex steroids have profound effects on bone metabolism. The current perception is that estrogen decreases bone resorption and androgen increases bone deposition. To investigate the potential for androgens to directly modulate bone resorption, we have examined avian osteoclast and human and mouse osteoclast-like cells for androgen responsiveness. There was a dose-dependent decrease in resorption activity in response to α-dihydrotestosterone (α-DHT), β-DHT, testosterone, or the synthetic androgen RU1881. This decrease was blocked by cotreatment with the specific androgen antagonist hydroxyflutamide. Further examination of avian osteoclasts revealed that the cells exhibited specific and saturable nuclear binding of tritiated RU1881 and that α-DHT stimulated the activity of the androgen response element as measured by using a chloramphenicol acetyltransferase reporter plasmid. In addition, avian osteoclasts responded to androgen treatment with elevated production and secretion of transforming growth factor β, a well documented response to androgen exposure in other cell systems. Treatment with either α-DHT or β-DHT for 24 hours resulted in a significant dose-dependent decrease in secretion of cathepsin B and tartrate-resistant acid phosphatase. This response to β-DHT, a stereoisomer of α-DHT that is inactive in other androgen receptor-dependent systems, supports the hypothesis that the osteoclast androgen receptor has unusual ligand-binding properties. Taken together, these results confirm the presence of functional androgen receptors in these cells and support the conclusion that osteoclasts are able to respond directly to androgens in vitro and thus are potential androgen target cells in vivo.
Androgens have long been recognized to play an important role in the normal development and physiology of bone and have profound influences on bone development and metabolism (1). The evidence published to date suggests that androgens exert their effects on bone metabolism through stimulation of bone formation by both indirect and direct effects on bone-forming osteoblasts derived from both males and females (2–4). Although the incidence of hip fractures in elderly men is less than in elderly women (17% in men versus 32% in women), osteoporosis in men is still a significant health problem with an aging population (5). Decreased androgen levels have been linked to lower bone density in men, yet there has been no definitive proof that there is a link between lower androgen levels and the increase in the incidence of hip fracture (6). There is, however, a strong correlation between hypogonadism in elderly men and hip fracture and spinal osteoporosis (5, 6). Although one study of males matched for age, race, and lifestyle has shown a direct correlation between hip fracture incidences and lower gonadal steroid levels (7), it has proven difficult in other studies to correlate serum androgen levels and risk for osteoporosis in elderly men, and it is likely that there are many other factors such as those of age, race, and lifestyles which are involved (8, 9). In support of the role of androgens in maintaining skeletal mass in adults, clinical studies have shown that castration and decreased testicular function result in osteoporosis in men and treatment with androgens prevents its occurrence (10). Moreover, androgen treatment of osteoporotic women has demonstrated that androgen therapy is effective in increasing bone density (10). This finding is not surprising given that androgen receptors and responses to nonaromatizable androgens have been reported in both males and females, and it has been well established that there are significant circulating levels of androgens in both men and women (11–14).
Mizuno et al. (15) have demonstrated androgen receptors in mouse osteoclast-like cells by using immunochemical localization, supporting that osteoclasts are targets for direct androgen action in vivo as well. We have therefore explored the influences of androgen on osteoclasts in vitro.
METHODS
Isolation of Cells.
Isolation of osteoclasts for resorption, gene expression, and lysosomal protein-secretion studies. Osteoclasts were isolated from the long bone of the chickens as described (16). An osteoclast-directed mAb, 121F (a gift from Philip Osdoby, Washington University, St. Louis), coupled to immunomagnetic beads obtained from Dynal (Great Neck, NY), was used to obtain cell populations that consist of at least 90% pure osteoclasts and 10% or less unidentified mononuclear cells (16). The purified osteoclasts exhibit all of the phenotypic attributes of osteoclasts, including multinucleation, attachment, and ruffled-border formation when cultured with bone particles and the ability to form resorption pits when cultured on slices of cortical bone.
Generation of mouse osteoclast-like cells.
Osteoclast precursors were co-cultured with ST2 support cells as by the technique of Udagawa et al. (17) and were purified as outlined by Wesolowski et al. (18).
Isolation of human osteoclast-like cells for resorption studies.
Multinucleated cells that similarly form resorption pits on cortical bone slices within 18 hr of culture were obtained from giant cell tumors of the bone as described (19). For all studies, cultures were carried out in phenol red-free α-modified minimal essential media (αMEM; GIBCO/BRL).
Quantitative Pit-Formation Assay.
Isolated osteoclasts or human or mouse osteoclast-like cells were resuspended in αMEM supplemented with 10% (vol/vol) charcoal-stripped fetal bovine serum (culture media) and plated on 1 mm2 slices of bovine cortical bone. Samples were treated with vehicle or the indicated steroid at the dose detailed in the figure legend. After 24 hr of culture, the slices were placed in 1% (vol/vol) paraformaldehyde in PBS. The number of osteoclasts per mm2 slice was determined for each slice as follows: the fixed slices were rinsed with water and stained for tartrate-resistant acid phosphatase (TRAP) activity by using a Sigma histochemical kit. Osteoclasts were identified as stained multinucleated cells. The number of pits per osteoclast was determined after removal of the cells. The pits, resulting from osteoclast activity, were stained with toluidine blue, counted by reflected light microscopy, and expressed as the number of pits per osteoclast as described (20).
Nuclear Binding Assay.
A sensitive nuclear binding assay was used to estimate the number of androgen receptors, i.e., those capable of being activated and bound to the nuclear sites. Because α-dihydrotestosterone (α-DHT) binds at low affinity to the estrogen receptor, we blocked with a 10-fold excess of cold 17β-estradiol for these binding studies. The nuclear binding assay for the cells has been detailed (21). Specific nuclear binding was calculated as fmol of receptor per mg of DNA to standardize for the variable number of nuclei in osteoclasts (22).
Transient Transfection and Analysis of Androgen Response Element Activity.
Isolated osteoclasts were transiently transfected with 10 μg of the reporter construct ARE-TK chloramphenicol acetyltransferase (CAT) (a gift from Ming Tsai, Baylor College of Medicine, Houston, TX) containing a progesterone/glucocorticoid/androgen response element linked to the CAT expression reporter by using Lipofectamine (GIBCO/BRL) according to the manufacturer’s instructions and as described (23). After transfection for 20 hr, cells were maintained in culture media in the presence of vehicle or 10−8 M α-DHT for 24 hr. Cells were harvested and whole-cell extracts were prepared by three freeze-thaw cycles in 0.04 M Tris⋅HCl (pH 7.4), 1 mM EDTA, and 0.15 M NaCl. To standardize for the cell number, aliquots of the cell pellet extracts were analyzed for protein content by using the methods of Bradford (Bio-Rad) with 100 mg of protein adjusted to equal volume used in each assay. CAT activity in cell extracts was determined after heat treatment at 60°C for 10 min to inactivate cellular deacetylation activity. The final reaction mixture was composed of 0.1 mCi (1 Ci = 37 GBq) [14C]chloramphenicol (New England Nuclear), 1 mM acetyl CoA (Sigma) in a final volume of 100 ml; incubation was for 150 min at 37°C. Acetylated [14C]chloramphenicol was separated from unacetylated chloramphenicol by using xylene extraction, and the β emissions were determined by scintillation counting using the method of Seed and Sheen (24). To normalize the transfection efficiency in each plate, 5 mg of a plasmid containing cytomegalovirus promoter enhancer sequences linked to a luciferase reporter system was cotransfected in each experiment. The luciferase activity was measured by using a substrate kit (Promega) and a Turner (Palo Alto, CA) luminometer. Values obtained for CAT activity (with background subtracted) were normalized for luciferase activity and are described in the figure legend and expressed as the ratio of androgen treated to control.
Analysis of the Secretion and Activation of TGF-β.
Isolated avian osteoclasts were cultured with 1 mg/106 cells of bone particles in medium in the presence of either vehicle or the indicated concentration of α DHT as detailed in the figure legend. Conditioned media were harvested following 24 hours of culture and analyzed for either total or active transforming growth factor-β (TGF-β) as described (25).
Lysosomal Enzyme Assays.
Conditioned media were assayed for secreted lysosomal enzyme levels. To standardize for cell number, aliquots of cell pellet extracts were analyzed for protein content by using the methods of Bradford (Bio-Rad protein detection system). TRAP activity was measured by using an assay based on the work of Brandenberger and Hanson (26) and Hofstee (27). The initial rate of hydrolysis of o-carboxyphenyl phosphate was determined by following the increase in absorbance at 300 nM resulting from the liberation of salicylic acid. One unit hydrolyzes one μmol of o-carboxyphenyl phosphate per minute at 24°C, pH 5.0. The assay was performed in the presence of 1 mM tartrate. Cathepsin B levels were measured by Nα-Cbz-Arg-Arg-4-methoxy-β-napthylamide hydrolysis as measured by 520 nM absorbance substrate as outlined by Barrett and Kirschke (28).
Statistical Analysis.
Unless otherwise indicated in the figure legends, the results represent the mean ± SEM of three separate experiments. The effect of treatment was compared with control values by one-way ANOVA; significant treatment effects were further evaluated by the Fisher’s least significant difference method of multiple comparisons in a one-way ANOVA. Tests were carried out by using Apple statview II, Abacus Concepts (Berkeley, CA).
RESULTS
Effects of Androgens on Resorption Activity.
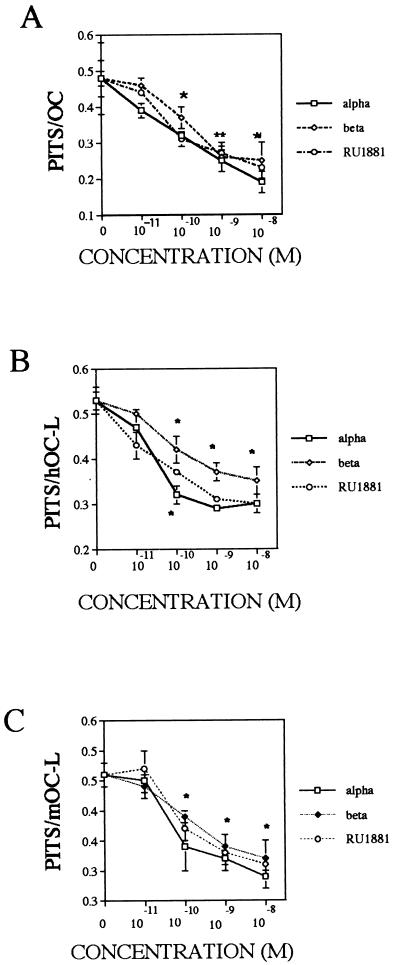
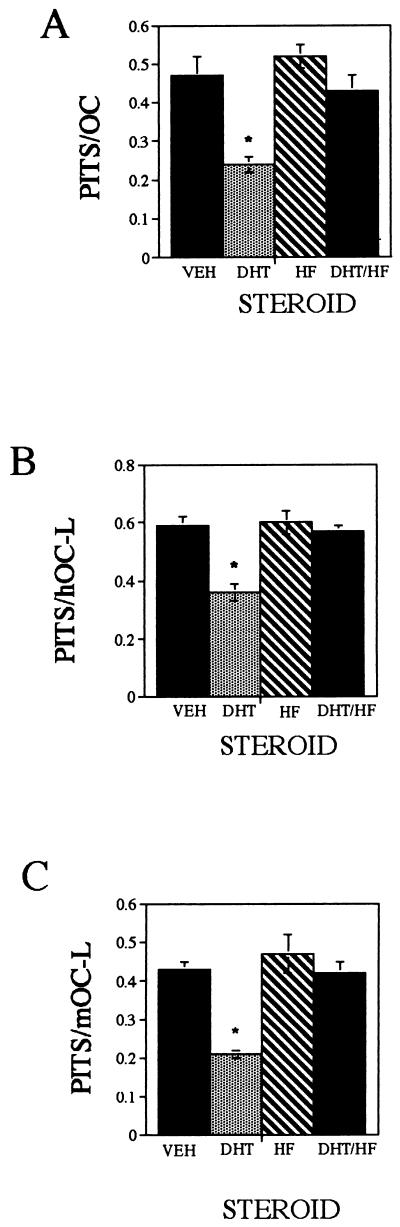
Isolated avian osteoclasts (Fig. 1A), human osteoclast-like cells (Fig. 1B), and mouse osteoclast-like cells (Fig. 1C) were cultured with either vehicle or with a broad range of α-DHT, β-DHT, testosterone, or R1881 (a synthetic, nonmetabolizable androgen analog) concentrations (10−11 M to 10−8 M) for 24 hr, and the level of resorption activity was monitored by using the quantitative pit-resorption assay. Treatment resulted in a steroid dose-dependent decrease in osteoclast resorption activity, which was detected at 10−10 M steroid. To examine the specificity of this response, isolated avian osteoclasts (Fig. 2A), human osteoclast-like cells (Fig. 2B), and mouse osteoclast-like cells (Fig. 2C) were treated for 24 hr with either 1 nM α-DHT, 100 nM hydroxyflutamide, or a combination of the two, and resorption activities were measured. As expected, treatment with α-DHT resulted in depression of resorption activity. Cultures that were treated with either hydroxyflutamide alone or α-DHT and hydroxyflutamide combined exhibited resorption activity comparable with control cultures.
Figure 1.
Effects of androgens on resorption activity. Isolated avian osteoclasts (A), human osteoclast-like cells (B), and mouse osteoclast-like cells (C) were cultured for 24 hr with either vehicle or the indicated concentration of α-DHT, β-DHT, or R1881 and analyzed for effects as detailed in Methods. ∗, P < 0.05; ∗∗, P < 0.01.
Figure 2.
Specificity of resorption response. Isolated avian osteoclast (A), human osteoclast-like cells (B), and mouse osteoclast-like cells (C) were cultured for 24 hr with either vehicle, 10−9 M α-DHT, 10−7 M hydroxyflutamide (HF), or with a combination of 10−9 M α-DHT and 10−7 M HF. ∗, P < 0.05.
Functional Androgen Receptor Protein in Avian Osteoclasts.
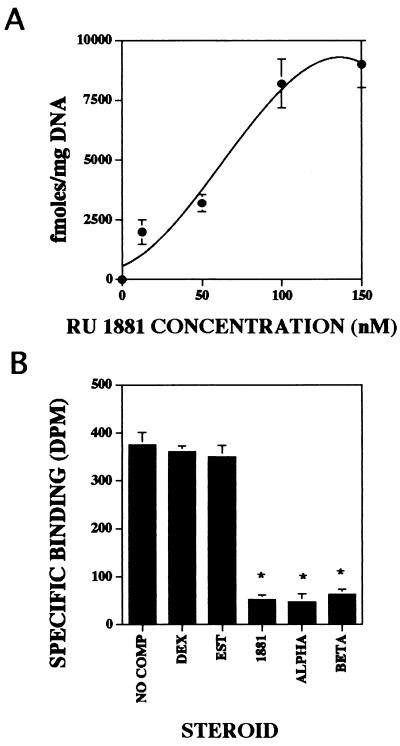
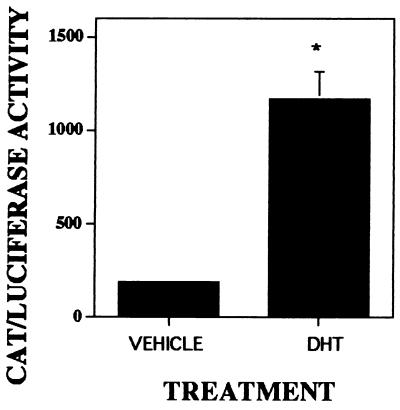
Steroid receptor nuclear binding in avian osteoclasts was examined by a well documented procedure that is able to detect the levels of functional nuclear bound steroid receptors from a small number of intact cells. By using this assay, isolated avian osteoclasts exhibited high levels of saturable specific binding of [3H]R1881 (a synthetic androgen) to the nucleus (Fig. 3A). These data demonstrate that osteoclasts exhibit saturable binding of this synthetic androgen. The data also show that saturation of the osteoclast receptor occurs at a significantly higher concentration than expected. To examine the steroid specificity of nuclear binding, osteoclasts were incubated with 10 nM [3H]R1881 alone or with 100-fold excess of unlabeled estrogen, dexamethasone, R1881, α-DHT, or β-DHT (Fig. 3B). Nonradiolabeled androgens R1881, α-DHT, and β-DHT compete with [3H]R1881 for nuclear binding whereas other steroids did not. To determine whether there were sufficient functional androgen receptors present in osteoclasts to modulate gene-transcription activity, we have examined the ability of endogenous androgen receptors to respond to α-DHT and regulate CAT expression, which is driven by an androgen response element in the reporter system ARE-TK CAT (Fig. 4). These data clearly demonstrate that there is sufficient functional androgen receptor present in avian osteoclasts to respond to androgen treatment and modulate gene expression.
Figure 3.
Androgen nuclear binding. (A) Isolated avian osteoclasts were incubated with 1–150 nM [3H]R1881 alone or in the presence of a 100-fold excess of unlabeled R1881 for total and nonspecific binding, respectively. (B) Isolated avian osteoclasts were incubated with 100 nM [3H]R1881 alone or with a 100-fold excess of unlabeled dexamethasone (Dex), estrogen (Est), R1881, α-DHT, or β-DHT.
Figure 4.
Androgen regulation of ARE-TK CAT expression. Isolated avian osteoclasts were cotransfected with a CAT reporter construct driven by androgen response elements and a cytomegalovirus promoter enhancer sequence with a luciferase reporter system to normalize for transfection efficiency. Cells were treated and harvested as described in Methods before assaying for reporter activity.
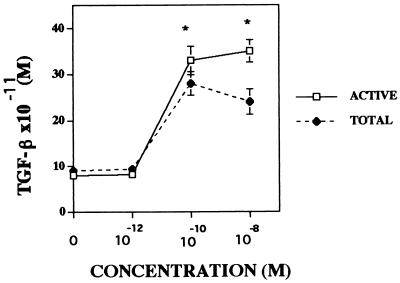
Osteoclasts produce, secrete, and activate TGF-β (25). Moreover, androgen modulates the production and secretion of TGF-β in many cell types (29, 30). To examine whether osteoclasts respond similarly to androgen, we have examined androgen effects on osteoclast TGF-β production. As shown in Fig. 5, although there was no detectable influence of 10−12 M α-DHT on either secretion or activation of TGF-β in these cultures, the amount of total TGF-β present in the conditioned media was significantly higher than control at 10−10 M. Higher α-DHT concentrations had no further effect. Although there was more “activated” TGF-β present with increasing α-DHT concentrations, the rate of increase of activation of TGF-β occurred at a slower pace than the rate of increase in the total concentration of TGF-β present.
Figure 5.
Androgen influences on secretion and activation of TGF-β. Isolated avian osteoclasts were cultured for 24 hr with either vehicle or the indicated concentration of α-DHT. The amount of active TGF-β (ACTIVE) and the total amount of TGF-β (TOTAL) in the conditioned media was determined as described. ∗∗, P < 0.01
Androgen Effects on Secretion of Lysosomal Enzymes.
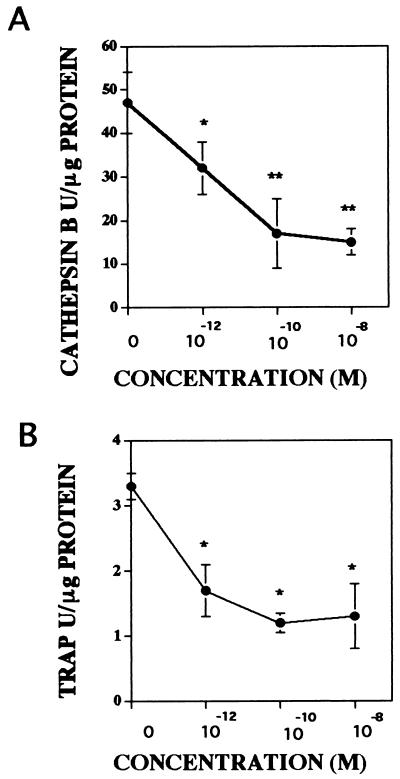
Osteoclast resorption activity can be inhibited by treatment of isolated osteoclasts with inhibitors of cathepsin B or TRAP, supporting that these lysosomal enzymes are likely to be involved in the process of bone resorption (31, 32). Because osteoclast resorption activity was inhibited by androgen treatment, we have investigated the influences of androgen on osteoclast lysosomal enzyme secretion (Fig. 6). Conditioned media from avian osteoclasts cultured with either vehicle or a broad range of α-DHT concentrations (10−12 M to 10−8 M) were analyzed for the levels of cathepsin B and TRAP activity. There was a significant decrease in the activity of these lysosomal enzymes present in the conditioned media detectable at 10−12 M α-DHT.
Figure 6.
Influence of androgen on secreted lysosomal enzyme activity levels. Isolated avian osteoclasts were cultured for 24 hr with either vehicle or the indicated α-DHT concentration. The levels of cathepsin B (A) and TRAP (B) activity released into the media were determined as described. ∗, P < 0.05; ∗∗, P < 0.01.
DISCUSSION
Studies in animal models have suggested an essential role for androgens in both skeletal growth and bone maintenance. Androgen deficiency in both young and old rats causes increased bone turnover and results in loss of both cancellous and cortical bone (33, 34). Although aromatization of androgen to estrogen may be responsible for some of these effects, α-DHT, which cannot be aromatized, has been shown to prevent bone loss in both young and aged orchidectomized male rats (33, 35). In vivo studies of androgen action have shown increased calcium and phosphate content of bone, increased alkaline phosphatase activity, and increased bone mineralization (34, 35). Additional actions on bone-forming osteoblasts may involve mitogenic action and increased differentiation from precursors (35).
Androgen effects on maintaining bone mass are thought to be both indirect through maintenance of muscle mass (and, therefore, loading on bone) and direct through action on bone-forming osteoblasts and on chondrocytes (6). Many studies have confirmed the presence of androgen receptors in osteoblasts and the ability of these cells to respond to androgens (36–41). It has been presumed that the androgen target cell involved in this alteration in bone metabolism is the osteoblast, but little is known concerning the potential for androgen action on osteoclast activity. The observation that mouse osteoclast-like cells contain androgen receptors (15) has led us to examine isolated avian osteoclasts and human and mouse osteoclast-like cells for androgen effects on activity. In this paper, osteoclasts are shown to have androgen receptors by demonstrating that isolated cells exhibit saturable and specific binding of a synthetic androgen and respond to androgen exposure by activation of a reporter system driven by androgen response elements. In addition, androgen was shown to modulate osteoclast TGF-β production. These are recognized classical androgen responses that have been observed in other cell types. Overall, these studies present data supporting that androgens are potent and specific bone-resorption inhibitors. Decreased secretion of cathepsin B and TRAP suggest that it is possible that this effect on resorption may be the result of repression of lysosomal enzyme secretion. It is possible that a substance that lowers activity level acts by decreasing viability of the target cell. Because androgen treatment elevated TGF-β secretion in these cultures, it is unlikely that androgen treatment lowered cell viability. Interestingly, androgen decreased the extent of osteoclast-mediated TGF-β activation. On the basis of enzyme inhibitor studies, we have shown that osteoclast-mediated activation of TGF-β in vitro is likely to be the result of lysosomal enzyme activity (25). It is possible that the lower level of TGF-β activation observed with androgen treatment was the result of the lower levels of lysosomal enzyme secretion, which we show here to result from androgen treatment. As with any primary culture system in which the cells are not clonally expanded, there is concern whether the effects are direct or mediated by a contaminating cell in low abundance. Because many of the responses reported herein were measured after up to 24 hr of treatment, it is possible that these responses may be indirect ones mediated by low-abundance contaminating cells. Until 100% pure cultures of osteoclasts are available, the issue of direct vs. indirect effects cannot be definitively answered, but the observation that mouse osteoclast-like cells contain androgen receptors increases the likelihood that at least some of the observed effects reported here are the result of direct effects on osteoclasts. This is an active area of investigation by many laboratories and, hopefully, will be resolved soon.
Although the predominant circulating sex steroid in men is testosterone and the predominant circulating sex steroids in women are estradiol and estrogen, androgens and estrogens circulate in both sexes (42). Thus, androgens may influence skeletal homeostasis in women, but estrogens are likely to be the more important factor in premenopausal bone maintenance when estrogen levels fall. When estrogen levels drop after menopause, it is possible that circulating androgens may become a significant influence on bone metabolism. In clinical studies of women, data have suggested that serum androgens may influence bone density in pre-, peri-, and postmenopausal women (11–13, 43–45). It is likely that aromatization of androgens to estrogens is an important component of this response. However, based on individual correlation of circulating steroid levels with bone density, androgen may play a direct role in maintenance of bone mass because normal physiological levels of endogenous androstenedione, estrogen, and testosterone protect the bone mass of young women (45). Further supporting this hypothesis, the specific antiandrogen hydroxyflutamide (a nonsteroidal competitive inhibitor) induces bone loss in female rats, thus indicating a direct influence of androgen in maintenance of skeletal mass in females (42). Both androgen and estrogen receptors have been demonstrated in cultures of normal human osteoblast-like cells obtained from both males and females (32). Biological responses to both of these steroids have confirmed that bone is a target tissue in both men and women for both androgens and estrogens (46). Moreover, we have shown that multinucleated cells from human giant cell tumors of the bone cells from both men and women respond to estrogen treatment by decreased bone resorption in vitro (19). Thus, in vivo and in vitro data strongly suggest that both men and women are capable of responding to both androgens and estrogens. There have been multiple studies that examined the influence of the loss of androgen on bone resorption rates in both animal models and humans. These have clearly shown that cessation of circulating androgen results in increased bone resorption (43, 44). Moreover, histomorphometric studies using the rat model have demonstrated an elevation in osteoclasts and bone-resorption surfaces following androgen withdrawal (33, 35, 46, 49–51). None of these types of studies are able to differentiate among androgen effects on osteoclasts formation, activity, or survival. Thus, this manuscript presents data that supports that osteoclasts are direct androgen targets and decrease bone-resorption activity.
It was unexpected that β-DHT was biologically indistinguishable from α-DHT and successfully competed for binding with [3H]R1881 because β-DHT has been shown clinically to not elicit the classical α-DHT physiological responses in reproductive tissues. Our observations could be the result of contaminating α-DHT in the β-DHT reagent. We have examined this possibility by testing the effects of our β-DHT on fetal human osteoblast-like cells that have been stably transfected with an androgen receptor cDNA and are α-DHT responsive. Treatment of these cells has resulted in no detectable responses to β-DHT (S. Khosla, personal communication). This is an unexpected finding and suggests several possibilities: (i) the osteoclast androgen receptor differs from the classical androgen receptor found in other target tissues, (ii) osteoclasts are capable of converting β-DHT to α-DHT, or (iii) β-DHT cross-reacts with other steroid receptors in these tissues. Understanding how androgens influence osteoclast activity should help us develop new strategies for the prevention and treatment of osteoporosis in both males and females. It is hoped that a better understanding of these processes will greatly facilitate prevention and treatment of metabolic disorders of bone.
Acknowledgments
We thank Dr. K. Unni for assistance with the giant cell tumors. This research was supported by Grant AR41114 from the National Institutes of Health.
ABBREVIATIONS
- TRAP
tartrate-resistant acid phosphatase
- DHT
dihydrotestosterone
- TGF
transforming growth factor
- CAT
chloramphenicol acetyltransferase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Oursler M J, Kassem M, Riggs B L, Spelsberg T C. In: Osteoporosis. Marcus, Feldman, Kelsey, editors. New York: Academic Press; 1996. pp. 237–260. [Google Scholar]
- 2.Kopera H. Acta Endocrinol. 1985;271:11–18. doi: 10.1530/acta.0.109s00011. [DOI] [PubMed] [Google Scholar]
- 3.Celotti F, Cesi N. J Steroid Biochem Mol Biol. 1992;43:469–477. doi: 10.1016/0960-0760(92)90085-w. [DOI] [PubMed] [Google Scholar]
- 4.Anderson D C. Br Med J. 1992;305:489–490. doi: 10.1136/bmj.305.6852.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orwoll E S, Klein R F. Endocr Rev. 1995;16:87–116. doi: 10.1210/edrv-16-1-87. [DOI] [PubMed] [Google Scholar]
- 6.Seeman E, Melton L J., III Am J Med. 1983;75:977–983. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- 7.Murphy S, Khaw K T, Cassidy A, Compston J E. Bone Miner. 1993;20:133–140. doi: 10.1016/s0169-6009(08)80022-0. [DOI] [PubMed] [Google Scholar]
- 8.Meier D E, Orwoll E S, Keena E J, Fagerstrom R M. J Am Geriatr Soc. 1987;35:189–197. doi: 10.1111/j.1532-5415.1987.tb02308.x. [DOI] [PubMed] [Google Scholar]
- 9.McElduff A, Wilkinson M, Ward P, Posen S. Bone. 1988;9:281–283. doi: 10.1016/8756-3282(88)90010-5. [DOI] [PubMed] [Google Scholar]
- 10.Dequeker J, Guesens P. Endocrinologica. 1985;271:45–52. doi: 10.1530/acta.0.109s0045. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg K K, Freni-Titulaer L W, DePeuy E G, Miller D T, Sgoutas D S, Coralli C H, Philip D L, Rogers T N, Clark R V. J Clin Endocrinol Metab. 1989;69:533–539. doi: 10.1210/jcem-69-3-533. [DOI] [PubMed] [Google Scholar]
- 12.Davidson B J, Riggs B L, Paganini-Hill A, Hammond G D, Siiteri P K, Judd L H. J Clin Endocrinol Metab. 1982;54:115–120. doi: 10.1210/jcem-54-1-115. [DOI] [PubMed] [Google Scholar]
- 13.Davidson B J, Riggs B L, Wahner H W, Judd L H. Obstet Gynecol. 1983;61:275–278. [PubMed] [Google Scholar]
- 14.Riggs B L R, Jowsey J, Goldsmith R S, Kelly P J, Hoffman D L, Arnaud C D. J Clin Invest. 1972;51:1659–1663. doi: 10.1172/JCI106967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuno Y, Hosoi T, Inoue S, Ikegami A, Kaneki M, Akedo Y, Nakamura T, Ouchi Y, Chan C, Orimo H. Calcif Tissue Int. 1994;54:325–329. doi: 10.1007/BF00295958. [DOI] [PubMed] [Google Scholar]
- 16.Collin-Osdoby P, Oursler M J, Webber D, Osdoby P. J Bone Miner Res. 1991;6:1353–1365. doi: 10.1002/jbmr.5650061213. [DOI] [PubMed] [Google Scholar]
- 17.Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin T, Suda T. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- 18.Wesolowski G, Duong L, Lakkakorpi P, Nagy R, Tezuka K, Tanaka H, Rodan G, Rodan S. Exp Cell Res. 1995;219:679–686. doi: 10.1006/excr.1995.1279. [DOI] [PubMed] [Google Scholar]
- 19.Oursler M J, Pederson L, Fitzpatrick L, Riggs B L, Spelsberg T C. Proc Natl Acad Sci USA. 1994;91:5227–5231. doi: 10.1073/pnas.91.12.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oursler M J, Pederson L, Pyfferoen J, Osdoby P, Fitzpatrick L, Spelsberg T C. Endocrinology. 1993;132:1373–1380. doi: 10.1210/endo.132.3.8440193. [DOI] [PubMed] [Google Scholar]
- 21.Kon O, Spelsberg T C. Endocrinology. 1980;111:1925–1932. doi: 10.1210/endo-111-6-1925. [DOI] [PubMed] [Google Scholar]
- 22.Oursler M J, Osdoby P, Pyfferoen J, Spelsberg T C. Proc Natl Acad Sci USA. 1991;88:6613–6617. doi: 10.1073/pnas.88.15.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pederson L, Kremer M, Foged N, Winding B, Ritchie C, Fitzpatrick L, Oursler M J. J Bone Miner Res. 1997;12:742–752. doi: 10.1359/jbmr.1997.12.5.742. [DOI] [PubMed] [Google Scholar]
- 24.Seed B, Sheen J Y. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 25.Oursler M J. J Bone Miner Res. 1994;8:443–452. doi: 10.1002/jbmr.5650090402. [DOI] [PubMed] [Google Scholar]
- 26.Brandenberger H, Hanson R. Helv Chim Acta. 1953;36:900. [Google Scholar]
- 27.Hofstee B H J. Arch Biochem Biophys. 1954;51:139. doi: 10.1016/0003-9861(54)90461-0. [DOI] [PubMed] [Google Scholar]
- 28.Barrett A, Kirschke H. Methods Enzymol. 1981;80:535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 29.Kasperk C, Fitzsimmons R, Strong D, Mohan S, Jennings J, Wergedal J, Baylink D. J Clin Endocrinol Metab. 1990;71:1322–1329. doi: 10.1210/jcem-71-5-1322. [DOI] [PubMed] [Google Scholar]
- 30.Benz D, Haussler M, Thomas M, Speelman B, Komm B. Endocrinology. 1991;128:2723–2730. doi: 10.1210/endo-128-6-2723. [DOI] [PubMed] [Google Scholar]
- 31.Zaidi M, Moss D, MacIntyre I. Biochem Biophys Res Comm. 1989;159:68–71. doi: 10.1016/0006-291x(89)92405-4. [DOI] [PubMed] [Google Scholar]
- 32.Ohsawa Y, Nitatori T, Higuchi S, Kominami E, Uchiyama Y. J Histochem Cytochem. 1993;41:1075–1083. doi: 10.1177/41.7.8515049. [DOI] [PubMed] [Google Scholar]
- 33.Vanderschueren D, Van Herck E, Suiker A M H, Visser W J, Schot L P C, Bouillon R. Endocrinology. 1992;130:2906–2916. doi: 10.1210/endo.130.5.1572302. [DOI] [PubMed] [Google Scholar]
- 34.Schoutens A, Verhas M, L’Hermite-Baleriauz M, L’Hermite M, Verschaeren A, Dourov N, Mone M, Heilporn A, Tricot A. Acta Endocrinol. 1984;107:428–432. doi: 10.1530/acta.0.1070428. [DOI] [PubMed] [Google Scholar]
- 35.Wakley G K, Schutte H D, Hannon K S, Turner R T. J Bone Miner Res. 1991;6:325–330. doi: 10.1002/jbmr.5650060403. [DOI] [PubMed] [Google Scholar]
- 36.Colvard D S, Eriksen E F, Keeting P E, Wilson E M, Lubahn D B, French F S, Riggs B L, Spelsberg T C. Proc Natl Acad Sci USA. 1989;86:854–857. doi: 10.1073/pnas.86.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasperk C, Fitzsimmons R, Strong D, Mohan S, Jennings J, Wergedal J, Baylink D. J Clin Endocrinol Metab. 1990;71:1322–1329. doi: 10.1210/jcem-71-5-1322. [DOI] [PubMed] [Google Scholar]
- 38.Kasperk C, Wakley G, Hierl T, Siegler R. J Bone Miner Res. 1997;12:464–471. doi: 10.1359/jbmr.1997.12.3.464. [DOI] [PubMed] [Google Scholar]
- 39.Kasperk C, Wergedal J, Farley J, Linkhart T, Turner R, Baylink D. Endocrinology. 1989;124:1576–1578. doi: 10.1210/endo-124-3-1576. [DOI] [PubMed] [Google Scholar]
- 40.Pilbeam C, Raisz L. J Bone Miner Res. 1990;5:1183–1188. doi: 10.1002/jbmr.5650051114. [DOI] [PubMed] [Google Scholar]
- 41.Bruch H-B, Wolf L, Budde R, Romalo G, Schweikert H-U. J Clin Endocrinol Metab. 1992;75:101–105. doi: 10.1210/jcem.75.1.1618995. [DOI] [PubMed] [Google Scholar]
- 42.Eriksen E F, Mosekilde L. In: Bone and Mineral Research. Heersche J N M, Kanis J A, editors. Amsterdam: Elsevier Biomedical; 1990. pp. 273–311. [Google Scholar]
- 43.Buchanan J R, Hospodar P, Myers C, Leuenberger P, Demers L M. J Clin Endocrinol Metab. 1988;67:937–943. doi: 10.1210/jcem-67-5-937. [DOI] [PubMed] [Google Scholar]
- 44.Haffner S M, Bauer R L. Int J Obes. 1992;16:869–874. [PubMed] [Google Scholar]
- 45.Ohta H, Ikeda T, Masuzawa T, Makita K, Suda Y, Nozawa S. Bone. 1993;14:111–116. doi: 10.1016/8756-3282(93)90236-4. [DOI] [PubMed] [Google Scholar]
- 46.Goulding A, Gold E. J Bone Miner Res. 1993;8:763–769. doi: 10.1002/jbmr.5650080615. [DOI] [PubMed] [Google Scholar]
- 47.Anderson F H, Francis R M, Peaston R, Wastell H J. J Bone Miner Res. 1997;12:472–478. doi: 10.1359/jbmr.1997.12.3.472. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Eyre D R, Clark R, Kleinberg D, Newman C, Iranmanesh A, Veldhuis J, Dudley R E, Berman N, Davidson T, et al. J Clin Endocrinol Metab. 1996;81:3654–3662. doi: 10.1210/jcem.81.10.8855818. [DOI] [PubMed] [Google Scholar]
- 49.Vanderschueren D, Van Herck E, Suiker A M H, Wisser W J, Schot L P C, Chung K, Lucas R S, Einhorn T A, Bouillon R. J Bone Miner Res. 1993;8:801–809. doi: 10.1002/jbmr.5650080705. [DOI] [PubMed] [Google Scholar]
- 50.Gunness M, Orwoll E. J Bone Miner Res. 1995;10:1735–1744. doi: 10.1002/jbmr.5650101117. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Jee S S, Ke H Z, Tang L Y, Ma Y F, Liang X G, Setterberg R B. J Bone Miner Res. 1995;10:66–73. doi: 10.1002/jbmr.5650100111. [DOI] [PubMed] [Google Scholar]