Abstract
Background
Lewy body disease is, after Alzheimer's disease, the second most common cause of senile degenerative dementia with progressive cognitive deterioration, fluctuation of cognitive and motoric functions and psychotic symptoms. It is characterized histologically by the occurrence of Lewy bodies in allocortical, neocortical and subcortical structures. The aim of this study was to measure the cortical glucose metabolism using FDG PET (2-[18F]fluoro-2-deoxy-D-glucose position emission tomography) compared to normal subjects.
Patients and Methods
Five patients (5 m, mean age 75 y) with clinically suspected diffuse Lewy body disease (DLB) were studied with FDG PET. PET studies of the head were performed with a Siemens ECAT-ART PET-scanner with attenuation correction using 137-Cs point sources.
Results
We found the same distribution pattern of diffuse glucose hypometabolism in the entire cortical region with relative sparing of the primary sensory-motor cortex in all the patients. The few cases reported in the literature so far describe findings similar to ours.
Conclusion
The pattern of diffuse glucose hypometabolism in the entire cortex including the occipital region seems to be a typical feature of DLB that is distinctive from dementia of Alzheimer's disease.
Keywords: Dementia, FDG PET, Lewy body disease
Background
Dementia with Lewy bodies (DLB) has been established as the second most common senile degenerative dementia after Alzheimer's disease (AD) [1-3]. Lewy bodies, first described in 1912 by F.H. Lewy are distinctive neuronal inclusions that may be seen in several different neurodegenerative processes [4]. Lewy bodies are eosinophilic structures located within the cytoplasm of neurons. They are characteristically circular with a dense protein core surrounded by a peripheral halo. They are thought to be the result of altered neurofilament metabolism and/or transport due to neuronal damage and subsequent degeneration, causing an accumulation of altered cytoskeletal elements. 5–10% of asymptomatic individuals have presumably insignificant numbers of Lewy bodies, usually located in the substantia nigra. [5]. Diagnostic criteria for DLB have been defined to allow clinical diagnosis [3]. Until quite recently, post mortem examination provided the definitive diagnosis. However, while these clinical criteria have high specificity (90–97%), they have low sensitivity (22–75%) [6]. The central clinical feature required for a diagnosis of DLB is a progressive and fluctuating cognitive decline with recurrent visual hallucinations, systematized delusions and spontaneous parkinsonian symptoms. Repeated falls, syncope, transient loss of consciousness and neuroleptic sensitivity are also clinically characteristic. In AD, in contrast, the progressive decline of memory is prominent and neuropsychiatric features usually occur in the later stages [7]. Moderate parkinsonian signs can be observed during both AD and DLB evolution [8]. Thus, in some cases, the clinical distinction of patients with DLB from those with AD may be difficult because of overlapping symptoms such as cognitive decline, psychiatric signs and parkinsonism. When the initial presentation of DLB is characterized by impaired cognition, the disease can mimic AD [5,9].
Differentiation of the two diseases from each other can be achieved by neuropsychological evaluation, which can disclose impaired performance of similar severity in both diseases with regard to attention, frontal lobe function and motor sequencing in DLB and AD [10]. In practice, differential diagnosis by the use of this method is most likely to be realized in specialized centers of neurology and psychiatry. A previous study has shown that noninvasive imaging modalities such as PET or SPECT may be useful methods for the diagnosis of dementing disorders, especially in the differential diagnosis of dementia and depression of the elderly [11]. In another study, it was found that in depression, symmetrically metabolic changes are found in the prefrontal cortex, and these changes improve with treatment [12], which was not typical for dementia.
In AD, the abnormal pattern of regional cerebral blood flow (rCBF) that is characteristic of the disease is bilaterally decreased perfusion in the temporal and parietal regions [13]. However, heterogeneous patterns of rCBF deficits have been seen in AD on examination with SPECT. This heterogeneity may reflect either different stages of the disease or cognitive subtypes [14]. In DLB, temporoparietal hypoperfusion has been shown to be associated with occipital hypoperfusion, which could explain the visual hallucinations in this illness [15].
The aim of this study was to evaluate FDG-PET brain imaging in assessing clinically manifested DLB by measuring regional glucose metabolism.
Methods
Five patients (5 males, mean age 75 y, age range 69–79) with fulfilled clinical criteria for DLB were referred to our department for PET examination. The disease duration was 3 ± 1 y. Every patient underwent a psychological examination including MMSE (mini mental state examination). Magnetic resonance imaging (MRI) of the brain was performed in all the patients. The data gathered from the patients were compared to the PET brain images from a normal data base in our department (six normal subjects, 2 males, 4 females, mean age 63, age range 45–79). The normal subjects had no history of neurological or psychiatric disease. The PET images were obtained 30 minutes after i.v. injection of 150 MBq FDG using a Siemens ECAT-ART (CTI, Knoxville, TN, USA). Attenuation correction of the images was made with an additional transmission scan using two 137-Cs point sources [16]. Reconstruction was performed using the filtered back projection method (FBP) applying a Gaussian filter (FWHM 4.0 mm) and a zoom factor of 2.50. An acquisition matrix size of 128 × 128 was chosen. Six regions of interest (ROI) were drawn on a 10 mm thick transaxial brain slice at 4.5 cm parallel to the cantomeatal line (CML) and two ROIs in the central region, and the SUVs of these regions were measured. Z scores for each patient were calculated according to the formula of Albin et al [17], as follows:
Z score = (patient regional SUV - controls regional SUV (mean value))/ controls regional SUV standard deviation).
A Z score of ≤ -2 was considered to be significant.
Results
Diffuse reduced glucose uptake in the entire cortical region with relative sparing of the central region (Figure 1), but including the occipital cortex was determined in all the patients, which was less remarkable in patient 2. The mean SUV in the patient group was 3.30 ± 0.30 vs. 6.10 ± 0.70 in the normal subjects. The detailed Z-scores of the patients' cerebral metabolic patterns are shown in Table 1. MRI of the brain showed a mild degree of cortical atrophy in three of the patients without further specific pathologic findings.
Figure 1.
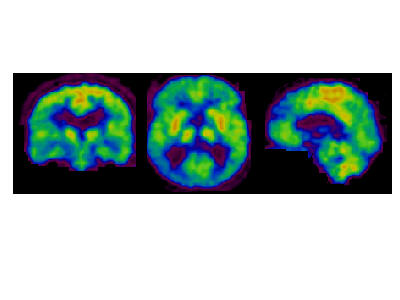
m, 75 y, Brain FDG-PET shows a diffuse glucose hypometabolism with relative sparing of the central region
Table 1.
Z scores of patient regional mean SUV compared to controls
| Patient Nr. | r.fr. | l.fr. | r.tp. | l.tp. | r.oc. | l.oc. | r.c. | l.c. |
| P1 | -2.7 | -3.1 | -3.1 | -3.4 | -3.0 | -3.1 | -1.4 | -2.3 |
| P2 | -1.8 | -2.1 | -2.3 | -2.0 | -2.9 | -3.1 | -1.4 | -0.6 |
| P3 | -2.9 | -3.2 | -2.6 | -3.4 | -2.6 | -2.9 | -1.8 | -1.9 |
| P4 | -2.3 | -2.7 | -2.3 | -3.1 | -2.3 | -2.7 | -1.9 | -1.7 |
| P5 | -2.6 | -3.0 | -2.7 | -3.5 | -2.7 | -3.1 | -2.2 | -2.1 |
r. = right, l. = left, fr. = frontal, pt. = temporoparietal, oc. = occipital, c. = central
Discussion
Postmortem examination remains the only way to definitively confirm the diagnosis of DLB, which is based on evidence of Lewy bodies in the cortex, the subcortical regions (nucleus basalis of Meynert) and the brain stem (substantia nigra and locus coeruleus) [5,18,19]. With the promise of neuroprotective treatments, early diagnosis is increasingly important. A more extensive cholinergic deficit has been observed in DLB compared with AD [9]. This observation explains the beneficial effects of cholinergic therapy in DLB (cholinesterase inhibitor), which has been shown to improve impaired cognitive functions [20]. This implies that early diagnosis would greatly improve the effectiveness of the treatment.
Although clinical criteria have been defined for DLB [3], development of objective measures to confirm clinical findings would be helpful in clinical routine. Recent studies have suggested that functional imaging with dopaminergic presynaptic ligands and postsynaptic D2 receptors like 123I-FP-CIT and 123I-iodobenzamide help to distinguish DLB from AD, since there are loss of dopaminergic neurons in DLB [21-23]. Another study demonstrated reduced vesicular monoamine transporter type 2 expression in patients with DLB indicating degeneration of nigrostriatal projections [24]. The patients described in this report fulfilled the clinical criteria for DLB. The PET examination revealed that all patients had glucose hypometabolism in the entire cortex with relative sparing of the primary sensory-motor cortex. In addition there was marked hypometabolism in the occipital cortex, which corresponds with results from other studies [1,25]. This characteristic pattern of cortical hypometabolism including the occipital areas could be a result of diaschisis due to disruption of intracortical connections. Diaschisis is defined as depression of regional neuronal metabolism and cerebral blood flow caused by dysfunction in anatomically separate but functionally related neuronal regions [26].
The results in this study were obtained in routine hospital work. We did not measure the arterial input function, as performed by Albin et al, since we consider it too invasive and inconvenient to the patient for routine use.
Conclusion
With the limitation that occipital hypometabolism may be manifested not only in DLB [27], we propose the use of a combination of FDG-PET and striatal dopamine terminal imaging as a supplement to the clinical examination to establish the final diagnosis of DLB and as the basis for a differentiated therapy.
Competition interests
None declared.
Authors' contribution
SM designed the study, performed the statistical analysis and drafted the manuscript.
PK performed the quality control of the examination procedure.
HK participated in the design of the study.
TB participated in the design of the study and preparation of the manuscript.
All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Siroos Mirzaei, Email: siroos.mirzaei@nuk.wil.magwien.gv.at.
Peter Knoll, Email: peter.knoll@nuk.wil.magwien.gv.at.
Horst Koehn, Email: horst.koehn@nuk.wil.magwien.gv.at.
Thomas Bruecke, Email: thomas.bruecke@neu.wil.magwien.gv.at.
References
- Byne EJ, Lennox G, Godwin-Austen R. Diffuse Lewy body disease: clinical features in 15 cases. J Neurol Neurosurg Psychiatry. 1989;52:709–717. doi: 10.1136/jnnp.52.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, Thal L, Pay MM, Hofstetter R, Klauber M, et al. The Lewy body variant of Alzheimer's disease: a pathological and clinical entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Lewy FH. Paralysis agitans. In: Lewandowsky M, editor. Pathologische Anantomie. Handbuch der Neurologie. Berlin: Springer Verlag; 1912. pp. 920–933. [Google Scholar]
- Hansen LA, Noville RL. Lewy body disease. Curr Opin Neurol Neurosurg. 1992;5:889–894. [PubMed] [Google Scholar]
- Ransmayr G, Wenning GK, Seppi K, Jellinger K, Poewe W. Demenz mit Lewy-Körperchen. Nervenarzt. 2000;71:929–935. doi: 10.1007/s001150050689. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Soininen H, Laulumaa V, Helkala EL, Hartikainen P, Riekkinen PJ. Extrapyramidal signs in Alzheimer's disease: a 3-year follow-up study. J Neural Transm Park Dis Dement Sect. 1992;4:107–119. doi: 10.1007/BF02251474. [DOI] [PubMed] [Google Scholar]
- Foerstl H, Burns A, Luther P, Cairns N, Levy R. The Lewy body variant of Alzheimer's disease: clinical and pathological findings. Br J Psychiatry. 1993;162:385–392. doi: 10.1192/bjp.162.3.385. [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Byrne EJ, Thornton A, Sambrook MA, Bannister P. Motor and cognitive function in Lewy body dementia: comparison with Alzheimer's and Parkinson's diseases. J Neurol Neurosurg Psychiatry. 1997;62:243–252. doi: 10.1136/jnnp.62.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DC, Pilowsky LS, Ell PJ. Nuclear Medicine in neurology and psychiatry. Lancet. 1999;354:1107–11. doi: 10.1016/S0140-6736(99)06095-X. [DOI] [PubMed] [Google Scholar]
- Goodwin GM. Functional imaging, affective disorder and dementia. Br Med Bull. 1996;52:495–512. doi: 10.1093/oxfordjournals.bmb.a011563. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Shields RA, Burjan AWI, Northen B. Single photon emission tomography using 99mTc-HMPAO in the investigation of dementia. J Neurol Neurosurg Psychiatry. 1987;50:1101–1109. doi: 10.1136/jnnp.50.9.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldemar G, Bruhn P, Kristensen M, Johnsen A, Paulson OB, Lassen NA. Heterogeneity of neocortical cerebral blood flow deficits in dementia of the Alzheimer type: a 99mTc-d,1-HMPAO SPECT study. J Neurol Neurosurg Psychiatry. 1994;57:285–295. doi: 10.1136/jnnp.57.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnemiller E, Heilmann J, Wenning GK, Berger W, Decristoforo C, Moncayo R, Poewe W, Ransmayr G. Brain perfusion scintigraphy with 99mTc-HMPAO or 99mTc-ECD and 123I-β-CIT single-photon emission tomography in dementia of the Alzheimer-type and diffuse Lewy body disease. Eur J Nucl Med. 1997;24:320–325. doi: 10.1007/s002590050060. [DOI] [PubMed] [Google Scholar]
- Mirzaei S, Knoll P, Köhn H. Comparison of different methods for attenuation correction in brain PET. Nuklearmedizin. 2000;39:N95. [PubMed] [Google Scholar]
- Albin RL, Minoshima S, D'Amato CJ, Frey KA, Kuhl DA, Sima AA. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47:462–466. doi: 10.1212/wnl.47.2.462. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Luthert PJ, Janota I, Lantos PL. Cortical Lewy body dementia: clinical features and classification. J Neurol Neurosurg Psychiatry. 1989;52:185–192. doi: 10.1136/jnnp.52.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree – a new disease? Clin Neuropathol. 1984;3:185–192. [PubMed] [Google Scholar]
- Lebert F, Souliez L, Pasquier F. Tacrine and symptomatic treatment in Lewy body dementia. In: Perry R, McKeith I, Perry E, editor. Dementia with Lewy bodies. Clinical, Pathological and Treatment Issues. Cambridge: Cambridge University Press; 1996. pp. 439–448. [Google Scholar]
- Walker Z, Costa DC, Ince P, McKeith IG, Katona CLE. In-vivo demonstration of dopaminergic degeneration in dementia with Lewy bodies. The Lancet. 1999;354:646–647. doi: 10.1016/S0140-6736(99)01178-2. [DOI] [PubMed] [Google Scholar]
- Walker Z, Costa DC, Livingston G, Walker RW, Jansen A, Katona CL. Lewy body dementia: the study of post-synaptic dopaminergic receptors with 123I-IBZM SPET. Eur J Nucl Med. 1997;24:609–614. doi: 10.1007/s002590050094. [DOI] [PubMed] [Google Scholar]
- Walker Z, Costa DC, Walker RW, Shaw K, Gacinovic S, Stevens T, Livingston G, Ince P, McKeith IG, Katona CL. Differentiation of dementia with Lewy bodies from Alzheimer's disease using a dopaminergic Presynaptic ligand. J Neurol Neurosurg Psychiatry. 2002;73:134–140. doi: 10.1136/jnnp.73.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Desmond TJ, Albin RL, Frey KA. Striatal monoaminergic terminals in Lewy body and Alzheimer's dementias. Ann Neurol. 2002;51:767–771. doi: 10.1002/ana.10186. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Foster NL, Sima AA, Frey KA, Albin RL, Kuhl DE. Alzheimer's disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50:358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- Brunberg J, Frey K, Horton J, Kuhl Crossed cerebellar diaschisis: occurence and resolution demonstrated with PET during carotid temporary ballon occlusion. AKNR. 1992;13:58–61. [PMC free article] [PubMed] [Google Scholar]
- Levine DN, Lee JM, Fischer CM. The visual variant of Alzheimer's disease: a clinicopathologic case study. Neurology. 1993;43:305–313. doi: 10.1212/wnl.43.2.305. [DOI] [PubMed] [Google Scholar]


