Abstract
Background
Ultrasonography is able to detect adult Wuchereria bancrofti worms in scrotal lymphatic vessels of infected men on account of the characteristic pattern of adult worm movements, known as the filarial dance sign. Furthermore, the technique is able to delineate associated pathology, such as hydrocoele and lymphoedema, which can be diagnosed in early stages. Ultrasonography is also useful in the assessment of macrofilaricidal effects of antifilarial medication.
The purpose of this study was to evaluate the usefulness of scrotal ultrasonography, in combination with a new method of digital documentation, in men infected with Wuchereria bancrofti.
Methods
Ultrasonography of the scrotal areas was carried out in 33 male patients from an endemic area in Ghana using a hand-carried ultrasound system and a linear array transducer at 7.5 MHz. Wuchereria bancrofti infection was also assessed by quantification of night blood microfilaraemia and semi-quantitative detection of circulating filarial antigen. Ultrasound findings were documented by print outs and by Digital Video sequences directly exported from the ultrasound machine which were edited in Final Cut Pro 3® and exported, using QuickTime® Pro, as MPEG-1 video.
Results
Worm nests, i.e. dilated lymphatic vessels with the characteristic movement patterns of worms, were found in all patients, and typical examples of larger as well as smaller nests are presented through MPEG-1 video in b- and m-modes as well as Colour Doppler and Pulse Wave Doppler images.
Conclusion
In this study, the filarial dance sign is being made available on the Internet to readers through MPEG-1 video. This method allows for demonstration of movement patterns rather than static images. In addition, the pathologic ultrasonographic signs of filariasis can be rapidly relayed over great distances and may be helpful to other investigators or clinicians in the diagnosis of patients infected with Wuchereria bancrofti.
Keywords: Wuchereria bancrofti, lymphatic filariasis, scrotal ultrasound, Filaria dance sign
Background
Lymphatic filariasis is a devastating and disfiguring disease that is a major public health problem with socioeconomic impacts in Africa, Asia, the Western Pacific and the Americas. 120 million people are estimated to be infected and at least 40 million are disabled both physically and psychosocially [1]. 1.2 billion people are at risk of acquiring the infection in more than 80 endemic countries. 90% of these infections are caused by Wuchereria bancrofti, and the remainder by Brugia spp. Humans are the only host for W. bancrofti [2]. Approximately 10–30% of the infected individuals develop clinical findings such as hydrocoele, adenolymphangitis and lymphoedema that can progress to elephantiasis.
Several studies have shown that adult W. bancrofti can be detected in the scrotal area of infected men by ultrasound [3-6]. It is reported that these adult worms are found mostly in dilated intrascrotal lymphatic vessels, and that the locations of the worm nests are stable over time [4]. The typical movement of these filariae, called the filarial dance sign (FDS), provides an opportunity to observe the adult worms in vivo. At the same time, ultrasound examination offers a method to detect pathological changes, such as the degree of dilatation in the lymphatic vessels, early and advanced stages of hydrocoele, and the number of worm nests over time.
Examination by ultrasound is a non-invasive tool that will become increasingly important in the long-term observation of lymphatic filariasis. For this reason, documentation of the findings is essential for scientific research. Up to now, the documentation has been published as print outs [4], typically showing a dilated lymphatic vessel with adult worms. Additionally, Amaral et al., [3] and, in higher resolution, Faris et al., [6] documented the movements of adult worms by Pulse Wave Doppler, seen as an undulating band. The objective of this study was to show, for the first time, the typical worm movements to the reader as MPEG-1 video, using a digital camcorder to record the dilated lymphatic vessels containing adult worms exhibiting the filaria dance sign.
Material and Methods
Study site
The study was carried out in cooperation with the Health Administration of the Nzema East district at Axim (Western Region), the Kumasi Center for Collaborative Research (KCCR), and the Kwame Nkrumah University of Science and Technology (KNUST) in Kumasi, Ghana. It was approved by the committee on human research and ethics of the School of Medical Science at KNUST, Ghana. The study conformed to the principles of the Helsinki Declaration of 1975 (as revised 1983 and 2000) [7]. Informed consent was obtained from all participants.
The study took place in six villages in the Nzema East district in the Western Region of Ghana. The villages are located on the coast where three rivers Ankobra, Ebi and Fia flow into the Gulf of Guinea. Several lagoons are located in this area near to the villages offering breading sites for the mosquito vector, and 10–15% of inhabitants are microfilaraemic. The primary occupation of male villagers is as fishermen and farmers.
Male residents in the six villages were initially screened for microfilariae (mf) by night blood examination between 9 pm and 11 pm by thick smear.
31 men were found to be positive for mf and these, along with 2 other individuals who were mf negative, but presented with hydrocele, were enrolled in the study (n = 33, range 17 – 56 years, median 32 years).
A patient questionnaire was completed for each participant with patient number, village number, age and history of antifilarial drug intake during the last two years. All patients were clinically examined for sequela of lymphatic filariasis such as hydrocele, lymphoedema, ulcers and swelling of inguinal and/or femoral lymph nodes.
29 patients consented to collection of venous blood, which was used to more accurately quantify mf counts (This was collected between the hours of 10 pm and 12 am). Briefly, mf was assessed after filtering 0.1 – 7 ml of venous blood (depending on the mf load screened in the thick smear) through a Whatman Membrane Polycarbonate 25 mm Nucleopore 5 μm filter (Eurolab Merck, Darmstadt / Germany). The filters were Giemsa stained for mf-counting.
Semi-quantitative detection of circulating adult filarial antigen was determined by use of an Og4C3 ELISA test kit (M/S TropBio® kit, Tropical Biotechnology Pty Ltd, Townsville, Queensland, Australia) on 50 μl of serum (diluted 1:4). Colour intensity for a given serum sample was compared by eye to pre-diluted standards from the manufacturer (levels: 32000, 8192, 2048, 512, 128, 32, <10 antigen units (AU), corresponding to titer groups 7, 6, 5, 4, 3, 2, 1 whereby level 32000 = titer group 7, and so on until <10 = titer group 1). Titer group 3 is considered equivocal, groups 2 and 1 are considered antigen negative.
Ultrasound examination
Ultrasound examination was carried out between 2 pm and 7 pm in a darkened room using a SonoSite 180 PLUS hand-carried ultrasound system (SonoSite Inc.; Washington, USA http://www.sonosite.com/) equipped with an L 38 mm 5–10 MHz linear array transducer. For scrotal ultrasound a frequency of 7.5 MHz was used. The men were examined in a supine position in order to reduce interference by movements of the patients themselves. Ultrasound gel was kept at room temperature to reduce interference from the cremasteric muscle.
In each patient the scrotum was first scanned in transverse sections of the right and the left side and then in longitudinal sections. The transducer was positioned in panorama-mode at each section to provide more information and better localization of the worm nests. The examiner took print outs and Digital Video sequences of the worm nests by keeping the transducer in a 90° angle to the skin surface in transverse and longitudinal sections.
Every worm nest detected by the typical movements of the adult worms (FDS) in two-dimensional b-mode search was confirmed by one-dimensional m-mode imaging. The m-mode is defined as a single beam of ultrasound whereby the reflected signals are displayed as dots of varying intensities over time.
Colour Power Doppler (CPD) and Pulse Wave Doppler (PWD) imaging were used as additional tools to differentiate the filaria dance sign of adult worms in lymphatic vessels from blood flow in veins and arteries. The Colour Power Doppler shows the movements of lymphatic fluids induced by the worms as irregular red signals in different parts of the worm nest, in contrast to pulsating signals due to blood flow in arteries. Before using the PWD, the nests were first visualized by b-mode imaging. The caliper was positioned where the filaria dance sign was seen. After the switch to the Pulse Wave Doppler mode, the signals on top of the neutral line were seen as the movement of the worms in direction to the transducer, while the signals below the neutral line were the movements of the worms away from the transducer.
Hydrocele was defined according to the criteria in the WHO-issued manual of diagnostic ultrasound [8], as fluid in the scrotum surrounding the testis with an echo free region, varying in size and position. The dimensions of hydrocele were recorded by freezing the b-mode image and measured in transverse and longitudinal diameter. In cases of very big hydrocele, where the hydrocele was larger than the length of the transducer (3.8 cm), the hydrocele was documented by taking print outs and videos of the part of the fluid that surrounded the testis (see additional file 1,2,3,4,5,6,7,8,9).
Documentation method
Ultrasound findings were documented by print outs of each worm nest in all patients in b- and m-mode as well as by print outs of the hydroceles. Digital Video sequences of each worm nest were recorded in b-, m-mode and Colour Power Doppler on mini DV-tapes, using a SONY-PC 120E PAL Handycam® (Sony Corporation, Japan http://www.sony.com/) which was directly connected to the ultrasound machine. Digital Video sequences were edited using Final Cut Pro 3® (Apple Computer Inc., California, USA http://www.apple.com/finalcutpro/) and exported, using QuickTime® Pro (Apple Computer Inc., California, USA http://www.apple.com/quicktime/upgrade/), as MPEG-1 video (Moving Picture Experts Group http://mpeg.telecomitalialab.com/).
At the end of the study all patients were treated with ivermectin (4 tablets à 3 mg). Shortly thereafter, there was the onset of mass treatment with ivermectin and albendazole in the district.
Results
Study participants
33 men between the ages of 17 and 56 years (median 32 years) participated in the study.
In 27 (81.8%) men, mf counts were found with a range of 10 – 10760 mf/ml (median 320 mf/ml). Four men (12.1%) were mf-negative. The remaining two (6.1%) patients were only willing to undergo ultrasound examination, but were known to be mf-positive eight months earlier.
8 (24.2%) of the participants of this study had taken ivermectin (4 tablets à 3 mg) eight months before ultrasound and blood examination, whereas 25 (75.8%) patients had not been treated for filariasis before examination.
The results of the Og4C3 ELISA are shown in Table 1.
Table 1.
Distribution of patients in titer group (as measured by Og4C3 ELISA) (AU = Antigen Units)
| Titer group | 7 (32000 AU) | 6 (8192 (AU) | 5 (2048 AU) | 4 (512 AU) | 3 (128 AU) | 2 (32 AU) | 1 (< 10 AU) |
| No. patients (n = 29) | 24 (82.8%) | 2 (6.9%) | 1 (3.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (6.9%) |
There was no clear correlation between the number of worm nests detected by ultrasonography and the level of circulating adult filarial antigen (as measured by Og4C3 ELISA), nor between mf count and the number of worm nests.
Patients with hydrocele
In four men (12.1%) a hydrocele was suspected based on findings during clinical examination. Upon ultrasound examination, 9 (27.3%) out the 33 patients were found to have hydroceles.
Two men with hydrocele were amicrofilaraemic, six patients with hydrocele were mf positive and one patient (who declined to be tested for mf) with hydrocele was known to be mf positive eight months previously. Mf counts ranged from 248 to 4640 mf/ml (median 1493 mf/ml). In patients with hydrocele we found 25 worm nests (Table 3). None of the patients had lymphoedema or ulcera of the lower extremities.
Table 3.
Number of adult worm nests detected in patients who had been previously treated with ivermectin before ultrasound examination and in untreated patients.
| Treatment | All patients / No. nests | Hydrocele patients / No. nests |
| Ivermectin | 8 / 19 (24.2 % / 20.2 %) | 3 / 7 (33.3 % / 28.0 %) |
| Untreated | 25 / 75 (75.8 % / 79.8 %) | 6 / 18 (66.6 % / 72.0 %) |
| Total | 33 / 94 (100 % / 100 %) | 9 / 25 (100 % / 100 %) |
Ultrasound findings
Worm nests
In ultrasonography, at least 1 worm nest was detected in each participant, characterized by a dilated lymphatic vessel with 1 or more worms exhibiting the filaria dance sign [3,4,6]. The number of worm nests per individual varied from 1 to 5 (median 3). A total of 94 worm nests were detected: 39 (41.5%) worm nests in the right scrotum and 55 (58.5%) in the left scrotum. 37 (39.4%) nests were located in supra-testicular, 17 (18.1%) in mid-testicular and 40 (42.5%) in infra-testicular position (Table 2 &3).
Table 2.
Distribution of adult worm nest locations as revealed by scrotal ultrasound
| Position | Supra-testicular | Mid-testicular | Infra-testicular | Total |
| Right scrotum | 14 (14.9%) | 9 (9.6%) | 16 (17%) | 39 (41.5%) |
| Left scrotum | 23 (24.5%) | 8 (8.5%) | 24 (25.5%) | 55 (58.5%) |
| Total | 37 (39.4%) | 17 (18.1%) | 40 (42.6%) | 94 (100%) |
Measurement of dilated lymphatic vessels
The diameters of the worm nests were measured in cm using m-mode imaging. The high quality of the m-mode imaging in the Sonosite 180 Plus shows the phasic motion of adult worms within the dilated lymphatic vessel, allowing a precise measurement since the wall of the vessel could be determined exactly. In every worm nest the examiner took the largest diameter of the vessel in transverse sections. The diameter of the 94 worm nests detected varied between 0.2 cm and 2 cm (median 0.6 cm).
Representative examples of worm nests
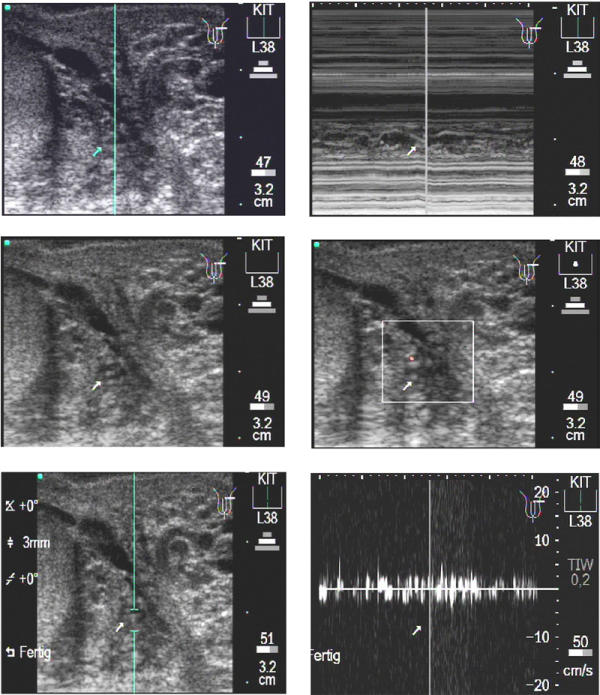
Figure 1 shows the procedure of documentation performed for every worm nest. The upper left image displays, in b-mode, a worm nest with a large volume of free lymphatic fluid, followed by the positioning of the cursor for the m-mode function. The upper right image shows the m-mode image where the adult worms are seen as wavy bands. The middle left image shows again the b-mode image of the same worm nest, followed by the middle right image which displays a Colour Power Doppler image. In this mode the movements of the worms can be detected easily, causing an irregular red Doppler signal at different locations within the lymphatic vessel, due to the moving of fluid by the worm(s).
Figure 1.
Transverse scan of a patient's left testis. Upper left: In para-testicular position an enlarged lymphatic vessel with one or more adult worms can be seen (arrow). The blue line depicts the cursor position of the following m-mode, upper right. The corresponding video image can be seen as Additional File 1: Movie1A.mpg. Middle left: The same worm nest as seen above. Middle right: The use of the Colour Power Doppler shows that this worms nests contains much free lymphatic fluid which induces a red signal due to the worm movements in different parts of the worm nest. Different to vessels, the signal is non-rhythmic and non-pulsating. The corresponding video image can be seen as Additional File 2: Movie1B.mpg. Lower left: The same worm nests as seen above. The caliper marks the position at which the Pulse Wave Doppler (PWD) is performed. Lower right: After switch to the PWD mode the filaria dance sign (FDS) is seen as an undulating band as a function of time, with sharp, irregular peaks (turquoise arrowhead). The corresponding video image can be seen as Additional File 3: Movie1C.mpg.
The lower left image shows, in b-mode, the position of the caliper for the Pulse Wave Doppler which is shown in the lower right image. In this mode, the movements of the adult worms can be seen as undulating bands with sharp peaks (the turquoise arrowhead depicts an example).
In Figure 1, images depict transverse sections and the worm nest is located in para-testicular position.
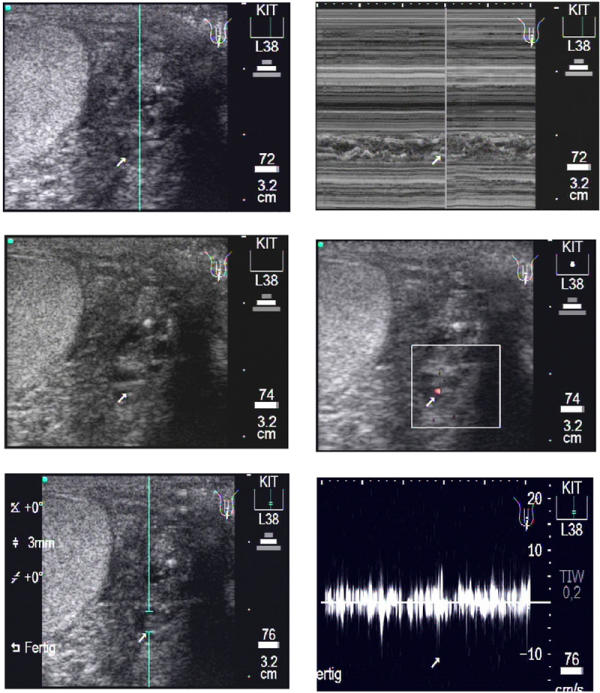
Figure 2 presents a smaller worm nest with less lymphatic fluid around the adult worm than in Figure 1. This worm nest is documented in transverse sections. According to our method of documentation we first (upper left) recorded the b-mode image of the worm nest followed by the m-mode image (upper right). In this m-mode image the body wall of the adult worm can be visualized as double layers in form of wavy bands. The Colour Power Doppler in the middle right image presents the irregular movements of the adult worms which makes it easy to differentiate this pattern from regularly pulsating arteries or veins. The lower left image shows the position of the caliper for the Pulse Wave Doppler. In the lower right image, after the switch to this mode an undulating band as function of worm movement over time is shown.
Figure 2.
Transverse scan of a patient's left testis. Upper left: The b-mode image shows the worm nest (arrow) in para-testicular location with the cursor (blue line) positioned for the following m-mode image. Upper right: In the m-mode image the body wall (arrow) of the adult worms is detectable as double layers in form of wavy bands. The corresponding video image can be seen as Additional File 4: Movie2A.mpg. Middle left: The same worm nests as above. In contrast to the worm nest seen in fig. 1 this worm nest is smaller and contains less lymphatic fluid. Middle right: The Colour power Doppler-mode shows clearly less red signals than in Fig. 1 (middle right, or movie 1B). The corresponding video image can be seen as Additional File 5: Movie2B.mpg. Lower left: The same worm nests as above. The caliper for the PWD is positioned where the worm nest is located. Lower right: The following PWD confirms the fact that this worm nest is smaller than the one Fig. 1, as documented by lower peaks, again characterized by the irregular undulating band in contrast to pulsating amplitudes caused by arteries. The corresponding video image can be seen as Additional File 6: Movie2C.mpg.
Below and to the left of the main nest there is clearly another, smaller, nest to be seen.
Figure 3 follows the same method of documentation as in Figures 1 and 2. It shows longitudinal sections of a para-testicular worm nest. The body of the adult worm is again seen as double layers in m-mode imaging. This worm nest is medium sized. In Colour Power Doppler mode there is less lymphatic fluid that causes nearly no color signal. The Pulse Wave Doppler image an irregular undulating band with smaller amplitudes than in Figure 1, which confirms that this lymphatic vessel is less dilated than the one seen in Figure 1, but also contains one or more worms.
Figure 3.
Longitudinal scan of a patient's left testis. Upper left: A medium sized para-testicular worm nest (arrow) is presented in this b-mode image. Upper right: The following m-mode section is positioned at the location of the largest diameter of the worm nest. The corresponding video image can be seen as Additional File 7: Movie3A.mpg. Middle left: The same worm nest as above. Middle right: The Colour Doppler-mode presents very few red signals as sign of less lymphatic fluid moved by the adult worms in this dilated lymphatic vessel. The corresponding video image can be seen as Additional File 8: Movie3B.mpg. Lower left: The same worm nest as above. The caliper for the PWD is positioned where the worm nest is located. Lower right: The Pulse Wave Doppler-mode confirms the medium sized worm nest by the irregular undulating band caused by the typical movements of the adult worms. The corresponding video image can be seen as Additional File 9: Movie3C.mpg.
Discussion
Several recent studies have shown the value of ultrasonography in the diagnosis of lymphatic filariasis as a non-invasive and cost-effective tool [3,4,6,9-12]. Ultrasonography offers a suitable method to detect adult worm nests as well as the degree of dilatation in the lymphatic vessels [5].
In the present study the filarial dance sign was observed in all microfilaraemic patients, which is higher than that reported by Faris et al., [6] and by Norões et al., [13] who described that in 37% and 80% of the microfilaraemic men respectively, worm nests were detected.
Increased sensitivity of ultrasonography in our study may be related to higher intensities of infection in Ghana and / or a higher tropism of adult Wuchereria bancrofti worms for the scrotal area in these patients related to unknown host factors. By using the Pulse Wave Doppler we were able to verify even less enlarged lymphatic vessels as worm nests, by the characteristic pattern of irregular worm movements in this mode, which in a few cases may not have been detected when using b- or m-mode imaging only. Conversely, in vessels below a diameter of 0.2 cm it becomes increasingly difficult without a PWD to clearly differentiate between dancing worms and the flow of arteries or veins because the typical pulsations are harder to detect in b-mode only.
In this study, two patients were included with ultrasound findings, but found to be negative for circulating antigen, (as measured by Og4C3 ELISA). These patients were also amicrofilaraemic (0 mf / 7 ml blood filtered). This confirms results of recent studies [12] where worm nests in the scrotal area were detected in patients who tested amicrofilaraemic at the time point of examination. Our study also confirms that the sensitivity of the Og4C3 assay may be low in persons who are microfilaria-negative or have a mf density of < 1 mf/ml [14,15]. The data also show that the administration of ivermectin 8 months prior to ultrasound examination in a subgroup of patients did not affect the number of worm nests, as expected from the action of this drug (Table 3).
b- and m-mode imaging
There are different methods to measure and to present the worm nests [4,6]. However, the adult worms are detected by b-mode imaging first and can then be confirmed by m-mode imaging, where the worms are seen as wavy bands, and / or by using further Doppler techniques. In our study by using the m-mode imaging, the body wall of the adult worms of W. bancrofti could be visualized as double layers within the dilated lymphatic vessels. The diameter of the lymphatic vessel could be measured easily in this mode.
Colour Power Doppler
Using the Colour Power Doppler (CPD) it is shown that in dilated lymphatic vessels the movement of the lymphatic fluid can be visualized in form of a red color signal. This contributes to the movements of the adult worms in large worm nests with a lot of lymphatic fluid around the adult worms. However, there is a clear difference in the CPD signal of adult worms compared to arteries and veins since the worm signals do not follow a pulsating rhythm but show irregular red signals within the dilated lymphatic vessel.
Pulse Wave Doppler
An innovative additional method originally published by Amaral et al. [3] and corroborated by Faris et al. [6], is the Pulse Wave Doppler (PWD) technique where the worm nests are first detected by b-mode imaging. After the switch to the Pulse Wave Doppler mode, the signals on top of the neutral line in this mode are seen as the movements of the worms in direction to the transducer while the signals below the neutral line are movements of the worms away from the transducer. Faris et al., described in detail the advantage of the PWD as a technique which facilitates the examination by allowing differentiation of scrotal lymphatic vessels from veins and arteries that have characteristic appearance due to unidirectional flow [6].
In the present study we used both Doppler techniques in order to compare the images and to get unequivocal findings. A pulsating rhythm was seen in arteries, whereas the worm movements in both Doppler-modes appear to be irregular. The data confirm earlier studies that the PWD can be an effective additional tool to differentiate exactly between blood vessels and adult worms moving in dilated lymphatic vessels.
If one has to make a choice between either one of the two Doppler modes, we would clearly favor the PWD which allows to monitor these movements over time and thus, in print version only. In addition, the PWD technique could bring a major benefit in the future by facilitating the monitoring of worm movement speed, which may slow down when the worms degenerate as a result of treatment with a macrofilaricidal drug. The extra cost of this device amounts to approx. 20% of the machine.
Conclusion
The purpose of this study was to provide a digital method of documenting scrotal ultrasonographic findings in lymphatic filariasis by making "real time" MPEG-1 video from scrotal ultrasound examinations available on the Internet. In particular in the b-mode and the Colour Doppler mode, the film sequences offer an impression of the filaria dance sign within the lymphatic vessels. While the m-mode imaging shows the phasic motion of the adult worms as wavy bands presenting the body wall of the adult worm as a double layer structure, the Pulse Wave Doppler mode shows the worm movements as a function of time seen as an undulating band.
This digital method of documentation offers the possibility to compare, over long distances, pathological findings in the lymphatic vessels of those infected with Wuchereria bancrofti.
Authors' contributions
S. Mand recruited the patients, did the ultrasound examination, obtained blood samples, compiled the data and wrote the paper draft. Y. Marfo-Debrekyei performed patient management during recruitment and ultrasound examinations. M. Dittrich participated with supervision of the ultrasound techniques and interpreted the ultrasound findings. K. Fischer performed the microfilaraemia analysis and Og4C3 ELISA tests. O. Adjei did preparatory studies for proper selection of villages, negotiations with the village elders, and performed the ethical clearance. A. Hoerauf designed and supervised the study, participated with patient recruitment and ultrasound examinations, compiled the data and edited the final manuscript version.
Supplementary Material
Transverse scan of left testis. In para-testicular position an enlarged lymphatic vessel can be seen (arrow). One or more adult worms are detectable by their typical movements (filaria dance sign, FDS). The following m-mode imaging presents the dilated lymphatic vessels, containing moving adult worms, as a section marked by the cursor in the b-mode image.
Transverse scan of left testis. The same worm nest as seen in Figure 1A in b-mode imaging. The following use of the Colour Power Doppler shows that this worms nests contains much free lymphatic fluid which induces an irregular red signal due to the worm movements in different parts of the worm nest.
Transverse scan of left testis. The same worm nests as seen in movie 1A and 1B. The caliper marks the position at which the PWD is performed. After switch to the Pulse Wave Doppler-mode the filaria dance sign (FDS) is seen as an undulating band as a function of time, with sharp, irregular peaks (turquoise arrowhead).
Transverse scan of left testis. The b-mode image shows the worm nest (arrow) in para-testicular location with the cursor positioned for the following m-mode image (below and to the left of the main nest, there is another, smaller, nest visible). In the m-mode image the body wall (arrow) of the adult worms is detectable as double layers in form of wavy bands.
Transverse scan of left testis. The same worm nests as seen in movie 2A. In contrast to the worm nest seen in movie 1A-C this worm nest is smaller and contains less lymphatic fluid. The Colour power Doppler-mode shows clearly less red signals than movie 1B.
Transverse scan left normal testis. The same worm nest as seen in movie 2A. The caliper is positioned where the worm nest is located. The following Pulse Wave Doppler-mode confirms the fact that this worm nest is smaller than the one in movie 1A-C as documented by lower peaks, again characterized by the irregular undulating band in contrast to pulsating amplitudes caused by arteries.
Longitudinal scan left testis. A medium sized para-testicular worm nest (arrow) is presented in this b-mode image. The following m-mode section is marked by the positioned cursor at the location of the largest diameter of the worm nest.
Longitudinal scan left testis. The same worm nest as seen in movie 3A. The Colour Doppler-mode presents very few red signals as sign of less lymphatic fluid moved by the adult worms in this dilated lymphatic vessel.
Longitudinal scan left testis. The same worm nest seen as in movie 3A. The Pulse Wave Doppler-mode confirms the medium sized worm nest by the irregular undulating band caused by the typical movements of the adult worms.
Acknowledgments
Acknowledgements
We would like to thank the individuals of the District Health Management Nzema East, Western Region, Ghana, for their cooperation. We would like to thank Dr Schueler, Bernhard-Nocht-Institute, Hamburg, for help with the preparing the real time MPEG-1 movies. We are obliged to the members of the KCCR for excellent support in our study.
We are grateful for the financial support by the German Research Foundation (DFG grant Ho 2009/1-3), the European Commission (grant ICA4-CT-2002-10051), the VW-Foundation (grant I/73952) and the Caritas Charity.
Contributor Information
Sabine Mand, Email: sabinemand@hotmail.com.
Yeboah Marfo-Debrekyei, Email: kccr@africaonline.com.gh.
Matthias Dittrich, Email: dittrich@kinder.klinik.uni-mainz.de.
Kerstin Fischer, Email: kfischer@bni.uni-hamburg.de.
Ohene Adjei, Email: oadjei@africaonline.com.gh.
Achim Hoerauf, Email: hoerauf@bni.uni-hamburg.de.
References
- Ottesen EA. The Global Programme to Eliminate Lymphatic Filariasis. Trop Med Int Hlth. 2000;5:591–594. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- Anonymous The Global Alliance for the Elimination of Lymphatic Filariasis - Epidemiology. http://www.filariasis.org/ 2002.
- Amaral F, Dreyer G, Figueredo-Silva J, Norões J, Cavalcanti A, Samico SC, Santos A, Coutinho A. Live adult worms detected by ultrasonography in human Bancroftian filariasis. Am J Trop Med Hyg. 1994;50:753–757. doi: 10.4269/ajtmh.1994.50.753. [DOI] [PubMed] [Google Scholar]
- Dreyer G, Amaral F, Norões J, Medeiros Z. Ultrasonographic evidence for stability of adult worm location in bancroftian filariasis. Trans R Soc Trop Med Hyg. 1994;88:558. doi: 10.1016/0035-9203(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Dreyer G, Addiss D, Roberts J, Norões J. Progression of lymphatic vessel dilatation in the presence of living adult Wuchereria bancrofti. Trans R Soc Trop Med Hyg. 2002;96:157–161. doi: 10.1016/s0035-9203(02)90288-9. [DOI] [PubMed] [Google Scholar]
- Faris R, Hussain O, El Setouhy M, Ramzy RM, Weil GJ. Bancroftian filariasis in Egypt: visualization of adult worms and subclinical lymphatic pathology by scrotal ultrasound. Am J Trop Med Hyg. 1998;59:864–867. doi: 10.4269/ajtmh.1998.59.864. [DOI] [PubMed] [Google Scholar]
- World Medical Association The Helsinki Declaration. http://www.wma.net/e/policy/17-c_e.html. 2000.
- Palmer P, editor. Manual of Diagnostic Ultrasound. World Health Organisation in collaboration with the World Federation for Ultrasound in Medicine and Biology; 1995. [Google Scholar]
- Dreyer G, Norões J, Amaral F, Nen A, Medeiros Z, Coutinho A, Addiss D. Direct assessment of the adulticidal efficacy of a single dose of ivermectin in bancroftian filariasis. Trans R Soc Trop Med Hyg. 1995;89:441–443. doi: 10.1016/0035-9203(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Dreyer G, Amaral F, Norões J, Medeiros Z, Addiss D. A new tool to assess the adulticidal efficacy in vivo of antifilarial drugs for bancroftian filariasis. Trans R Soc Trop Med Hyg. 1995;89:225–226. doi: 10.1016/0035-9203(95)90506-5. [DOI] [PubMed] [Google Scholar]
- Dreyer G, Addiss D, Norões J, Amaral F, Rocha A, Coutinho A. Ultrasonographic assessment of the adulticidal efficacy of repeat high-dose ivermectin in bancroftian filariasis. Trop Med Int Health. 1996;1:427–432. doi: 10.1046/j.1365-3156.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- Dreyer G, Santos A, Norões J, Amaral F, Addiss D. Ultrasonographic detection of living adult Wuchereria bancrofti using a 3.5-MHz transducer. Am J Trop Med Hyg. 1998;59:399–403. doi: 10.4269/ajtmh.1998.59.399. [DOI] [PubMed] [Google Scholar]
- Norões J, Addiss D, Amaral F, Coutinho A, Medeiros Z, Dreyer G. Occurrence of living adult Wuchereria bancrofti in the scrotal area of men with microfilaraemia. Trans R Soc Trop Med Hyg. 1996;90:55–56. doi: 10.1016/s0035-9203(96)90478-2. [DOI] [PubMed] [Google Scholar]
- Rocha A, Addiss D, Ribeiro ME, Norões J, Baliza M, Medeiros Z, Dreyer G. Evaluation of the Og4C3 ELISA in Wuchereria bancrofti infection: infected persons with undetectable or ultra-low microfilarial densities. Trop Med Int Health. 1996;1:859–864. doi: 10.1111/j.1365-3156.1996.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Simonsen PE, Dunyo SK. Comparative evaluation of three new tools for diagnosis of bancroftian filariasis based on detection of specific circulating antigens. Trans R Soc Trop Med Hyg. 1999;93:278–282. doi: 10.1016/s0035-9203(99)90022-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transverse scan of left testis. In para-testicular position an enlarged lymphatic vessel can be seen (arrow). One or more adult worms are detectable by their typical movements (filaria dance sign, FDS). The following m-mode imaging presents the dilated lymphatic vessels, containing moving adult worms, as a section marked by the cursor in the b-mode image.
Transverse scan of left testis. The same worm nest as seen in Figure 1A in b-mode imaging. The following use of the Colour Power Doppler shows that this worms nests contains much free lymphatic fluid which induces an irregular red signal due to the worm movements in different parts of the worm nest.
Transverse scan of left testis. The same worm nests as seen in movie 1A and 1B. The caliper marks the position at which the PWD is performed. After switch to the Pulse Wave Doppler-mode the filaria dance sign (FDS) is seen as an undulating band as a function of time, with sharp, irregular peaks (turquoise arrowhead).
Transverse scan of left testis. The b-mode image shows the worm nest (arrow) in para-testicular location with the cursor positioned for the following m-mode image (below and to the left of the main nest, there is another, smaller, nest visible). In the m-mode image the body wall (arrow) of the adult worms is detectable as double layers in form of wavy bands.
Transverse scan of left testis. The same worm nests as seen in movie 2A. In contrast to the worm nest seen in movie 1A-C this worm nest is smaller and contains less lymphatic fluid. The Colour power Doppler-mode shows clearly less red signals than movie 1B.
Transverse scan left normal testis. The same worm nest as seen in movie 2A. The caliper is positioned where the worm nest is located. The following Pulse Wave Doppler-mode confirms the fact that this worm nest is smaller than the one in movie 1A-C as documented by lower peaks, again characterized by the irregular undulating band in contrast to pulsating amplitudes caused by arteries.
Longitudinal scan left testis. A medium sized para-testicular worm nest (arrow) is presented in this b-mode image. The following m-mode section is marked by the positioned cursor at the location of the largest diameter of the worm nest.
Longitudinal scan left testis. The same worm nest as seen in movie 3A. The Colour Doppler-mode presents very few red signals as sign of less lymphatic fluid moved by the adult worms in this dilated lymphatic vessel.
Longitudinal scan left testis. The same worm nest seen as in movie 3A. The Pulse Wave Doppler-mode confirms the medium sized worm nest by the irregular undulating band caused by the typical movements of the adult worms.



