Abstract
Background
The ability to detect sinusoidal vibrations on the skin surface is dependent on the activation of two classes of receptors. The density of such receptors varies across the skin surface and is a factor in determining the sensory acuity of each skin area. However, the acuity of many sensory systems is known to deteriorate with advancing age. The aim of this study was to determine if vibrotactile sensibility of several skin surfaces deteriorated equally with advancing age.
Methods
Vibration detection thresholds for two frequencies of vibration (30 Hz and 200 Hz) were determined using a method of limits protocol, in two groups of healthy adults, one group aged 17 to 27 years and the other aged 55 to 90 years. Sinusoidal vibrations were generated by a computer and delivered to the skin surface via the probe (diameter = 2 mm) of a mechanical vibrator. Four skin sites (palmar surface of the tip of the middle finger, volar surface of the forearm, lateral aspect of the shoulder, cheek just caudal to the zygoma) were tested.
Results
The fingertip was the most sensitive site for vibrotactile detection at both frequencies in a substantial majority of subjects. The older group of subjects showed significantly higher detection thresholds for both frequencies at all sites, except the fingertip, when compared to young subjects.
Conclusion
The study confirms the deterioration of vibrotactile acuity at several skin sites previously reported in the literature. However, there appears to be no significant reduction in vibrotactile detection at the fingertips in older subjects. This may reflect the high receptor density of this area, or the functional importance of vibrotactile sensibility of the fingertips or some combination of both of these factors.
Background
Inputs arising from various groups of peripheral receptors play a role in vibrotactile sensibility. Four separate channels have been proposed to mediate the sense of touch from the glabrous skin of humans [1]. The ability to detect high frequency (60–1000 Hz) vibration principally depends on the P channel, associated with Pacinian corpuscles (PC). However, by manipulating stimulus parameters a second channel the NPI channel, may also be responsive to vibrotactile stimuli in this range. The NPII channel, associated with Meissner's corpuscles in glabrous skin and hair follicle receptors in the hairy skin, and their rapidly adapting (RA) afferents fibres, appear to best respond to low frequency (2–100 Hz) vibration. The fourth channel, the NPIII channel responds best to frequencies in the range of 0.4–100 Hz [1]. There are considerable differences in the density of receptors and receptive field sizes among the glabrous skin locations, the hands and the lips being most densely populated [2,3] and having small receptive fields [3,4]. Even within the hand there is a significant increase in the density of receptors when moving from the proximal part of the finger to the fingertip [2,5], which accounts for the outstanding sensitivity of this region. The receptor density is also associated with cortical magnification, i.e. densely populated body regions having larger cortical representation [6].
The hairy skin shows location specific variations in receptor density and field size, presumably reflecting the functional roles played by the afferent input from these skin surfaces. The hairy skin of the forearm has a predominance of slowly adapting afferent units, with rapidly adapting units being only sparsely distributed [7]. The skin of the face also has a predominance of slowly adapting mechanoreceptive units, some fast adapting type I or RA units with receptive field sizes similar to those seen in the hand, and an absence of fast adapting type II or PC units [3]. The hairy skin surfaces examined in this study were chosen for their proximity on the somatosensory homunculus to the area for the glabrous hand [8]. Alterations to the somatosensory input to one area on the homunculus are known to affect the sensitivity of surrounding areas [9].
Deteriorating effects of advancing age have been demonstrated for most sensory modalities [10,11]. Vibrotactile sensibility has also been reported to show age related changes [12-20]. Although initial studies (for review see [14]) were implemented using rather crude stimulating devices, some of the more recent methodologies allowed quantitative evaluation of this sensory modality [14,15,20,21]. However, most of these quantitative studies also had limitations. They either tested a single location or skin type [14-17] and/or used a single vibration test frequency [16,17,19,20], which did not allow the assessment of separate afferent channels involved in vibrotactile signalling. In this study our aim was to investigate the effects of aging on vibrotactile sensibility in various body regions and, by using different frequencies, determine the changes in vibration information processing signalled through separate channels of afferent units.
Methods
Subjects
Testing was performed on 22 young adults (11 females and 11 males; 17–27 years old, μ ± δ, 20.2 years ± 2.2) and 22 elderly adults (11 females and 11 males; 55–90 years old, μ ± δ, 68.6 years ± 10.6). All subjects were in good general health, without any history of upper limb nerve lesions, peripheral vascular disease or diabetes mellitus. Testing was carried out in a temperature-controlled room, no measures were made of skin temperature. The study was approved by the Human Ethics Committee of the University of Sydney and conforms to the guidelines for human experimentation of the National Health and Medical Research Council of Australia.
Apparatus
Sinusoidal vibration was delivered perpendicular to the skin surface at each test site via a 2 mm diameter perspex© probe attached to the shaft of a mechanical vibrator (GWV4). The vibrator was mounted on an isolated rigid trunnion (Gearing and Watson, T4), in turn attached to a series of adjustable levers allowing the vibrator to be positioned at a range of heights and orientations. Sinusoidal waveforms were generated by a computer equipped with the Labview® application, passed to a linear power amplifier (Gearing and Watson, PA30) and then delivered to the mechanical vibrator. The use of the Labview® application allowed instantaneous alterations in the frequency and amplitude of the sinusoids. Although online measurements of the dampening effect of applied loads on displacement of the vibrator probe were not made, offline measurements using a linear displacement transducer (OD4, Schlumberger Industries) indicated that there was no reduction in the peak-to-peak amplitude of the sinusoid under loads equivalent to those experienced during data collection. As the mechanical vibrator did not emit any audible sound at the frequencies and amplitudes used in this series of experiments auditory masking was not required.
Experimental procedure
Subjects were tested at four sites bilaterally:
i) palmar surface of the distal one third of the distal pad of the middle finger,
ii) anterior surface of the forearm, in the midcubital line, at the junction of the proximal and middle one third of the forearm,
iii) lateral aspect of the shoulder at the junction of the proximal and distal halves of the deltoid muscle, and
iv) immediately inferior to the zygomatic bone on the face.
Subjects were seated with the upper limb supported, foam blocks were used to stabilise the upper limb and to hold the forearm and hand in a supine position. At each site, the apparatus was positioned so that the probe tip was making contact with the skin surface. The tip projected 2 mm through a 6 mm diameter hole in a rigid perspex© plate (300 mm2) suspended from the rigid trunnion. The plate was positioned parallel to the skin surface and acted as a guard to maintain a constant indentation of the probe into the skin of the testing site and to limit the spread of surface waves across the skin. The probe and the rigid surround were separated by a gap of 2 mm on each side.
Two different frequencies of vibrotactile stimulation were selected for preferential activation of separate afferent classes, 30 Hz to preferentially activate rapidly adapting (RA) units and 200 Hz to activate the Pacinian (PC) units.
At each site and at each frequency subjects were initially allowed to experience an amplitude of vibration above the threshold for detection. The amplitude of the test frequency was then gradually decreased (descending mode) and subjects were asked to indicate the point at which the vibration could no longer be detected. The amplitude of the sinusoidal waveform was then gradually increased from a subthreshold level (ascending mode) and subjects were asked to indicate the point at which vibration could again be detected. Sinusoidal vibration amplitude in both the ascending and descending modes was incremented and decremented respectively, in steps of 0.17 μm for 200 Hz vibration and 1.05 μm for 30 Hz vibration. On average, three step changes in amplitude were made during each second. Threshold was determined by averaging the results of six trials (three descending and three ascending). This method of limits procedure has been shown to be as reliable as, and more time efficient than the two-alternative forced-choice procedure in a group of adult subjects aged 22 to 56 years [22]. Furthermore, in four elderly subjects we studied the vibration detection thresholds for 30 Hz and 200 Hz vibration on the tip of the index finger and on the thenar eminence using both methods. A t-test comparison revealed no significant differences in the detection thresholds obtained using the method of limits and the two-alternative forced-choice procedure (fingertip 30 Hz p = 0.48, fingertip 200 Hz p = 0.70, thenar eminence 30 Hz p = 0.32, thenar eminence 200 Hz p = 0.28). As the time course of the testing procedure was much shorter with the method of limits, taking into account the number of sites tested and the age of elderly subjects, this method was considered to be more suitable for the study.
Statistical Analysis
For each group of subjects, group means and standard errors were calculated for the detection threshold of each test frequency at each site. To analyse the effects of aging on vibrotactile sensibility, a three way ANOVA with repeated measures on two variables, formatted as A × B × C with repeated measures on variables B and C, was used to analyse all the data. Variable A was defined as age, firstly young vs. elderly, and then as old (55–64 years, n = 9) vs. older (65–74 years, n = 6) vs. oldest (over 75 years, n = 7), variable B was defined as frequency (30 Hz vs. 200 Hz) and variable C was defined as test site.
Results
In the substantial majority of subjects tested, the detection thresholds for both low and high frequency vibration were lowest in the glabrous skin of the fingertip, an observation that reflects the high innervation density at this site [2]. At 30 Hz vibration, the fingertip was the most sensitive site for detection in forty-one subjects, while in the remaining three subjects (all within the young group) the cheek was the most sensitive site. At 200 Hz vibration the fingertip was the most sensitive site for detection in forty subjects. In the remaining subjects, the cheek was the most sensitive site in three (2 young, 1 elderly) and the shoulder in one young subject. The least sensitive site for vibration detection was the shoulder in twenty subjects, the forearm in seventeen subjects, and the cheek in seven subjects. At the group level, the threshold of vibration detection at the fingertip at both 30 and 200 Hz was significantly (p < 0.001) lower than detection threshold at each of the other sites. Pooled results for all subjects at each site are shown in Table 1.
Table 1.
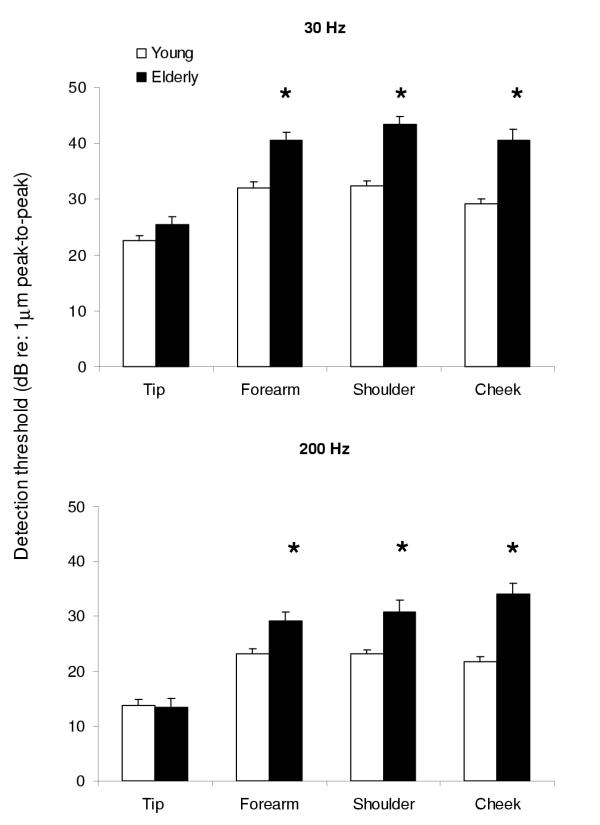
Vibration detection thresholds (dB) form the four sites tested in all young and elderly subjects
| Stimulus and age group | Group size | Fingertip | Forearm | Shoulder | Cheek | ||||
| n | μ | SE | μ | SE | μ | SE | μ | SE | |
| 30 Hz | |||||||||
| young | 22 | 22.58 | 0.88 | 32.09 | 0.92 | 32.35 | 0.90 | 29.10 | 0.94 |
| elderly | 22 | 25.51 | 1.34 | 40.59 | 1.32 | 43.46 | 1.43 | 40.65 | 1.79 |
| 200 Hz | |||||||||
| young | 22 | 13.80 | 1.02 | 23.20 | 0.89 | 23.12 | 0.76 | 21.78 | 0.86 |
| elderly | 22 | 13.33 | 1.62 | 29.12 | 1.76 | 30.80 | 2.23 | 34.03 | 2.11 |
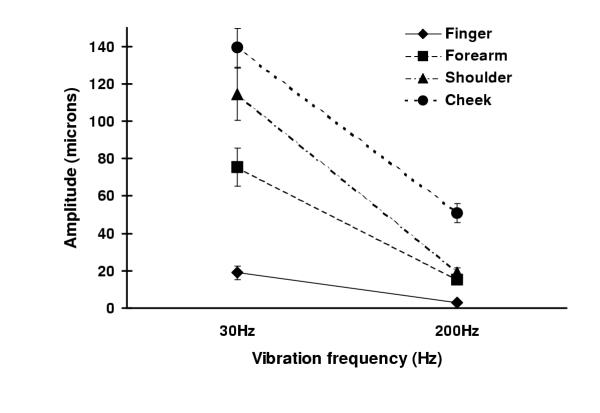
In the glabrous skin, i.e. fingertips, the detection thresholds for high frequency vibration were lower than those for low frequency vibration in all subjects. These results conform with previous observations on frequency tuning curves obtained from both psychophysical human and electrophysiological animal studies (for review see [23]). The hairy skin locations also showed similar frequency sensitivities, i.e. a greater sensitivity to high frequency vibration, however as expected the detection thresholds were significantly higher than the glabrous skin. Figure 1 shows the results obtained from an individual subject at both frequencies for all sites tested. The threshold levels shown at various sites are representative of the results obtained from the majority of subjects.
Figure 1.
Detection thresholds at several body sites for two frequencies of vibration (30 and 200 Hz) in one elderly subject (male, aged 79 years). Detection thresholds for two frequencies of vibration (30 and 200 Hz). The y-axis represents the peak to peak amplitude (microns) of vibration. The results for this single subject are typical for results from all subjects in the older group.
Although in general, the vibration detection thresholds at the fingertip and at the forearm were lower in the descending mode compared to the ascending mode, these differences were not statistically significant. At the shoulder and at the cheek, the vibration detection thresholds were lower in the ascending mode compared to the descending mode, but again these differences were not statistically significant. Accordingly, data obtained from the ascending and the descending modes were combined for further analysis.
Effect of age on vibration detection thresholds
ANOVA revealed statistically significant main effects for age (F 1,42 = 15.57, p < 0.001), for site (F 1,42 = 31.33, p < 0.001) and for frequency (F 1,42 = 39.04, p < 0.001). Follow up t-tests within the elderly group revealed significantly elevated thresholds for the detection of vibration at both frequencies and at all sites, except the fingertip (p < 0.01). When data obtained from young and elderly subjects were further analysed, those differences that were significant appeared to be the result of deterioration in detection thresholds at all sites, except the fingertip, with aging. The differences in sensitivity between elderly fingertips and other sites were significantly greater, (F 1,42 = 15.65, p < 0.001), than the differences between young fingertips and other sites. Furthermore, it appeared that this discrepancy was further affected by frequency (F 1,42 = 12.47, p < 0.01), with greater deterioration of thresholds for 30 Hz vibration at all sites, except the cheek. Figure 2 shows the comparison of vibration detection thresholds between young and elderly subjects at each site. For all sites, apart from the fingertips, the elderly group showed higher detection thresholds at both frequencies. It should be noted that at 30 Hz vibration there was considerable variability within the elderly group. For this frequency at the fingertip, although the elderly group had higher detection threshold values overall, the statistical analysis did not reveal any significant differences between the two groups.
Figure 2.
Comparison of vibration detection thresholds in young and elderly subjects, for two frequencies of vibration at several body sites. The histograms show the mean and standard error of vibration detection thresholds of all young (open columns) and all elderly subjects (filled columns) at each site tested, for two frequencies of vibration (30 Hz – upper panel, 200 Hz – lower panel). The y-axis represents the peak to peak amplitude of vibration detection thresholds (dB re: 1 μm peak-to-peak). Statistically significant comparisons are indicated by an asterisk (*).
Analysis of data from the elderly group
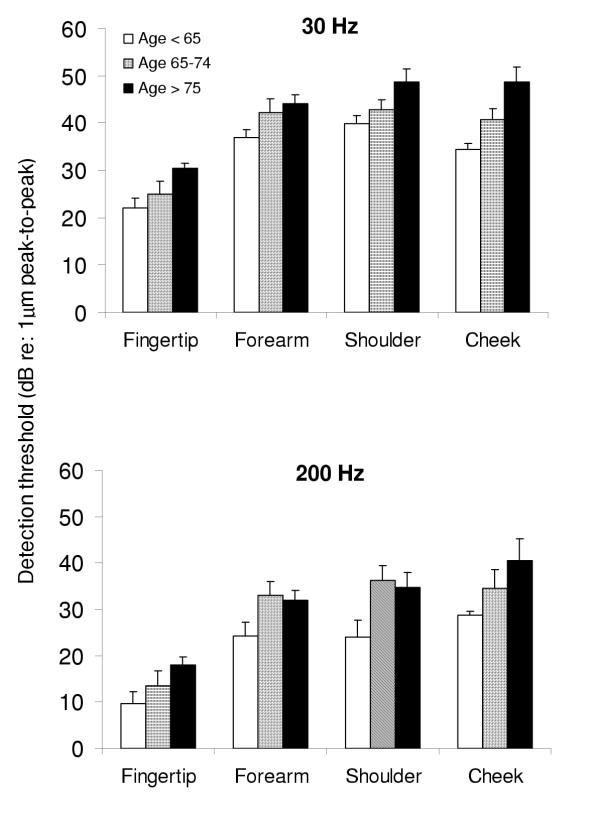
Figure 3 shows the group means and standard errors for vibration detection thresholds at each site for the three subgroups of elderly subjects. Analysis of these data revealed the same main effects for frequency (F1,19 = 38.30, p < 0.001) and for site (F1,19 = 34.92, p < 0.001) within the elderly group, as that revealed by the analysis of data from young versus elderly groups. A main effect for age (F 1,19 = 7.53, p < 0.02) was seen, with thresholds for vibration detection in two of the elderly groups (older, aged 65–74 and oldest, aged 75–90) being significantly (p < 0.04) elevated when compared to the thresholds in the remaining group (old, aged under 65), for both frequencies and at all sites, except the fingertip. At the fingertip, although overall there was an increase in the detection threshold values with increasing age, there were no statistically significant differences between those aged under 65 and those in the older (aged 65–74) and oldest (aged 75–90) groups (p = 0.06 - 0.24).
Figure 3.
Comparison of vibration detection thresholds in all elderly subjects, for two frequencies of vibration at several body sites. The histograms show the mean and standard error of vibration detection thresholds of all elderly subjects – subjects aged under 65 (open columns), subjects aged between 65 and 74 (hatched columns) and subjects aged over 75 (filled columns) at each site tested, for two frequencies of vibration (30 Hz – upper panel, 200 Hz – lower panel). The y-axis represents the peak to peak amplitude of vibration detection thresholds (dB re: 1 μm peak-to-peak).
When the fingertips of the older (aged 65–74) group were compared to those of the oldest (aged 75–90) group, there were no significant differences for the 200 Hz vibration detection threshold (p = 0.32), but at 30 Hz the fingertip detection thresholds of the oldest group were significantly (p = 0.02) higher than those of the older group. At the remaining sites (forearm, shoulder and cheek) there was greater deterioration for the detection of 30 Hz vibration compared to detection of 200 Hz with progressive aging. This effect was more pronounced at the shoulder and at the cheek, than at the forearm.
Discussion
Comparison to previous work
The overall results of this study are in agreement with previous work, the hairy skin locations demonstrate higher detection thresholds for both 30 Hz and 200 Hz vibration [24], the hairy skin of the cheek showing almost none of the U-function described by Verrillo and further demonstrated by Barlow [25].
Direct comparisons of absolute thresholds reported here and those reported by others are difficult for two reasons. Firstly, the contactor size (0.04 mm2) used in the current study is smaller than that reported by most other groups. The detection threshold for high frequency vibrations is known to be affected by contactor size. Spatial summation in the Pacinian system in the presence of a large contactor leads to lowered detection thresholds [21]. However, detection thresholds for low frequency vibrations appear to be somewhat independent of contactor size, even for very large contactors [14,21,26]. Secondly, the gap between the contactor and the rigid surround used here was larger than most other studies (2 mm vs 1 mm). A larger gap has been shown to elevate detection threshold for low frequency vibrations due the enhanced sensitivity of this class of receptors to the presence of an edge in their receptive field [27]. The relatively small contactor and larger contactor-rigid surround gap used here may account for the generally higher detection thresholds seen in this study in comparison to others.
For the glabrous skin of the fingertip of young subjects, detection thresholds for 30 Hz vibration reported here are comparable to those reported by Mountcastle et al, 1972 [28], Ferrington et al, 1977 [29], Barlow, 1987 [25] and Horch et al, 1992 [18]. While each of these groups used different contactor sizes to the present study, the critical similarity appears to be the size of the contactor-surround gap. Both the current work and the Ferrington group used relatively large gaps, while the Mountcastle and Horch groups used no surround. The thresholds reported by other groups [26,21,30,31] using similar or larger contactor sizes and a smaller contactor-rigid surround gap are considerable lower. Detection thresholds for 200 Hz vibration at the fingertip are comparable to the work of Gescheider et al, 1994 [26] using a smaller contactor and a similar gap. However, most other studies [21,24,28,30,31] used larger contactor sizes, known to significantly improve detection for high frequency vibration [21].
For the hairy skin locations, the detection thresholds for 30 Hz vibration are higher than those previously reported for the forearm [24,32], but considerably lower than those reported for the cheek [25]. Each of these groups used larger contactors and smaller contactor-surround gaps than the present study. For 200 Hz vibration the thresholds reported here are comparable to previous reports for the forearm [24,32] and considerably lower than those for the cheek [25]. There are no previous reports of vibrotactile detection thresholds for the hairy skin of the shoulder region.
Comparison of detection thresholds between young and elderly subjects
The overall results of this study are in agreement with previous studies [13-21], that aging has a significant deteriorating effect on vibration sensibility, and that greater deterioration is observed on the proximal sites tested. However, a number of previous studies reported that only a small percentage (5%–24%) of subjects showed deterioration in vibration sensibility with advancing age [33-35]. Even though these impairments were particularly prominent beyond the eighth decade of life, the vibrotactile sensibility appeared to be normal on the upper extremities compared to the lower extremities [35]. Our results also appear to be in agreement with these studies, as we were unable to demonstrate a deterioration of vibrotactile detection at the fingertips in elderly subjects. The reasons for the differential effect of aging on detection threshold at various body regions are not clear. Several hypotheses have been put forward to account for the mechanism of aging related deterioration in mechanoreception. These include, changes in the mechanical properties of the skin, decrease in density and alterations in the morphology of mechanoreceptors, decline in the number of spinal root afferent fibres, changes and irregularities in internodal distances as a result of demyelination and remyelination that may influence the input transmission synchrony, and diminished circulation and ischaemia at the peripheral and spinal cord levels (for review see [15]). Some or all of these factors may be responsible for the age related changes observed in vibrotactile sensibility at various body regions.
Similar methodology to that described here has been used by other groups to measure the detection thresholds of the glabrous skin of young and older subjects. Both Goble [21] and Gescheider's [26] groups found a deterioration of detection thresholds at the fingertip for both high and low frequency vibrations in older subjects, with greater deterioration of thresholds for high frequency vibrations. Increasing the size of the contactor caused small improvements in the ability to detect low frequency vibrations, but made an insignificant difference to the thresholds for high frequency vibrations. Verrillo [14] found a deterioration of detection thresholds at the thenar eminence for high frequency vibration, but no change in low frequency detection thresholds, with aging. Similarly, Gescheider et al, 1994 [26] demonstrated age related changes in detection thresholds for high frequency vibrations at the thenar eminence (low frequency thresholds were not reported in this paper). Each of these latter studies used a very large contactor. However, in the present study we were unable to demonstrate a deterioration in detection thresholds for high and low frequency vibrations at the fingertip. There is a 3–6 fold increase in the RA and PC type mechanoreceptive unit density between the palm of the hand and the fingertip [2]. Furthermore, a recent study on the distribution of Pacinian corpuscles in the hand of human cadavers reported that while 44–60% of corpuscles of the hand are found in the fingers, only 8–18% are located in the thenar and hypothenar regions [5]. Therefore, the different results reported for vibrotactile sensibility in the upper extremities may be related to the variability in receptor density within this body part. If aging affects a proportion of afferent units and their associated receptors in a given area, it is likely that the densely populated regions, such as the fingertips, may retain their sensitivity compared to less densely populated areas, such as the thenar eminence.
Vibration detection threshold differences amongst elderly subjects
Our analysis of data obtained from elderly subjects indicates a more significant deterioration of detection thresholds in the older (age 65–74) and oldest (age >75) groups compared to the old (age <65) group at all sites tested, except the fingertip. Therefore, it appears that on proximal body sites there is a progressive decline in the sensitivity to vibration with increasing age. Such progressive changes with aging have previously been reported [14]. Verrillo [14] described a more prominent decrease in sensitivity to high frequency vibration but not to low frequencies, measured on the thenar eminence, in a group with a mean age of 65 years. Possible mechanisms for this deterioration include reduction in the number of Pacinian corpuscles, and more favourably, structural changes in the corpuscle morphology [14]. Either of these hypotheses would be particularly relevant for body regions less densely populated with receptors. In our results, measurement of both high and low frequency vibration detection thresholds on proximal body sites also indicated a progressive deterioration with aging. However, at the fingertip, particularly at 200 Hz, no significant difference could be established between values obtained from the three elderly groups. This conflicting outcome is likely to be due to the different hand regions tested in the present and in Verrillo's studies [14]. It is most likely that gradual changes in receptor density and/or structure throughout aging would result in progressive deterioration in vibrotactile sensibility, more profoundly so in proximal regions where receptor densities are lower.
In addition to these possible peripheral mechanisms, such as the afferent unit density, central mechanisms may also play a role for vibration detection sensibility to be retained at the fingertips. There are an increasing number of studies indicating that the adult central nervous system is capable of undergoing plastic changes (for review see [36]). Alteration of inputs to the central nervous system may occur not only as a result of peripheral or central lesions, but also following increased stimulation of a particular body region. Evidence also appears to suggest that active forms of peripheral stimulation involving attention are most effective in inducing changes in the central representation. It has been shown that when monkeys perform a vibration frequency discrimination task with a particular finger, the cortical representation for the trained finger increases compared with control fingers [36,37]. Mapping of the cortical representation of the trained skin location revealed a significantly greater (1.5–3 times) area of representation compared to equivalent skin locations on control fingers of the same or opposite hemisphere, and on fingers of passively stimulated control monkeys [37]. Evidence from human studies also suggests similar consequences. For example, results from a neurophysiological imaging study indicate that the sensorimotor cortical representation of the left hand of string players is greater than the right hand representation and the representations in non-violin playing control subjects [38]. In another study, it has been reported that the cortical representation of the fingertip in Braille readers is larger than the representation of the fingertip of sighted control subjects [39].
The functional implications of a larger cortical representation can be assessed by means of psychophysical methods. The preliminary results of a study from our laboratory suggest lower thresholds for vibration detection in the string playing fingers of adult violinists. Previously, Stevens, Foulke & Patterson [40] assessed the tactile acuity in blind and age-matched sighted subjects, and reported that blind subjects showed better performance at the fingertips than sighted subjects. Although this study further suggested that the acuity at the fingertip declined in both blind and sighted subjects as a function of age, it should be noted that the tests applied in this study were based on discriminatory aspects of tactile sensation rather than vibrotactile detection measurements. It may well be the case that the afferent channels conveying different aspects of mechanoreception are differentially affected during aging. It is therefore possible that, as the most frequently and attentively used body part, especially for texture discrimination, grasp and slip detection, the cortical representations of fingertips may be functionally retained throughout life. Such central changes may occur through various mechanisms, such as expansion of representations or synaptic strengthening of neural connections.
Conclusions
In summary, the results of this study confirm the changes in vibration detection sensibility throughout aging. However, there appears to be no significant deterioration of this function at the fingertips. The preservation of detection thresholds at the fingertips reflects the functional importance of the skin of the fingertip throughout life in exploration and manipulation of the environment, and is consistent with central mechanisms of plasticity likely to result from such use. The exact mechanisms of this process are not clear at present, but may be established in the near future with more advanced techniques, such as magnetic resonance imaging, that can detect central representational changes.
Competing interests
None declared.
Authors' contributions
MS participated in data collection, analysis and preparation of the manuscript. BT conceived of the study and participated in its design and preparation of the manuscript, JS participated in data collection and analysis, NW participated in data collection and subject recruitment, VN participated in data collection and subject recruitment. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This research was supported by Sydney University Internal Mechanism B Grant 32 304 024 and Sydney University Research Grant S000 4645.
Contributor Information
Meg Stuart, Email: M.Stuart@fhs.usyd.edu.au.
A Bulent Turman, Email: B.Turman@fhs.usyd.edu.au.
Jacqueline Shaw, Email: jacquiehiller@bigpond.com.
Natalie Walsh, Email: natalie@anatomy.usyd.edu.au.
Vincent Nguyen, Email: V.Nguyen@fhs.usyd.edu.au.
References
- Bolanowski SJ, Jr, Gescheider GA, Verrillo RT, Checkosky CM. Four channels mediate the mechanical aspects of touch. Journal of the Acoustical Society of America. 1988;84:1680–1694. doi: 10.1121/1.397184. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. Journal of Physiology. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Trulsson M, Olsson KA, Westberg K-G. Mechanoreceptor activity from the human face and oral mucosa. Exp Brain Res. 1988;72:204–208. doi: 10.1007/BF00248518. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Spatial properties of the population of mechanoreceptive units in the glabrous skin of the human hand. Brain Research. 1980;184:353–366. doi: 10.1016/0006-8993(80)90804-5. [DOI] [PubMed] [Google Scholar]
- Stark B, Carlstedt T, Hallin RG, Risling M. Distribution of human Pacinian corpuscles in the hand. A cadaver study. J Hand Surg [Br] 1998;23:370–372. doi: 10.1016/s0266-7681(98)80060-0. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Central nervous mechanisms in mechanoreceptive sensibility. In: Brookhart JM, Mountcastle VB, editor. Handbook of Physiology: The Nervous System Sensory Processes, part 2. III. Maryland, USA: American Physiological Society; 1984. pp. 789–878. [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J, Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. Journal of Physiology. 1995;483:783–795. doi: 10.1113/jphysiol.1995.sp020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The cerebral cortex in man: a clinical study of localisation of function New York. Macmillan. 1950.
- Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM. Progressive improvement in discriminative abilities in adult owl monkeys performing a tactile frequency discrimination task. Journal of Neurophysiology. 1992;67:1015–1030. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- Corso JF. Sensory processes and age effects in normal adults. Journal of Gerontology. 1971;26:90–105. doi: 10.1093/geronj/26.1.90. [DOI] [PubMed] [Google Scholar]
- Verrillo RT, Verrillo V. Sensory and perceptual performance. In: Charness N, editor. Aging and Human Performance. Chichester: John Wiley and Sons; 1985. pp. 1–33. [Google Scholar]
- Perret E, Regli F. Age and the perceptual threshold for vibratory stimuli. Europ Neurol. 1970;4:65–76. doi: 10.1159/000114011. [DOI] [PubMed] [Google Scholar]
- Potvin AR, Syndulko K, Tourtellotte WW, Lemmon JA, Potvin JH. Human neurologic function and the aging process. Journal of the American Geriatrics Society. 1980;28:1–9. doi: 10.1111/j.1532-5415.1980.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Verrillo RT. Age related changes in the sensitivity to vibration. Journal of Gerontology. 1980;35:185–193. doi: 10.1093/geronj/35.2.185. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR. Somesthetic sensitivity in young and elderly humans. Journal of Gerontology. 1986;41:732–742. doi: 10.1093/geronj/41.6.732. [DOI] [PubMed] [Google Scholar]
- Merchut MP, Toleikis SC. Aging and quantitative sensory thresholds. Electromyography and Clinical Neurophysiology. 1990;30:293–297. [PubMed] [Google Scholar]
- Wiles PG, Pearce SM, Rice PJS, Mitchell JMO. Vibration perception threshold: influence of age, height, sex, and smoking, and calculation of accurate centile values. Diabetic medicine. 1990;8:157–161. doi: 10.1111/j.1464-5491.1991.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Horch K, Hardy M, Jimenez S, Jabaley M. An automated tactile tester for evaluation of cutaneous sensibility. J of Hand Surg [Am] 1992;17:829–837. doi: 10.1016/0363-5023(92)90452-u. [DOI] [PubMed] [Google Scholar]
- de Neeling JN, Beks PJ, Bertelsmann FW, Heine RJ, Bouter LM. Sensory thresholds in older adults: reproducibility and reference values. Muscle & Nerve. 1994;17:454–461. doi: 10.1002/mus.880170414. [DOI] [PubMed] [Google Scholar]
- Williams G, Gill JS, Aber V, Mather HM. Variability in vibration perception threshold among sites: a potential source of error in biothesiometry. British Medical Journal. 1988;296:233–235. doi: 10.1136/bmj.296.6617.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble AK, Collins AA, Cholewiak RW. Vibrotactile threshold in young and old observers – the effects of spatial summation and the presence of a rigid surround. Journal of the Acoustical Society of America. 1996;99:2256–2269. doi: 10.1121/1.415413. [DOI] [PubMed] [Google Scholar]
- Gerr FE, Letz R. Reliability of a widely used test of peripheral cutaneous vibration sensitivity and a comparison of two testing protocols. Br J Ind Med. 1988;45:635–639. doi: 10.1136/oem.45.9.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I. The sense of touch: performance and peripheral neural processes. In: Brookhart JM, Mountcastle VB, editor. Handbook of Physiology Section I: The Nervous System Sensory Processes, part 2. III. Maryland, USA: American Physiological Society; 1984. pp. 739–788. [Google Scholar]
- Verrillo RT, Gescheider GA. Perception via the sense of touch. In: Summers IR, editor. Tactile aids for the hearing impaired. London: Whurr Publishers; 1992. [Google Scholar]
- Barlow SM. Mechanical frequency detection thresholds in the human face. Experimental Neurology. 1987;96:253–261. doi: 10.1016/0014-4886(87)90044-6. [DOI] [PubMed] [Google Scholar]
- Gescheider GA, Bolanowski SJ, Hall KL, Hoffman KE, Verrillo RT. The effects of aging on information-processing channels in the sense of touch: 1. Absolute sensitivity. Somatosensory & Motor Research. 1994;11:345–357. doi: 10.3109/08990229409028878. [DOI] [PubMed] [Google Scholar]
- Verrillo RT. The effect of surface gradients on vibrotactile thresholds. Sensory Processes. 1979;3:27–36. [PubMed] [Google Scholar]
- Mountcastle VB, Lamotte RH, Carli G. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibres innervating the monkey hand. Journal of Neurophysiology. 1972;35:122–136. doi: 10.1152/jn.1972.35.1.122. [DOI] [PubMed] [Google Scholar]
- Ferrington DG, Nail BS, Rowe M. Human tactile detection threshold: modification by inputs from specific tactile receptor classes. J Physiol. 1977;272:415–433. doi: 10.1113/jphysiol.1977.sp012052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LE, Schechter MB, Goldstein MH. Child and adult vibrotactile thresholds for sinusoidal and pulsatile stimuli. J Acoust Soc Ame. 1986;80:118–124. doi: 10.1121/1.394172. [DOI] [PubMed] [Google Scholar]
- Donahue AM, Letowski T. Vibrotactile performance by normal and hearing-impaired subjects using two commercially available vibrators. Audiology. 1985;24:362–373. doi: 10.3109/00206098509078354. [DOI] [PubMed] [Google Scholar]
- Verrillo RT. Vibrotactile thresholds for hairy skin. Journal of Experimental Psychology. 1966;72:47–50. doi: 10.1037/h0023321. [DOI] [PubMed] [Google Scholar]
- Plumb CS, Meigs JW. Human vibration perception. Part I, Vibration perception at different ages (normal values). Arch Gen Psychiat. 1961;4:611–614. doi: 10.1001/archpsyc.1961.01710120081009. [DOI] [PubMed] [Google Scholar]
- Steinberg FU, Graber AL. The effect of age and peripheral circulation on the perception of vibration. Archives of Physical Medicine and Rehabilitation. 1963;44:645–650. [PubMed] [Google Scholar]
- Cosh JA. Studies on the nature of vibration sense. Clin Sci. 1953;12:131–151. [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WJ, Grajski KA, Dinse HR. Topographic reorganisation of the hand representation in cortical area 3b of owl monkeys trained in a frequency-discrimination task. Journal of Neurophysiology. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM. Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. Journal of Neurophysiology. 1992;67:1057–1070. doi: 10.1152/jn.1992.67.5.1057. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Weinbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers in the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Foulke E, Patterson MQ. Tactile acuity, aging and Braille reading in long-term blindness. Journal of experimental psychology: applied. 1996;2:91–106. doi: 10.1037//1076-898X.2.2.91. [DOI] [Google Scholar]