Abstract
Ribosomes can be programmed to shift from one reading frame to another during translation. Hepatitis C virus (HCV) uses such a mechanism to produce F protein from the −2/+1 reading frame. We now report that the HCV frameshift signal can mediate the synthesis of the core protein of the zero frame, the F protein of the −2/+1 frame, and a 1.5-kDa protein of the −1/+2 frame. This triple decoding function does not require sequences flanking the frameshift signal and is apparently independent of membranes and the synthesis of the HCV polyprotein. Two consensus −1 frameshift sequences in the HCV type 1 frameshift signal facilitate ribosomal frameshifts into both overlapping reading frames. A sequence which is located immediately downstream of the frameshift signal and has the potential to form a double stem-loop structure can significantly enhance translational frameshifting in the presence of the peptidyl-transferase inhibitor puromycin. Based on these results, a model is proposed to explain the triple decoding activities of the HCV ribosomal frameshift signal.
Fidelity of ribosomes is critical for ensuring accurate synthesis of proteins from their respective mRNAs. Translational errors, therefore, are relatively uncommon and are suppressed, for example, by various proofreading mechanisms. Among different types of translational errors, frameshift errors are believed to be the least common, occurring at rates much lower than 5 × 10−5 per amino acid incorporation (6). However, it has become clear that various prokaryotic and eukaryotic systems in fact utilize frameshifting to regulate the synthesis of various proteins (6). In addition, many viruses have been found to specifically program such frameshifting to generate viral proteins. By “programming” such frameshift events, these viruses utilize the alternate reading frames at rates that are much higher than would be expected by chance alone. Examples of these viruses are retroviruses (11, 12), coronaviruses (1, 3), and astroviruses (13). More recently, a flavivirus, hepatitis C virus (HCV), also joined the list (28).
HCV is known to cause severe liver diseases in human, including liver cirrhosis and hepatocellular carcinoma (15, 18, 21). It is estimated that HCV currently infects about four million people in the United States alone (18). HCV is an RNA virus with a genome that encodes a polyprotein of about 3,010 amino acids in length (9). This protein is translated from the viral genomic RNA in a cap-independent manner, using an internal ribosomal entry site, which encompasses most of the 5′ untranslated region (UTR) and the first few codons of the HCV coding sequence (20). After its synthesis, the HCV polyprotein is cleaved to generate at least 10 individual viral proteins. Recently, others and we have discovered another HCV protein (26, 28) which is expressed by programmed ribosomal frameshift (28). The translation of this 11th HCV protein initiates from the 5′ end of the viral coding sequence. During translation, however, ribosomes shift from the normal (i.e., zero) reading frame to the −2/+1 reading frame to generate a 17-kDa protein product. This protein has been subsequently named F protein to indicate frameshifting. Importantly, antibodies to this protein have been detected in HCV patients, indicating the production of the F protein during natural HCV infection (26, 28).
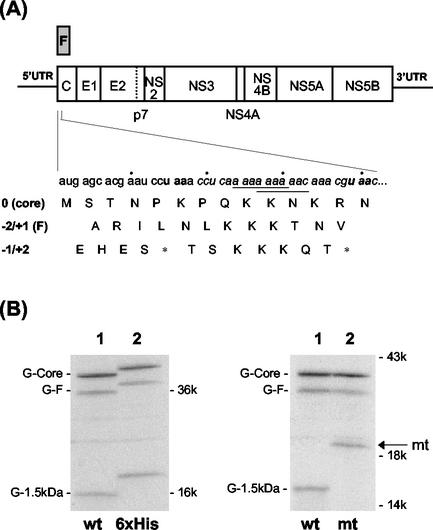
The 5′ end of the HCV polyprotein coding sequence codes for the p21 core protein. Previously, we discovered that a short, adenosine-rich sequence (codons 8 to 14) near the 5′ end of the core protein coding sequence was sufficient to induce F protein production in vitro as well as in cell cultures (see Fig. 1A) (28). Radiosequencing of the in vitro-labeled F protein indicated that the frameshift junction likely occurred at codons 9 to 11 of the core protein coding sequence. The results also suggested that this was likely caused by a −2 ribosomal frameshift event. However, minor protein sequence heterogeneities were also detected, suggesting the possible involvement of multiple frameshifting events. Interestingly, the HCV frameshift signal, as shown below in Fig. 1A, contains the sequences A AAA AAA and A AAA AAC, which are consistent with the consensus −1 ribosomal frameshift signal, X XXY YYZ, where X, Y, and Z can be any nucleotides (1, 11). It has been proposed that a −1 frameshift on this consensus sequence will allow the two tRNAs occupying the P site and the A site to maintain two base pairs with the codons in the new reading frame. The observation that the HCV frameshift signal also contains these signals raises a possibility that the HCV A-rich sequence may also mediate −1 ribosomal frameshift.
FIG. 1.
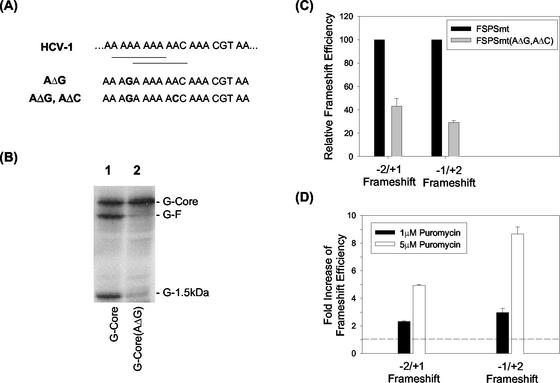
HCV genome and ribosomal frameshifts. (A) HCV genomic organization. Codons 8 to 14, which contain the frameshift signal, are italicized. Bolded letters indicate termination codons in the −1/+2 reading frame. The two consensus −1 ribosomal frameshift sequences are underlined. The amino acids encoded by all three reading frames are also shown. Asterisks denote the locations of stop codons. The HCV-1 sequence is used for the figure (22). (B) Protein expression from pCMV-G-Core (lane 1) and pCMV-6xHis-G-Core (lane 2). The latter contains the insertion of the hexahistidine tag at the 5′ end of the G-core coding sequence. (C) Protein expression from pCMV-G-Core (lane 1) and pCMV-G-Coremt (lane 2). The latter contains the mutation that removed the termination codon at codon 14 of the −1/+2 reading frame. RNA was synthesized from DNA constructs by using T7 RNA polymerase and translated in vitro, using rabbit reticulocyte lysates in the presence of [35S]methionine. The proteins synthesized were analyzed on a polyacrylamide gel. The arrow denotes the G-1.5-kDa protein made from the pCMV-G-Coremt construct.
In this report, we have further investigated the molecular mechanisms that regulate the HCV translational frameshifting. Our results indicate that the HCV frameshift signal indeed has a dual function, mediating not only −2/+1 frameshift but also −1/+2 frameshift. In addition, we have located a double stem-loop structure, immediately downstream of the frameshift signal sequence, and we demonstrate that this region facilitates frameshifting in the presence of a peptidyl-transferase inhibitor, puromycin. Furthermore, we demonstrate that the consensus −1 ribosomal frameshift signals (see Fig. 1A), which are absent in the sequences of many HCV isolates, facilitate but are not essential for the expression of the −1/+2 reading frame and the −2/+1 reading frame products. Based on these results, a model is proposed to explain the observed activities of the HCV ribosomal frameshift signal.
MATERIALS AND METHODS
Construction of DNA plasmids.
pCMV-Core contains nucleotides (nt) 252 to 936 of the HCV type 1 (HCV-1) sequence, corresponding to a segment of the 5′ UTR and the entire core protein coding sequence (28). A termination codon has been inserted at the end of the core protein coding sequence for translation termination. This plasmid expresses the HCV-1 core protein, using the immediate early promoter of cytomegalovirus (CMV). Note that all numbers refer to the nucleotide number of the HCV-1 genome, starting from the beginning of the 5′ UTR. pCMV-G-Core was identical to the above construct except that a human α-globin sequence (G) was inserted in frame, between the initiation codon and the rest of the core protein coding sequence. pCMV-6xHis-G-Core further contained a hexahistidine (6xHis) sequence, directly inserted in frame between the initiation codon and the G sequence in pCMV-G-Core. pCMV-G-Coremt was derived from pCMV-G-Core and contained a T-to-G mutation at nt 389 to remove a termination codon, TAA, located in the −1 reading frame at codon 14 of the HCV-1 coding sequence. pCMV-G-Core(AΔG) was created by mutating A to G at nt 376. pCMV-G-CoreΔ harbored a deletion of nt 393 to 500 in the pCMV-G-Core construct. This deletion removed a putative double stem-loop region found at codons 15 to 50 and fused the flanking sequences in frame to each other. All plasmid modifications were made by standard PCR techniques and were verified by nucleotide sequencing. Resulting gene products were cloned into pRc/CMV by HindIII and XbaI sites in the polylinker region, under the control of both CMV and T7 promoters. pGEM-9ACore contained the HCV-1 core protein coding sequence with a 1-nt deletion in the 10-adenosine region to create a −2/+1 frameshift mutation (28). Expression of this sequence was under the control of a T7 promoter. pEF-9ACore and pEF-9ACoreE1 expressed the same mutated HCV-1 core protein sequence under the control of the eukaryotic elongation factor 1α promoter, either without (9ACore) or with (9ACoreE1) the downstream E1 protein sequence.
The construction of pEF-FSLuc plasmids has been described previously (28). pEF-FSmtLuc constructs were identical to the above constructs except for a T at nt 389 of the HCV-1 core protein coding sequence that was mutated to C, to remove the termination codon located at codon 14, in the −1/+2 reading frame. For these and all other reporter constructs, the luciferase gene was fused to three different reading frames, for example, to generate pEF-FSmt(0)Luc, pEF-FSmt(−1/+2)Luc, and pEF-FSmt(−2/+1)Luc. pEF-FSPSmtLuc was similar to the pER-FSmtLuc reporter constructs except that codons 1 to 53 of the core protein coding sequence were used instead of codons 1 to 14. To allow determination of the −1/+2 frameshift efficiency, two termination codons that occurred in the −1/+2 reading frame, at codons 14 and 43 of the core sequence, were removed by mutating T to C at nt 389 and T to G at nt 476. pEF-FSPS(AΔG, AΔC)Luc constructs contained codons 1 to 53 of the HCV core protein coding sequence, with A-to-G and A-to-C substitutions at nt 376 and 382, respectively. pEF-FSPSmt(AΔG, AΔC)Luc constructs were the same as the pEF-FSPS(AΔG, AΔC)Luc constructs except that the two termination codons, located in the −1/+2 reading frame, were removed by T-to-C and T-to-G mutations at nucleotide positions 389 and 476, respectively. pCMV-FSLuc constructs were generated by cloning the FS(0)Luc, FS(−1/+2)Luc, and FS(−2/+1)Luc sequences from the pEF-FSLuc constructs into pRc/CMV to allow in vitro expression of these frameshift reporter constructs. Similarly, pCMV-FSmtLuc constructs were obtained by cloning the respective FSmtLuc sequences from the pEF-FSmtLuc constructs into pRc/CMV. Frameshift reporter gene sequences were all preceded by a partial β-globin leader sequence, 5′AACCTCAAACAGACACC3′, unless indicated otherwise (i.e., HCV 5′ UTR).
Cell transfection and frameshift reporter analysis.
Human hepatoma cells (Huh7) were maintained in Dulbecco's modified essential medium supplemented with 10% fetal bovine serum. The luciferase reporter constructs were transfected into Huh7 cells by the calcium phosphate precipitation method. pXGH, a human growth hormone-expressing plasmid, was cotransfected into Huh7 cells, and the amount of human growth hormone released into the cell culture medium was determined by using a commercial radioimmunoassay kit (Nichols Diagnostic) to monitor transfection efficiencies. After 42 to 48 h, cells were harvested and analyzed for their luciferase activity, using a luciferase assay kit (Promega). Relative frameshift efficiency was then calculated, using the following equation: frameshift efficiency = [(LucX − LucB)/TEX]/[(Luc0 − LucB)/TE0] × 100, where LucX is luciferase activity, LucB is the background luciferase activity, Luc0 is the luciferase activity of the zero-frame luciferase constructs, TEX is the transfection efficiency, and TE0 is the transfection efficiency of the zero-frame luciferase constructs. Background luciferase activity was determined by using pCDEF-transfected Huh7 lysates.
In vitro transcription, translation, and radioimmunoprecipitation.
Linearized DNA templates were transcribed in vitro using T7 RNA polymerase (Promega Corporation) as described elsewhere (28). Briefly, 5 to 10 μg of linearized DNA was typically incubated in a reaction mixture consisting of 40 mM Tris (pH 7.9), 6 mM MgCl2, 2 mM spermidine, 10 mM NaCl, 0.8 U of RNasin (Promega)/μl, 5 mM dithiothreitol, 0.5 mM rNTP mix, and 7.5 U of T7 RNA polymerase/μl at 37°C for 1 h. At the end of the incubation, DNA templates were removed with RNase-free DNase I (Amersham-Pharmacia) and the RNA products were phenol-chloroform extracted, ethanol precipitated, and resuspended in water for translation. Translation reactions were carried out by using rabbit reticulocyte lysates or wheat germ extracts (Promega Corporation), in the presence of 20 to 40 μCi of [35S]methionine (>1,000 Ci/mmol; ICN) with or without ∼50 μCi of [3H]lysine (∼70 Ci/mmol; Amersham-Pharmacia), according to the manufacturers' protocols. Some reaction mixtures also contained canine microsomal membranes (Promega Corporation) or 0.1 to 5 μM puromycin (Sigma-Aldrich). For radioimmunoprecipitation, translation products were incubated with antiglobin (Dako Corporation), anti-p21 core (16), anti-E1 (gift of M. Selby, Chiron Corporation), or anti-F (28) antibodies in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris [pH 7], 15 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]). Gamma-bind (Amersham-Pharmacia) was subsequently added, and the resulting immune complexes were washed extensively with RIPA buffer, boiled at 95°C in 2× Laemmli buffer (0.135 M Tris-HCl [pH 6.8], 20% glycerol, 6% SDS, 10% β-mercaptoethanol), and then analyzed by gel electrophoresis, followed by autoradiography.
Western blot analysis.
Huh7 cells were transfected with pCDEF, pCDEF-9ACoreE1HA, or pCDEF-9ACore for ∼42 to 48 h, lysed in Laemmli buffer, analyzed on a 12.5% SDS-polyacrylamide gel, and Western blotted, using the rabbit anti-core primary antibodies (16) and horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (Boehringer-Mannheim).
EIA.
The −1 frameshift protein, MSTNPKPQKKKQT, was synthesized at the USC Microchemical Core Facility according to the sequence predicted from the HCV-1 genome. The enzyme immunoassay (EIA) was carried out according to the manufacturer's protocol, using a kit supplied by Bethyl Laboratories. Briefly, each well of a microtiter plate was coated with 0.5 μg of the peptide and incubated with 10 μl of human serum and 90 μl of diluent at room temperature for 1 h. The wells were washed and subsequently incubated with 100 μl of a 1:10,000 dilution of a horseradish peroxidase-conjugated goat anti-human antibody (Pierce) at room temperature for 1 h. The wells were washed again and allowed to react with 3,3′,5,5′-tetramethylbenzidine for color development. The reaction was stopped by adding 100 μl of 2 M H2SO4 and analyzed at A450.
Statistical analysis.
Data are presented as the mean ± standard error of the mean (SEM), unless indicated otherwise. Data were analyzed using Student's t test or a one-way analysis of variance, followed by Student-Newman-Keul's or Tukey's test. A P value of <0.05 was considered significant.
RESULTS
The HCV core protein coding sequence directs −1/+2 frameshifting in addition to −2/+1 frameshifting in vitro.
As shown in Fig. 1A, the HCV frameshift signal contains the sequences A AAA AAA and A AAA AAC, which are consistent with the consensus −1 frameshift signal X XXY YYZ. We have therefore performed the in vitro translation experiments using the coding sequence of the HCV core protein to investigate whether the HCV frameshift signal may also mediate the −1 ribosomal frameshift. The −1/+2 reading frame of the HCV core protein coding sequence, unlike the −2/+1 reading frame, contains numerous stop codons, with one of them located at codon 14, shortly after the A-rich frameshift signal for F protein production (Fig. 1A). Thus, the occurrence of the −1/+2 frameshift between codons 8 and 14 of the core protein coding sequence would generate a protein product about 1.5 kDa in size, which would be difficult to detect by gel electrophoresis. In order to facilitate the detection of this putative −1/+2 frameshift product, the human α-globin sequence (G) was fused to the 5′ end of the HCV core protein coding sequence, immediately after the initiation codon, AUG. This fusion would be expected to increase the sizes of p21 core, F protein (∼17 kDa), and the putative −1/+2 frameshift protein product by approximately 14 kDa. When this fused sequence was expressed in vitro by using T7 RNA polymerase and rabbit reticulocyte lysates, three discrete protein bands were detected (Fig. 1B, lane 1). The two upper bands corresponded to the expected sizes of G-core and G-F fusion proteins, which were ∼35 and ∼31 kDa, respectively. The third protein band was approximately 16 kDa in size. The 35-kDa protein could be immunoprecipitated with anticore antibodies and the 31-kDa protein was immunoprecipitated with antibodies raised against the F protein, while all three proteins could be precipitated with antiglobin antibodies (data not shown). This indicated that the three protein bands might represent G-core, G-F, and the globin-1.5-kDa fusion protein (G-1.5-kDa).
In order to examine whether the third protein band was indeed the −1/+2 frameshift product, a hexahistidine tag was introduced between the 5′ end of the globin sequence and the initiation codon in the zero reading frame. If the translation of this protein initiated from the initiation codon of the zero frame, this tag should be incorporated into the protein and increase its size by approximately 1 kDa. Indeed, its size as well as the sizes of both G-core and the G-F fusion proteins were slightly increased, as expected (Fig. 1B). Next, we investigated whether this protein terminated in the −1/+2 reading frame. The first stop codon in the −1/+2 reading frame occurred only five codons away from the initiation codon, at codon 6 (Fig. 1A), and the second stop signal was found at codon 14. The next termination codon occurred at codon 43. It was conceivable that the translation of the −1/+2 frameshift protein terminated at codon 14, shortly after the consensus −1 ribosomal frameshift sequences in the A-rich region. Therefore, the stop codon, located at codon 14 in the −1/+2 reading frame, was mutated from TAA to CAA to allow continued translation until the next stop codon. This mutation did not introduce any termination codon in the zero or the −2/+1 reading frame. If the −1/+2 frameshift product indeed terminated at codon 14, this mutation should increase its size by slightly more than 3 kDa without affecting the synthesis of G-core and G-F proteins. As shown in Fig. 1C, while this mutation did not affect the synthesis of G-core and G-F proteins, it clearly increased the size of the G-1.5-kDa protein.
These experiments indicated, first of all, that the synthesis of the 16-kDa protein band initiated in the zero reading frame but terminated in the −1/+2 reading frame and, secondly, that the termination occurred at codon 14, immediately after the putative −1 frameshift signals. More importantly, these results indicated that the HCV core protein coding sequence was capable of directing −2/+1 as well as −1/+2 frameshift. Hence, the HCV ribosomal frameshift signal appears to possess a triple decoding function for the expression of proteins from all three reading frames. By taking into consideration the number of methionine residues in each of the three protein products, the −2/+1 frameshift efficiency was determined to be about 12 to 15% and the −1/+2 frameshift efficiency was around 30 to 45%, with the remaining fraction for the zero frame.
The HCV core protein coding sequence directs −1/+2 frameshifting in Huh7 cells.
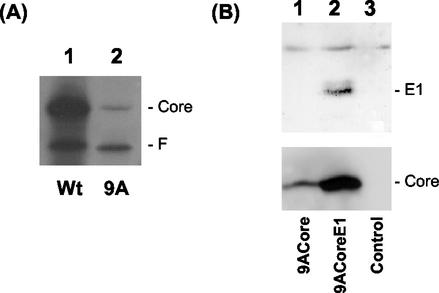
To further investigate whether this −1/+2 ribosomal frameshift could also occur in cells, a single adenosine residue was deleted from the stretch of 10 As located at codons 8 to 11. This deletion (9ACore) fuses the first 10 codons of the core protein coding sequence to the F protein coding sequence. The translation from this fused sequence would produce predominantly the F protein. The core protein could only be expressed by a −1/+2 ribosomal frameshift, which would restore the reading frame of the core coding sequence. This mutated core sequence, as well as the wild-type core sequence, were first expressed in vitro, using wheat germ extracts. As shown in Fig. 2A, the translation of the wild-type sequence led to the production of more p21 core protein than F protein, whereas the translation of the 9ACore sequence produced more F protein than p21 core protein. The identity of these protein bands was confirmed by immunoprecipitation experiments using p21 core and F antibodies (data not shown). The ability of this mutated sequence to direct the synthesis of the core protein confirmed the occurrence of the −1/+2 frameshift. To determine whether this −1/+2 ribosomal frameshift could also take place in cells, expression plasmids that contained the mutated core sequence, without (pEF-9ACore) or with (pEF-9ACoreE1) the following E1 sequence, were transfected into Huh7 hepatoma cells. As shown in Fig. 2B, the core protein could be detected in cells transfected by either pEF-9ACore or pEF-9ACoreE1 by Western blot analysis. The E1 protein could only be detected in cells transfected by pEF-9ACoreE1 (Fig. 2B). These results indicated that the −1/+2 ribosomal frameshift also occurred in Huh7 cells and that it was independent of the downstream E1 sequence.
FIG. 2.
Analysis of the −1/+2 frameshift from the 9A-Core sequences. (A) p21 core and 9A core RNAs, synthesized from pCMV-Core (lane 1) and pGEM-9ACore (lane 2) constructs, were translated by using wheat germ extracts in the presence of [35S]methionine and [3H]lysine and analyzed by gel electrophoresis, as described in Materials and Methods. [3H]lysine was included as the label to enhance the signal of the core protein synthesized from the 9ACore sequence, which otherwise would be difficult to detect (see Fig. 4). RNA amounts used in lanes 1 and 2 were not equivalent. (B) pEF-9ACore (lane 1), pEF-9ACoreE1 (lane 2), and the pCDEF control vector (lane 3) were transfected into Huh7 cells and analyzed by Western blot analysis about 42 h posttransfection, using antibodies against p21 core protein (bottom panel) or E1 envelope protein (upper panel). The electrophoretic mobility of the p21 core protein produced from the 9ACore constructs was indistinguishable from that expressed from the wild-type core protein construct (data not shown).
The −1/+2 and the −2/+1 frameshifts are mediated by the same RNA sequence.
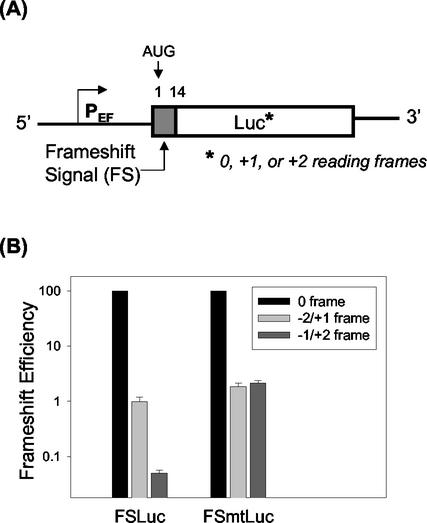
In order to further determine whether the A-rich region served as the dual frameshift signal for the −1/+2 and −2/+1 frameshifts, we generated reporter constructs that enabled us to assay both −1/+2 and −2/+1 frameshifts in vivo. We had previously fused the first 14 codons of the HCV core protein coding sequence to the zero frame [FS(0)Luc], the −2/+1 frame [FS(−2/+1)Luc], and the −1/+2 reading frame [FS(−1/+2)Luc] of the luciferase coding sequence (28). In these reporter constructs, FS(0)Luc would express the luciferase gene fused in frame to the core protein sequence, whereas FS(−2/+1)Luc would express luciferase sequence only by a −2 or a +1 frameshift. FS(−1/+2)Luc, because of the aforementioned termination codon in the −1/+2 reading frame at codon 14, only served as a negative control (Fig. 3A). In order to analyze the −1/+2 frameshift efficiency, we removed this stop codon by mutating it from TAA to CAA to allow the −1/+2-frameshifted ribosomes to continue into the luciferase coding sequence. Thus, a new set of reporter constructs, FSmt(0)Luc, FSmt(−2/+1)Luc, and FSmt(−1/+2)Luc, harboring this point mutation, were created (Fig. 3A). These DNA constructs were then transfected into Huh7 cells along with a human growth hormone reporter plasmid that was used to monitor the transfection efficiency. As shown in Fig. 3B, FSmt(−2/+1)Luc yielded luciferase activity that averaged 1.9% ± 0.3% of the FSmt(0)Luc construct. This value was similar to the −2/+1 frameshift efficiency of FS(−2/+1)Luc, indicating that the point mutation did not affect the −2/+1 frameshift in Huh7 cells. Using the FSmt(−1/+2) construct, the −1/+2 frameshift efficiency was determined to be about 2.2% ± 0.2%. The negative control luciferase activity, assessed using FS(−1/+2)Luc, remained at around 0.05%, which was near the background value and significantly lower than the −1/+2 and −2/+1 frameshift efficiencies (P < 0.001). The ∼2% ribosomal frameshift efficiencies detected are similar to what has been reported for other viral systems (7, 19). These data demonstrated that the HCV frameshift signal had a relatively unique function, supporting both −1/+2 and −2/+1 frameshifts in human hepatoma cells.
FIG. 3.
Analysis of −1/+2 and −2/+1 frameshift efficiencies in Huh7 cells. (A) The first 14 codons of the core protein sequence, with (pEF-FSLuc) or without (pEF-FSmtLuc) the termination codon at codon 14, were fused to the three reading frames of the luciferase reporter (indicated by an asterisk). (B) Huh7 cells transfected by the reporter DNA plasmids were harvested 48 h after transfection for the luciferase assays. Transfection efficiency was monitored by cotransfection with a human growth hormone expression plasmid. Frameshift efficiencies, which are shown in the log scale, were calculated as described in Materials and Methods. Experiments were done either in duplicates or triplicates and repeated at least four times. Numbers represent the average of all experiments combined.
Similar experiments were also performed in vitro by fusing the first 14 codons of the HCV coding sequence to either the luciferase (pCMV-FSmtLuc) or the globin sequence. The translation of these fused RNA sequences showed that the first 14 codons of the HCV coding sequence were sufficient to induce both the −2/+1 and the −1/+2 frameshifts (data not shown). The frameshift efficiencies were about 10 to 13% for the −2/+1 frameshift and ∼30 to 35% for the −1/+2 frameshift, which were similar to those of the globin-core fusion constructs (Fig. 1) but significantly higher than those obtained in Huh7 cells (Fig. 3). Such differences between in vitro and in vivo frameshift efficiencies are common and may be attributed to the differences inherent to each system (7, 19).
Ribosomal frameshifts in the context of the entire HCV genome.
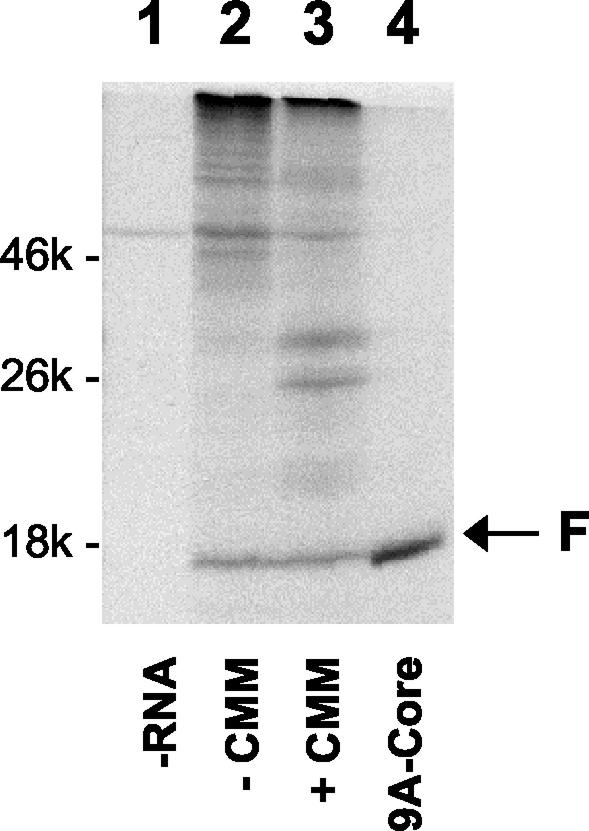
Although the above results indicated that codons 1 to 14 were sufficient to mediate HCV ribosomal frameshifts, it was unclear whether the frameshifts could occur in the context of the whole HCV genome and whether they could be affected by sequences flanking the frameshift signal. To investigate these possibilities, the HCV-1 genomic RNA was synthesized in vitro and translated using the rabbit reticulocyte lysates with and without the canine microsomal membranes. The protein products were then analyzed on an SDS-polyacrylamide gel. The F protein, with a size of about 17 kDa, was readily detected both with and without the microsomal membranes (Fig. 4). This protein band comigrated with the control F protein, produced from the 9ACore sequence, and could be precipitated with anti-F antibodies (data not shown). The −1/+2 frameshift product could not be detected due to its small size. These results indicated that the HCV frameshifts, at least for the −2/+1 frameshift, could occur in its natural context and that this activity was independent of membranes. Inclusion or exclusion of the HCV 5′ UTR, which contains the internal ribosomal entry site, and the sequence downstream of codon 14 did not significantly affect the −1/+2 or −2/+1 frameshifts in vitro or in Huh7 cells (data not shown), indicating that these flanking sequences were not important for the activities of the frameshift signal.
FIG. 4.
Synthesis of F protein by the HCV-1 genomic RNA. HCV-1 RNA was synthesized from pHCV-1 and then translated using rabbit reticulocyte lysates and labeled with [35S]methionine in the absence (lane 2) or presence (lane 3) of canine microsomal membranes (CMM). Protein products were analyzed on a 15% gel. The F protein synthesized from pGEM-9ACore was used as a marker (lane 4). p21 core protein was not readily detected in lane 4, as its detection usually required radiolabeling with [3H]lysine (see Fig. 2A). Lane 1 was the translation conducted in the absence of RNA.
Enhancement of frameshifting by codons 15 to 53 and puromycin.
Ribosomal frameshifts may be regulated by the downstream RNA secondary structures (6, 24) and, indeed, a double stem-loop structure could potentially be formed by codons 15 to 53 of the HCV sequence (see below), located immediately downstream of the frameshift signal. Although the HCV sequences downstream of codon 14 did not have apparent effects on ribosomal frameshifts (see above), the possibility that they might have such effects in the presence of other regulators could not be ruled out. Translational frameshifting has been shown to be regulated by antibiotic compounds (4, 8, 10). We therefore analyzed whether puromycin, an elongation inhibitor (25), could affect HCV frameshifts in the absence and the presence of codons 15 to 53.
First, Huh7 cells were transiently transfected with the FSmtLuc constructs and the human growth hormone expression plasmid, which served as a transfection control, and treated with different concentrations of puromycin for 24 h. Treating the cells with 1 μM puromycin did not significantly affect the expression levels of the zero-frame luciferase reporter or the human growth hormone internal control (data not shown), and treating the cells with 5 μM puromycin decreased the expression levels of the zero-frame luciferase reporter and the human growth hormone to approximately 25 to 50% of that of the untreated samples (data not shown). At either puromycin concentration, only marginal effects on −1/+2 and −2/+1 ribosomal frameshifts were detected (Fig. 5A).
FIG. 5.
Regulation of frameshifting by puromycin. (A) pEF-FSmtLuc and pEF-FSPSmtLuc constructs were transfected into Huh7 cells along with the growth hormone plasmid, which was used to monitor the transfection efficiency. After 24 h, cells were treated with puromycin for another 24 h, harvested, and analyzed for luciferase activity. The experiment was done in duplicates, which were repeated at least three times. Data represent the mean ± SEM of a typical set of experiments. The fold increase in frameshift efficiency was calculated by dividing the frameshift efficiency of puromycin-treated samples by that of the untreated samples. (B) pCMV-G-Core and pCMV-G-CoreΔ constructs were used for the synthesis of RNA, which was then translated in vitro, using wheat germ extracts in the presence of [35S]methionine and 0 to 5 μM puromycin. Products were analyzed by gel electrophoresis, followed by autoradiography. (C) Protein products shown in panel B were quantitated by densitometry. Frameshift efficiency was calculated by determining the intensity of G-F protein or G-1.5-kDa protein bands divided by that of the G-core protein at each puromycin concentration, taking into consideration the number of methionine residues in each protein. The fold changes in these frameshift efficiencies from that of the untreated control (0 μM puromycin) were then plotted against the puromycin concentration. Similar results were obtained using rabbit reticulocyte lysates (data not shown).
Next, we investigated whether the putative double stem-loop region located at codons 15 to 53 modulated puromycin-induced frameshifting in Huh7 cells. For this purpose, frameshift reporter constructs containing codons 1 to 53 of the core protein coding sequence (pEF-FSPSmtLuc) were employed. Puromycin had little effect on the frameshifting at a 1 μM concentration but elevated the −2/+1 and the −1/+2 frameshift efficiencies by approximately 7- and 8-fold, respectively, at the concentration of 5 μM (Fig. 5A). These results indicated that 5 μM puromycin could enhance the HCV ribosomal frameshifts in the presence of codons 15 to 53.
To confirm these effects of puromycin on both −1/+2 and −2/+1 frameshifts, the globin-core fused sequence with (G-Core) or without (G-CoreΔ) the putative double stem-loop region was expressed in vitro, in the presence of different concentrations of puromycin. As shown in Fig. 5B, puromycin suppressed the overall translation efficiencies of G-Core and G-CoreΔ in wheat germ extracts in a dose-dependent manner. This was accompanied by significant increases in both −1/+2 and −2/+1 frameshift efficiencies in the translation of the G-Core sequence but not the G-CoreΔ sequence (Fig. 5B and C). Since the deletion of the putative double stem-loop region at codons 15 to 53 led to marked decreases in the ability of puromycin to enhance frameshifting (Fig. 5B and C), these in vitro results further illustrated the importance of this region in the puromycin-induced frameshifting.
Role of the consensus −1 frameshift signals in HCV ribosomal frameshifts.
As shown in Fig. 1A and 6A, there are two putative −1 ribosomal frameshift signals between codons 8 and 14 of the core protein sequence. To understand their importance in the regulation of HCV ribosomal frameshifts, we substituted A376 with G376 (AΔG) to remove the upstream −1 frameshift consensus signal and investigated its effect on the ribosomal frameshift (Fig. 6A). This A-to-G substitution at nt 376 drastically decreased the −1/+2 frameshift efficiency, as evidenced by the reduction of the G-1.5-kDa protein signal in vitro (Fig. 6B). It also suppressed the −2/+1 ribosomal frameshift, which mediated the G-F protein synthesis. Nevertheless, both −1/+2 and −2/+1 frameshift products were still apparent. If the downstream −1 ribosomal frameshift consensus signal was also removed by the mutation of nt 382 from A to C (AΔG, AΔC), then the F protein was no longer detectable by gel electrophoresis (unpublished observation).
FIG. 6.
Analysis of the consensus −1 frameshift signals in ribosomal frameshift. (A) The A-rich HCV-1 frameshift signal. The two consensus −1 frameshift signals are underlined. The A-to-G substitution at nt 376 (AΔG) and the A-to-C substitution at nt 382 (AΔC) are also shown. (B) Effect of the AΔG substitution on ribosomal frameshift in vitro. G-Core and G-Core(AΔG) RNAs were synthesized from pCMV-G-Core and pCMV-G-Core(AΔG), respectively, translated in wheat germ extracts in the presence of [35S]methionine, and analyzed on a polyacrylamide gel. (C) Effect of the AΔG and AΔC double nucleotide substitutions on the frameshift efficiencies in Huh7 cells. pEF-FSPSmtLuc and pEF-FSPSmt(AΔG, AΔC)Luc constructs were transiently transfected into Huh7 cells along with the growth hormone plasmid, which monitored the transfection efficiency, and analyzed for luciferase activity at 42 to 48 h posttransfection. Experiments were done in either duplicates or triplicates. Experiments were repeated at least twice. Data represent the average of all experiments. pEF-FSPSmtLuc constructs gave frameshift efficiencies of 0.54% ± 0.07% for −2/+1 frameshift and 2.47% ± 0.18% for −1/+2 frameshift, which were taken as 100%. (D) pEF-FSPSmt(AΔG, AΔC)Luc constructs were transfected into Huh7 cells with human growth hormone plasmid for 24 h and then treated with 1 and 5 μM puromycin for another 24 h. Cells were harvested and analyzed for frameshift efficiencies by the luciferase assays. Experiments were done in duplicates and repeated three times. Data represent the mean ± SEM of a typical set of experiments. The fold increase of frameshift efficiency was calculated by dividing the frameshift efficiency of the puromycin-treated samples by that of the untreated samples.
Since our inability to detect the ribosomal frameshift products might be due to the low sensitivity of the protein gels, we also conducted luciferase reporter assays, using Huh7 cells. As shown in Fig. 6C, when the first 53 codons of the HCV core protein coding sequence with this double nucleotide substitution were fused to the luciferase reporter gene to create frameshift reporter constructs [pEF-FSPSmt(AΔG, AΔC)], −2/+1 and −1/+2 frameshifts could still be detected in Huh7 cells, although their efficiencies were reduced by approximately 60 and 70%, respectively. Interestingly, these frameshift efficiencies could also be significantly enhanced by puromycin treatment (Fig. 6D). The −2/+1 and the −1/+2 frameshifting efficiencies were increased two- to threefold by 1 μM puromycin and approximately five- and ninefold, respectively, by 5 μM puromycin. As the majority of HCV sequences contain these two nucleotide variations within the A-rich region (http://s2as02.genes.nig.ac.jp), these results suggested that HCV sequences other than HCV-1 could also support the dual frameshifting, although at lower efficiencies.
Detection of reactive antibodies in HCV patients to the −1 frameshift product.
To examine whether the −1 frameshift occurred during natural HCV infection, a peptide, MSTNPKPQKKKQT, corresponding to the predicted sequence of the −1 frameshift protein product was chemically synthesized and used for EIA to look for its reactive antibodies in four HCV patients and one hepatitis B virus (HBV) patient. As shown in Table 1, all four HCV sera showed significantly higher reactivity to the peptide than the HBV serum. These data suggest that the −1 frameshift product can be synthesized during natural HCV infection. However, as the −1 frameshift protein shares at least the first eight amino acids with the core protein of the zero reading frame and the F protein of the −2/+1 reading frame, one must be careful with the interpretation of these results due to the possible cross-reactivity with the core and F protein antibodies.
TABLE 1.
EIA for antibodies that react with the −1 frameshift proteina
| Patient no.b | OD450 | HCV |
|---|---|---|
| 1 | 1.24 ± 0.05c | + |
| 2 | 0.24 ± 0.04c | + |
| 3 | 0.45 ± 0.02c | + |
| 4 | 0.25 ± 0.01c | + |
| 5 | 0.12 ± 0.01 | − |
EIA was carried out using the peptide MSTNPKPQKKKQT. The experimental procedures are described in Materials and Methods.
Patients 1 to 4 were positive for HCV, whereas patient 5 was negative for HCV and positive for HBV. This HBV patient served as a negative control.
These samples had a positive-to-negative ratio of ≥2 and were considered positive for the antibodies against the −1 frameshift peptide. OD450, optical density at 450 nm.
DISCUSSION
In this report, we showed that the HCV ribosomal frameshift signal had a triple decoding function which led to the expression of three distinct protein entities, p21 core, F protein, and a 1.5-kDa protein, from each of the three reading frames of the HCV coding sequence. Ribosomal frameshift usually shows a directional specificity, supporting, for example, either −1 or +1 frameshift (6). The HCV frameshift signal, however, can apparently support both −1/+2 and −2/+1 frameshifts and, thus, is unique.
This special ability of HCV to support both −1/+2 and −2/+1 frameshifts is likely due to the particular slipperiness of its A-rich frameshift site. For instance, the stretch of 10 continuous As located at codons 8 to 11 will allow the ribosomes to slip either forward or backward at codons 9, 10, or 11 by one or two nucleotides and still allow the tRNAs to maintain at least two correct base pairings with the codons. It is interesting that there is at least one copy of the consensus −1 ribosomal frameshift signal X XXY YYZ in each of the three reading frames. This observation raises the possibility that the F protein, which overlaps the core protein in the −2/+1 reading frame, may also be produced by a double −1 frameshift mechanism. Such a mechanism is similar to that used by human T-cell leukemia virus-2 (HTLV-2) for the expression of its pol gene although, for HTLV-2, the two −1 ribosomal frameshift signals are separated by the pro sequence (5, 17). If this mechanism is indeed used by HCV, then the 1.5-kDa protein product might not have any particular functions and might simply be a byproduct of F protein synthesis.
Such slipperiness of the HCV-1 frameshift signal raises a possibility that the F protein might be synthesized through multiple frameshifting events at multiple sites. This would explain the sequence heterogeneity that we detected in our previous radiosequencing experiments (28). The mutation of A to G at nt 376 to remove the upstream X XXY YYZ consensus sequence in the zero reading frame, shown in Fig. 6A, significantly reduced but did not abolish the −1/+2 and the −2/+1 ribosomal frameshift efficiencies in vitro (Fig. 6B). Further mutation of nt 382 from A to C to remove the downstream X XXY YYZ consensus sequence (Fig. 6A) reduced the HCV frameshift efficiencies to a level that was undetectable by gel electrophoresis. However, this double mutation was found to reduce but not abolish the HCV ribosomal frameshifts in Huh7 cells when the more-sensitive luciferase reporter assays were conducted (Fig. 6C). The reduction of frameshift efficiencies caused by these two nucleotide mutations was likely the result of decreasing the number of potential ribosomal frameshift sites and hence the ribosomal frameshift incidents.
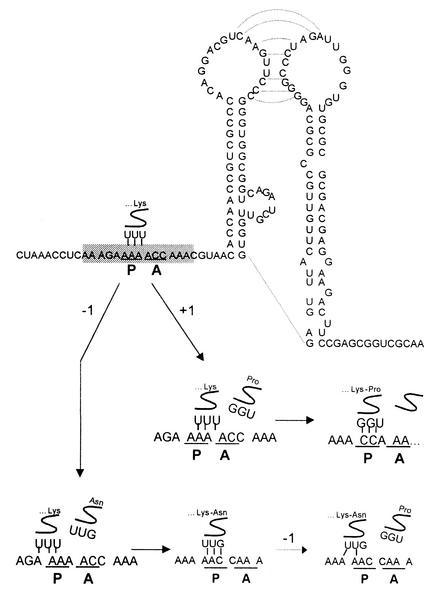
How the HCV-1 sequence can still mediate −1/+2 and −2/+1 ribosomal frameshifts without consensus −1 frameshift signals in the zero reading frame is intriguing. It would appear that the most likely scenario is that the 1.5-kDa protein and the F protein are produced by the slippage of tRNA at codon 10 by 1 nt, to the −1 and +1 reading frames, respectively. This hypothetical scheme is illustrated in Fig. 7. Alternatively, the F protein might be produced by two −1 frameshifts at codons 10 and 11, although, in this case, the second −1 frameshift will produce only two base pairs between the codon and the anticodon (Fig. 7). As there are no consensus −1 frameshift signals, these frameshifts would use mechanisms different from the simultaneous A and P site slippage model (11), such as the slippage of peptidyl tRNA at the P site while the A site is still empty (30).
FIG. 7.
Mechanisms of HCV ribosomal frameshifts in the absence of the consensus −1 frameshift signals. The RNA sequence and structure are based on the pH 77c sequence (29), which contains G and C residues at nt 376 and 382, respectively. The A-rich sequence is shaded. The two loops of the double stem-loop structure can potentially form base pairs to produce a pseudoknot structure. In this model, ribosomes, reaching the double stem-loop structure, will stall in the presence of puromycin or other factors, thus facilitating the slippage of peptidyl-tRNA at the P site either forward (+1) or backward (−1). The −1 slippage may occur one more time (dashed arrow), leading to the decoding of the F reading frame.
Note that the double mutation of A to G at nt 376 and A to C at nt 382 is found in a large fraction of the HCV sequences that have been compiled in the HCV database. As the double mutation did not abolish ribosomal frameshift in Huh7 cells (Fig. 6C), these HCV sequences are expected to support the dual frameshifting, although at efficiencies lower than those of the HCV-1 sequence. This observation is consistent with the previous findings that many HCV patients have antibodies to the F protein (26, 28). It is likely that these HCV sequences mediate the dual ribosomal frameshifting through the mechanisms proposed in Fig. 7.
In addition to the A-rich frameshift signal, codons 15 to 53 located immediately downstream significantly enhanced ribosomal frameshifting in the presence of puromycin. The sequence encompassing codons 15 to 53 has the potential to form a double stem-loop structure (23, 27). Our analysis indicated that nt 392 to 447 could form one stem-loop structure and nt 448 to 508 could form the other, with a combined free energy of more than −44.5 kcal (Fig. 7). Interestingly, this double stem-loop structure may be capable of forming a pseudoknot by base pairings of the two loops (Fig. 7). Our predicted upstream stem-loop is slightly different from those suggested by Smith and Simmonds (23), later referred to as loops V and VI by Wang et al. (27). The precise RNA structures and their importance in ribosomal frameshifts, however, should be experimentally determined.
Puromycin is a tyrosyl-tRNA analog that can compete with aminoacyl-tRNAs for the transfer of nascent peptide chain from the P site of ribosomes (25). This compound is one of the antibiotics that have been demonstrated to alter ribosomal frameshift efficiencies (8, 10) and, indeed, it changed the ratios of the F, 1.5-kDa, and p21 core proteins in our translational studies (Fig. 5B and C). This effect of puromycin is not likely due to the differences in the size of proteins that are being generated, because the G-Core and G-CoreΔ constructs failed to respond to puromycin to similar levels, despite similar differences in the length of their protein products (Fig. 5B and C). Furthermore, the same enhancement of frameshifting could be observed using luciferase reporter constructs, which produced protein products practically of the same size (Fig. 5A).
While the exact mechanism by which puromycin regulates frameshifting in the presence of codons 15 to 53 remains to be elucidated, the effect of puromycin likely involves the stalling of ribosomes at the double stem-loop structure, which then promotes the slippage of tRNA at the frameshift site located immediately upstream (Fig. 7). Although it is also possible that puromycin regulates frameshifting indirectly by inhibiting the synthesis of some cellular proteins in Huh7 cells, this scenario is not likely to be true in vitro (Fig. 5B and C), unless one of its three protein products can regulate frameshifting. Also, the effect of puromycin is clearly independent of the context of the shifting signal, as the A376-to-G376 and A386-to-C386 double nucleotide substitution decreased the basal frameshifting efficiency without affecting the fold enhancement induced by puromycin (Fig. 6D). The observation that puromycin could increase the frameshift efficiencies in the presence of codons 15 to 53 raises the possibility that the HCV ribosomal frameshift may also be regulated by other factors. It will be interesting to determine whether any HCV gene products interact with codons 15 to 53 to regulate ribosomal frameshift during HCV infection.
The HCV core protein has been suggested to have diverse biological functions, ranging from viral morphogenesis and modulation of host gene expression to the development of steatosis and hepatocellular carcinoma (2, 14). In view of the findings that the HCV core protein sequence can also mediate the synthesis of the F protein and the 1.5-kDa protein, one must now carefully reevaluate whether some of these functions of the core protein are in fact shared or mediated by these two frameshift products.
Acknowledgments
We thank Ben Yen for critical reading of the manuscript and members of J. Ou's laboratory for helpful discussions throughout this entire project.
This work was supported by postdoctoral fellowship PF-01-037-01-MBC to J.C. from the American Cancer Society and a grant from the National Institutes of Health.
REFERENCES
- 1.Brierley, I., P. Digard, and S. C. Inglis. 1989. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell 57:537-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi, J., W. Lu, and J.-H. Ou. 2001. Structure and functions of hepatitis C virus core protein. Recent Res. Dev. Virol. 3:105-120. [Google Scholar]
- 3.Denison, M. R., P. W. Zoltick, J. L. Leibowitz, C. J. Pachuk, and S. R. Weiss. 1991. Identification of polypeptides encoded in open reading frame 1b of the putative polymerase gene of the murine coronavirus mouse hepatitis virus A59. J. Virol. 65:3076-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinman, J. D., M. J. Ruiz-Echevarria, K. Czaplinski, and S. W. Peltz. 1997. Peptidyl-transferase inhibitors have antiviral properties by altering programmed −1 ribosomal frameshifting efficiencies: development of model systems. Proc. Natl. Acad. Sci. USA 94:6606-6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk, H., N. Mador, R. Udi, A. Panet, and A. Honigman. 1993. Two cis-acting signals control ribosomal frameshift between human T-cell leukemia virus type II gag and pro genes. J. Virol. 67:6273-6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farabaugh, P. J. 1996. Programmed translational frameshifting. Microbiol. Rev. 60:103-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grentzmann, G., J. A. Ingram, P. J. Kelly, R. F. Gesteland, and J. F. Atkins. 1998. A dual-luciferase reporter system for studying recoding signals. RNA 4:479-486. [PMC free article] [PubMed] [Google Scholar]
- 8.Honigman, A., H. Falk, N. Mador, T. Rosental, and A. Panet. 1995. Translation efficiency of the human T-cell leukemia virus (HTLV-2) gag gene modulates the frequency of ribosomal frameshifting. Virology 208:312-318. [DOI] [PubMed] [Google Scholar]
- 9.Houghton, M., A. Weiner, J. Han, G. Kuo, and Q. L. Choo. 1991. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology 14:381-388. [PubMed] [Google Scholar]
- 10.Irvine, J. H., J. A. Horsfield, C. Z. McKinney, and W. P. Tate. 1998. A novel strategy to interfere with human immunodeficiency virus type 1 propagation. N. Z. Med. J. 111:222-224. [PubMed] [Google Scholar]
- 11.Jacks, T., H. D. Madhani, F. R. Masiarz, and H. E. Varmus. 1988. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell 55:447-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacks, T., M. D. Power, F. R. Masiarz, P. A. Luciw, P. J. Barr, and H. E. Varmus. 1988. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature 331:280-283. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, B., S. S. Monroe, E. V. Koonin, S. E. Stine, and R. I. Glass. 1993. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc. Natl. Acad. Sci. USA 90:10539-10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai, M. M., and C. F. Ware. 2000. Hepatitis C virus core protein: possible roles in viral pathogenesis. Curr. Top. Microbiol. Immunol. 242:117-134. [DOI] [PubMed] [Google Scholar]
- 15.Lefkowitch, J. H., E. R. Schiff, G. L. Davis, R. P. Perrillo, K. Lindsay, H. C. Bodenheimer, Jr., L. A. Balart, T. J. Ortego, J. Payne, J. L. Dienstag, et al. 1993. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. Gastroenterology 104:595-603. [DOI] [PubMed] [Google Scholar]
- 16.Lo, S. Y., F. Masiarz, S. B. Hwang, M. M. Lai, and J. H. Ou. 1995. Differential subcellular localization of hepatitis C virus core gene products. Virology 213:455-461. [DOI] [PubMed] [Google Scholar]
- 17.Mador, N., A. Panet, and A. Honigman. 1989. Translation of gag, pro, and pol gene products of human T-cell leukemia virus type 2. J. Virol. 63:2400-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health. 1997. Management of hepatitis C. National Institutes of Health Consensus Development Conference Statement 15(3):1-41. [Google Scholar]
- 19.Parkin, N. T., M. Chamorro, and H. E. Varmus. 1992. Human immunodeficiency virus type 1 gag-pol frameshifting is dependent on downstream mRNA secondary structure: demonstration by expression in vivo. J. Virol. 66:5147-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rijnbrand, R. C., and S. M. Lemon. 2000. Internal ribosome entry site-mediated translation in hepatitis C virus replication. Curr. Top. Microbiol. Immunol. 242:85-116. [DOI] [PubMed] [Google Scholar]
- 21.Scheuer, P. J., P. Ashrafzadeh, S. Sherlock, D. Brown, and G. M. Dusheiko. 1992. The pathology of hepatitis C. Hepatology 15:567-571. [DOI] [PubMed] [Google Scholar]
- 22.Selby, M. J., Q. L. Choo, K. Berger, G. Kuo, E. Glazer, M. Eckart, C. Lee, D. Chien, C. Kuo, and M. Houghton. 1993. Expression, identification and subcellular localization of the proteins encoded by the hepatitis C viral genome. J. Gen. Virol. 74:1103-1113. [DOI] [PubMed] [Google Scholar]
- 23.Smith, D. B., and P. Simmonds. 1997. Characteristics of nucleotide substitution in the hepatitis C virus genome: constraints on sequence change in coding regions at both ends of the genome. J. Mol. Evol. 45:238-246. [DOI] [PubMed] [Google Scholar]
- 24.ten Dam, E. B., C. W. Pleij, and L. Bosch. 1990. RNA pseudoknots: translational frameshifting and readthrough on viral RNAs. Virus Genes 4:121-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vazquez, D. 1974. Inhibitors of protein synthesis. FEBS Lett. 40(Suppl.):S63-S84. [DOI] [PubMed] [Google Scholar]
- 26.Walewski, J. L., T. R. Keller, D. D. Stump, and A. D. Branch. 2001. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7:710-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, T. H., R. C. Rijnbrand, and S. M. Lemon. 2000. Core protein-coding sequence, but not core protein, modulates the efficiency of cap-independent translation directed by the internal ribosome entry site of hepatitis C virus. J. Virol. 74:11347-11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu, Z., J. Choi, T. S. Yen, W. Lu, A. Strohecker, S. Govindarajan, D. Chien, M. J. Selby, and J. Ou. 2001. Synthesis of a novel hepatitis C virus protein by ribosomal frameshift. EMBO J. 20:3840-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagi, M., R. H. Purcell, S. U. Emerson, and J. Bukh. 1997. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc. Natl. Acad. Sci. USA 94:8738-8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yelverton, E., D. Lindsley, P. Yamauchi, and J. A. Gallant. 1994. The function of a ribosomal frameshifting signal from human immunodeficiency virus-1 in Escherichia coli. Mol. Microbiol. 11:303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]