Abstract
Reactive oxygen species (ROS) are implicated in cardiovascular diseases. ROS, such as H2O2, act as second messengers to activate diverse signaling pathways. Although H2O2 activates several tyrosine kinases, including the epidermal growth factor (EGF) receptor, JAK2, and PYK2, in vascular smooth muscle cells (VSMCs), the intracellular mechanism by which ROS activate these tyrosine kinases remains unclear. Here, we identified two distinct signaling pathways required for receptor and nonreceptor tyrosine kinase activation by H2O2 involving a metalloprotease-dependent generation of heparin-binding EGF-like growth factor (HB-EGF) and protein kinase C (PKC)-δ activation, respectively. H2O2-induced EGF receptor tyrosine phosphorylation was inhibited by a metalloprotease inhibitor, whereas the inhibitor had no effect on H2O2-induced JAK2 tyrosine phosphorylation. HB-EGF neutralizing antibody inhibited H2O2-induced EGF receptor phosphorylation. In COS-7 cells expressing an HB-EGF construct tagged with alkaline phosphatase, H2O2 stimulates HB-EGF production through metalloprotease activation. By contrast, dominant negative PKC-δ transfection inhibited H2O2-induced JAK2 phosphorylation but not EGF receptor phosphorylation. Dominant negative PYK2 inhibited H2O2-induced JAK2 activation but not EGF receptor activation, whereas dominant negative PKC-δ inhibited PYK2 activation by H2O2. These data demonstrate the presence of distinct tyrosine kinase activation pathways (PKC-δ/PYK2/JAK2 and metalloprotease/HB-EGF/EGF receptor) utilized by H2O2 in VSMCs, thus providing unique therapeutic targets for cardiovascular diseases.
Reactive oxygen species (ROS), including superoxide anion and hydrogen peroxide (H2O2), are known to act as second messengers (21, 41). ROS activate a wide variety of serine-threonine and tyrosine kinases, which are key regulatory proteins of signal transduction pathways important in mediating cellular growth, apoptosis, survival, migration, and aging (22, 35). An increasing body of evidence suggests that ROS and tyrosine kinase play prominent roles in the development and progression of the cardiovascular remodeling associated with hypertension, atherosclerosis, and restenosis after balloon angioplasty (2, 29).
ROS activate nonreceptor tyrosine kinases JAK2 (1, 59), PYK2/CAKβ (25), and Src (11, 67) and receptor tyrosine kinases epidermal growth factor (EGF) receptor (23, 53, 67) and platelet-derived growth factor receptor (34) in vascular smooth muscle cells (VSMCs) as well as other cell lines. A few studies have shown an inhibition of ligand-stimulated receptor tyrosine kinase induced by ROS (33), suggesting a dominant role for ROS as a tyrosine kinase activator. Among tyrosine kinases activated by ROS, the EGF receptor and JAK2 are of particular interest in VSMCs. A G-protein-coupled receptor (GPCR) agonist, angiotensin II (AngII), has been shown to utilize ROS to activate the EGF receptor in VSMCs (23, 66). The activation of the EGF receptor by AngII or thrombin appears to be required for extracellular signal-regulated kinase (ERK) activation and the subsequent growth of VSMCs (15, 17, 19, 38). By contrast, ROS-dependent JAK2 activation is required for AngII-induced cytokine induction (56) and thrombin-induced heat shock protein induction (47) in VSMCs. Thus, ROS-dependent activation of the EGF receptor and JAK2 could mediate two distinct functions in VSMCs, such as growth and inflammatory responses, respectively. However, whether ROS activate the EGF receptor and JAK2 through distinct mechanisms remains unknown.
Recently, it has become apparent that the EGF receptor is also a part of the signaling networks activated by stimuli that do not directly interact with this receptor. These stimuli include agonists that specifically bind to other membrane receptors and environmental stressors (6). Collectively, EGF receptor transactivation by these factors is employed in a wide array of biological signaling responses (32, 45), which may participate in several disease processes (4, 42, 50). In this regard, EGF receptor transactivation is a current topic of signal transduction research.
ROS have been proposed to exert their effects through targeting the cysteine regions of the active sites of tyrosine phosphatases, which in turn activates tyrosine kinases (21). In fact, H2O2 has been shown to inhibit the dephosphorylation of the EGF receptor through the inhibition of a tyrosine phosphatase (39). Protein kinase C-δ (PKC-δ) is also implicated in ROS-dependent activation of tyrosine kinases, such as c-Abl and Src. H2O2 stimulates binding between PKC-δ and c-Abl where the activation of c-Abl is dependent on PKC-δ activation (61). Interestingly, H2O2-induced activation of PKC-δ is reported to be independent from tyrosine phosphatase inhibition (68). Alternatively, ROS may activate a tyrosine kinase by generating growth factors, such as heparin-binding EGF-like growth factor (HB-EGF), through metalloprotease cleavage. ROS production and metalloprotease-dependent HB-EGF generation are implicated in EGF receptor transactivation initiated through several GPCRs (9, 52). Our group has shown that both mechanisms are indispensable for EGF receptor transactivation induced by AngII in VSMCs (14, 23). In addition, a metalloprotease, ADAM17 (TACE), was reported to require PKC-δ activation to generate HB-EGF (37).
In this study, we examined the hypothesis that the activation of receptor and nonreceptor protein tyrosine kinases by ROS utilizes distinct signal transduction mechanisms involving a metalloprotease or PKC-δ. We found that a metalloprotease-dependent shedding of HB-EGF is required for H2O2-induced EGF receptor transactivation but not for JAK2 activation. By contrast, PKC-δ is required for H2O2-induced JAK2 activation but not for EGF receptor transactivation. The activation of JAK2 but not of the EGF receptor also requires PYK2 activation. Taken together, our findings provide a unique example of two distinct signaling pathways that mediate ROS-dependent tyrosine kinase activation in vascular cells.
MATERIALS AND METHODS
Reagents.
BB2116 was kindly provided by Helen Mills (British Biotech). CGS27023, GM6001, AG1478, and rottlerin were purchased from Calbiochem. H2O2, AngII, N-acetylcysteine, and poly[Glu80-Tyr20] were purchased from Sigma. Antibodies were purchased from the following sources: phospho-JAK2, phospho-EGF receptor, and phospho-PYK2, BioSource International; JAK2, Upstate Biotechnology; EGF receptor, PKC-δ, PKC-α, and PKC-β1, Santa Cruz Biotechnology; PYK2, Transduction Laboratories; and neutralizing human HB-EGF, R & D Systems.
Cell culture.
VSMCs were prepared from thoracic aortas of Sprague-Dawley rats (18). Subcultured cells from passages 3 to 12 were used and showed 99% positive immunostaining with smooth muscle α-actin antibody (Sigma). Human aortic VSMCs were obtained from Clonetics and subcultured according to the manufacturer's manual. For experiments, VSMCs at 80 to 90% confluency were used after serum depletion for 3 days.
Adenovirus transfection.
The generation of kinase-inactive PKC-δ, PKC-α, PKC-β1, and PYK2/CAKβ mutant-encoded adenovirus constructs is described in detail elsewhere (36, 48). VSMCs were infected with adenovirus for 2 days as previously described (17).
Immunoprecipitation and immunoblotting.
After stimulation, cells were lysed with ice-cold immunoprecipitation buffer (150 mM NaCl, 20 mM Tris [pH 7.5], 1% Triton X-100, 5 mM EDTA, 50 mM NaF, 10% [vol/vol] glycerol, 10 mg of leupeptin, 10 μg of aprotinin, and 10 μg of phenylmethylsulfonyl fluoride). The lysates were centrifuged, and the supernatant was immunoprecipitated with antibody and protein A/G agarose at 4°C for 16 h (19). Cell or immunoprecipitation lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and immunoblotted as described previously (19).
HB-EGF shedding assay.
To examine the release of soluble HB-EGF, COS-7 cells were transfected with the alkaline phosphatase (AP)-tagged HB-EGF (HB-EGF-AP) plasmid (63) by a transferrin receptor-operated transfer (8, 58) with TransFast transfection reagent (Promega). Forty-eight hours after transfection, the medium was changed to Dulbecco modified Eagle medium without phenol red and cells were stimulated with H2O2. HB-EGF-AP secreted into the medium was assessed by measuring AP activity (63).
PKC-δ kinase assay.
Kinase activity of PKC-δ was measured by an immune complex kinase assay as described previously (26, 61). After stimulation, cells were lysed with a buffer containing 50 mM HEPES (pH 7.5), 0.5% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1 mM NaF, 2 mM phenylmethylsulfonyl fluoride, and 10 μg each of pepstatin and leupeptin/ml. Cell lysates were centrifuged, and the supernatant was immunoprecipitated with anti-PKC-δ antibody for 2.5 h. The kinase assay was performed with a kinase assay buffer (20 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 2.5 μCi of [γ-32P]ATP, and a substrate, 200 μg of histone H1/ml) incubated for 15 min at 30°C.
PYK2 kinase assay.
PYK2 kinase activity was measured by an immune complex kinase assay as described previously (25). In brief, the cell lysates were centrifuged and the supernatant was immunoprecipitated with anti-PYK2 antibody for 2.5 h at 4°C. After being washed, the immune complexes were incubated with or without H2O2 for 10 min at room temperature in the kinase buffer (100 mM sodium HEPES [pH 7.6], 60 mM MgCl2, 2 mM MnCl2, 0.2 mM Na3VO4, 0.2% Triton X-100). Afterwards, the lysates were incubated at room temperature in kinase buffer containing 0.25 mg of poly[Glu80-Tyr20] and 2.5 μCi of [γ-32P]ATP for 15 min. The reaction mixture was spotted onto Whatman 3MM paper, washed, and then measured by liquid scintillation counting.
RESULTS
H2O2 stimulates EGF receptor activation and JAK2 activation.
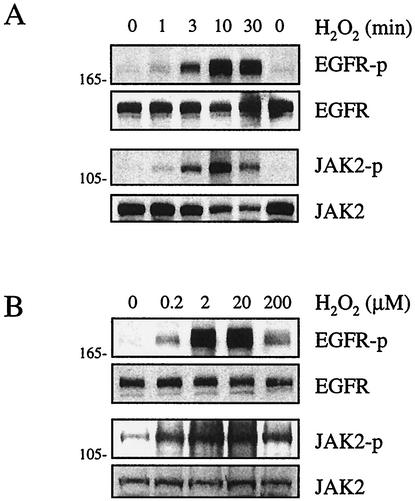
The activation of the EGF receptor by H2O2 was assessed by a phospho-specific antibody. This antibody selectively recognizes the EGF receptor only when Tyr1068 (a Grb2 binding site) is autophosphorylated. Also, the activation of JAK2 was assessed by a phospho-specific antibody that selectively recognizes Tyr1007/1008 dually phosphorylated JAK2. These tyrosine residues are believed to be autophosphorylation sites, with Tyr1007 phosphorylation being essential for JAK2 kinase activity (57). The specificities of these antibodies have been established previously (23). As shown in Fig. 1A, H2O2 time-dependently stimulated the phosphorylation of the EGF receptor and JAK2, with maximal phosphorylation occurring at 10 min. As shown in Fig. 1B, H2O2 concentration-dependently stimulated the phosphorylation of the EGF receptor and JAK2, with maximal phosphorylation occurring at H2O2 concentrations of 2 to 20 μM. These data suggest that the EGF receptor and JAK2 represent ROS-sensitive tyrosine kinases in VSMCs.
FIG. 1.
Activation of EGF receptor and JAK2 by H2O2 in VSMCs. Cells were stimulated with 20 μM H2O2 for the indicated time periods (A) or with various concentrations of H2O2 for 10 min (B). Cell lysates were immunoblotted by antibodies toward phospho-EGF receptor (EGFR-p), EGF receptor (EGFR), phospho-JAK2 (JAK2-p), and JAK2 as indicated. Numbers on the left are molecular weights.
Involvement of metalloprotease-dependent HB-EGF generation in H2O2-induced EGF receptor activation.
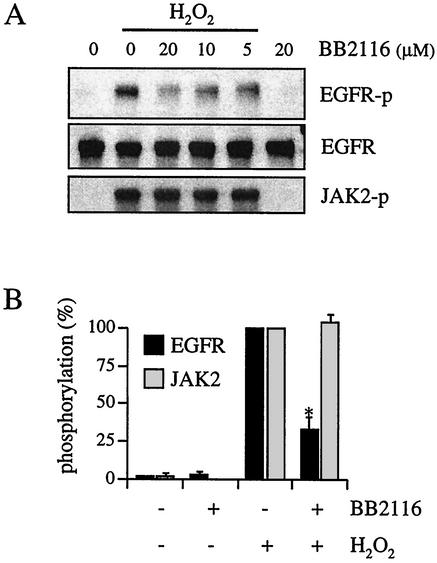
To determine whether EGF receptor transactivation by H2O2 requires metalloprotease-dependent generation of an EGF receptor ligand, VSMCs were pretreated with a metalloprotease inhibitor (BB2116) and stimulated with H2O2. BB2116 and a structurally related compound, batimastat, have been shown to selectively inhibit the processing of several EGF receptor ligand precursors (5, 12, 13). It has previously been shown that BB2116 has no nonspecific effect on EGF receptor signals stimulated by EGF (14). As shown in Fig. 2, H2O2-induced EGF receptor activation was concentration-dependently inhibited by BB2116 but JAK2 activation by H2O2 was unaffected by BB2116. Other metalloprotease inhibitors, CGS27023 and GM6001 (10 μM each, 30-min pretreatments), also inhibited H2O2-induced EGF receptor activation but had no effect on JAK2 activation (data not shown).
FIG. 2.
Effect of metalloprotease inhibitor on H2O2-induced activation of EGF receptor and JAK2. (A) VSMCs were pretreated with the indicated concentrations of BB2116 for 30 min and stimulated with H2O2 (20 μM) for 10 min. Cell lysates were immunoblotted by antibodies toward phospho-EGF receptor (EGFR-p), EGF receptor (EGFR), and phospho-JAK2 (JAK2-p). (B) Densitometric analysis of EGF receptor and JAK2 phosphorylation. VSMCs were pretreated with or without 20 μM BB2116 for 30 min and stimulated with H2O2 (20 μM) for 10 min. Results are the means ± standard errors of the means (SEM) (n = 4). Asterisk, P < 0.05 versus H2O2 stimulation.
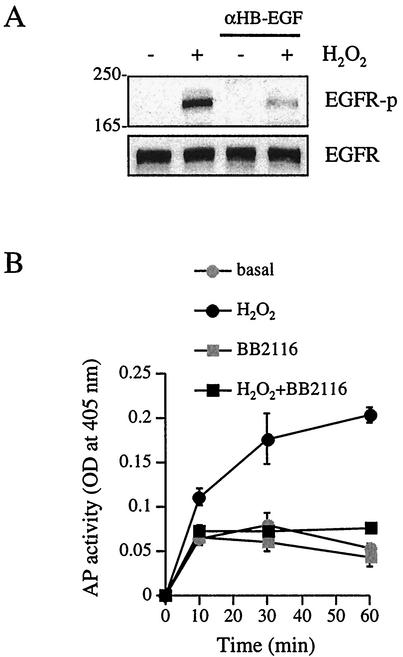
It has previously been shown that an HB-EGF neutralizing antibody effectively antagonizes AngII-induced EGF receptor transactivation in VSMCs (14). As shown in Fig. 3A, H2O2-induced EGF receptor activation was markedly inhibited by the HB-EGF neutralizing antibody. Since the detection of endogenous EGF receptor ligand generation has proven difficult (14), we utilized the HB-EGF-AP expression system, an established assay, to measure the ectodomain shedding of EGF receptor ligands. H2O2 time-dependently stimulated HB-EGF-AP release into the culture medium at as early as 10 min in COS-7 cells transfected with HB-EGF-AP plasmid, and BB2116 almost completely inhibited HB-EGF-AP generation in response to H2O2 (Fig. 3B). To confirm H2O2-induced EGF receptor tyrosine kinase activation, we examined the effect of AG1478, an EGF receptor kinase inhibitor (44). It has previously been shown that AG1478 specifically inhibits EGF receptor-mediated signal transduction in VSMCs (14, 15, 17, 19). AG1478 (250 nM, 30-min pretreatment) markedly inhibited H2O2-induced EGF receptor activation, whereas this inhibitor had no specific effect on JAK2 activation by H2O2 (data not shown). These data clearly implicate metalloprotease-dependent HB-EGF generation as a mechanism for H2O2-induced EGF receptor activation.
FIG. 3.
Involvement of HB-EGF in H2O2-induced EGF receptor activation. (A) Human VSMCs were pretreated with HB-EGF neutralizing antibody (αHB-EGF; 40 μg/ml) for 1 h and stimulated with H2O2 (20 μM) for 10 min. Cell lysates were immunoblotted by phospho-EGF receptor antibody (EGFR-p) and EGF receptor (EGFR) antibody. Numbers on the left are molecular weights. (B) After pretreatment with or without BB2116 (20 μM) for 30 min, COS-7 cells transfected with HB-EGF-AP plasmid were stimulated with H2O2 (20 μM). AP activity in the medium was determined. Results are the means ± SEM (n = 3). OD, optical density; basal, control sample.
JAK2 activation but not EGF receptor activation requires PKC-δ.
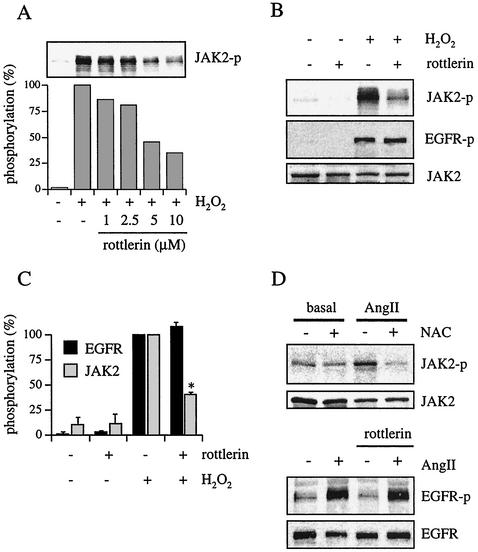
H2O2 has been shown to induce PKC-δ activation (40), which may in turn activate tyrosine kinases (61). PKC-δ is also implicated in the ectodomain shedding of HB-EGF (37). Given these findings, we investigated whether PKC-δ was involved in either JAK2 or EGF receptor activation by H2O2. VSMCs were pretreated with a PKC-δ inhibitor, rottlerin (31). The conditions required for the inhibition of PKC-δ function in VSMCs have been established previously (26). As shown in Fig. 4A, rottlerin (1 to 10 μM) concentration-dependently inhibited JAK2 phosphorylation by H2O2 in VSMCs. Figure 4B and C show that 10 μM rottlerin markedly inhibited H2O2-induced JAK2 activation but not H2O2-induced EGF receptor activation. A potent endogenous ROS inducer, AngII (30), produces intracellular H2O2 in the 10 to 100 nM range in VSMCs (64), which may mimic the exogenous addition of H2O2 as shown in Fig. 1A. Our group has previously reported that AngII-induced JAK2 activation requires PKC-δ (26) and is not blocked by a metalloprotease inhibitor, BB2116 (14), in VSMCs. Another study has shown that AngII-induced JAK2 activation requires ROS production in VSMCs (56). As shown in Fig. 4D, AngII-induced JAK2 activation was markedly inhibited by an antioxidant, N-acetylcysteine, in our VSMCs. Also, it has previously been reported that AngII-induced transactivation of the EGF receptor requires ROS (23) as well as HB-EGF production through a metalloprotease (14) in VSMCs. As shown in Fig. 4D, rottlerin had no inhibitory effect on AngII-induced EGF receptor transactivation, confirming that this pathway is independent from the JAK2 pathway activated by AngII. Taken together, these data suggest that AngII utilizes these two distinct pathways through ROS production in VSMCs, supporting the pathophysiological relevance of our findings.
FIG. 4.
Effects of rottlerin, a PKC-δ inhibitor, on H2O2-induced JAK2 and EGF receptor activation. (A) VSMCs were pretreated with various concentrations of rottlerin as indicated for 30 min and stimulated with H2O2 (20 μM) for 10 min. Cell lysates were immunoblotted by antibody toward phospho-JAK2 (JAK2-p). (B) VSMCs were pretreated with rottlerin (10 μM) for 30 min and stimulated with H2O2 (20 μM) for 10 min. Cell lysates were immunoblotted by antibodies toward phospho-JAK2, phospho-EGF receptor (EGFR-p), and JAK2. (C) Densitometric analysis of EGF receptor (EGFR) and JAK2 phosphorylation in the immunoblot shown in Fig. 4B. Results are the means ± SEM (n = 4). (D) VSMCs were pretreated with 20 mM N-acetylcysteine (NAC) for 90 min (upper panels) or 10 μM rottlerin for 30 min (lower panels) as indicated and stimulated with AngII (100 nM) for 3 min. Cell lysates were immunoblotted by antibodies toward phospho-JAK2 and JAK2 or phospho-EGF receptor and EGF receptor as indicated. Basal, control sample.
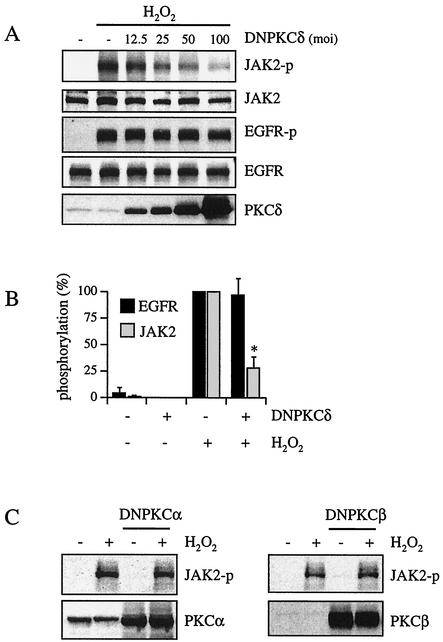
To further clarify the role of PKC-δ in ROS-dependent JAK2 activation, we transfected VSMCs with adenovirus encoding a kinase-deficient PKC-δ mutant that acts as a dominant negative PKC-δ (48). The specificity of this mutant has been shown previously (26). Several control studies using adenovirus encoding LacZ or vector alone also showed that the transfection of adenovirus (up to a multiplicity of infection [MOI] of 100) had no nonspecific effects in VSMCs (17, 24, 26). As shown in Fig. 5A and B, dominant negative PKC-δ transfection concentration-dependently inhibited H2O2-induced JAK2 activation but not H2O2-induced EGF receptor activation. Moreover, control studies using VSMCs transfected with kinase-inactive PKC-α and PKC-β1 revealed no inhibitory effect on H2O2-stimulated JAK2 phosphorylation (Fig. 5C). In addition, H2O2 (20 μM, 10-min stimulation) significantly stimulated PKC-δ activity in VSMCs (2.49 [±0.09]-fold increase in activity; P ≤ 0.05; n = 4) as measured by an immune complex kinase assay. These results strongly suggest that PKC-δ is required for H2O2-induced JAK2 activation but not for H2O2-induced EGF receptor activation.
FIG. 5.
Effects of dominant negative PKC-δ transfection on H2O2-induced JAK2 and EGF receptor activation. (A) Cells were transfected with the indicated amount of adenovirus encoding dominant negative PKC-δ (DNPKCδ) for 48 h and stimulated by H2O2 (20 μM) for 10 min. The cell lysates were immunoblotted by antibodies toward phospho-JAK2 (JAK2-p), JAK2, phospho-EGF receptor (EGFR-p), EGF receptor (EGFR), and PKC-δ. (B) Densitometric analysis of EGF receptor and JAK2 phosphorylation. Cells were transfected with adenovirus encoding dominant negative PKC-δ (MOI, 100) and stimulated with H2O2 (20 μM) for 10 min. Results are the means ± SEM (n = 3). (C) Cells were transfected with dominant negative PKC-α (DNPKCα) or dominant negative PKC-β1 (DNPKCβ) for 48 h and stimulated by H2O2 (20 μM) for 10 min. The cell lysates were immunoblotted by antibodies toward phospho-JAK2, PKC-α, or PKC-β1.
JAK2 activation but not EGF receptor activation requires PYK2.
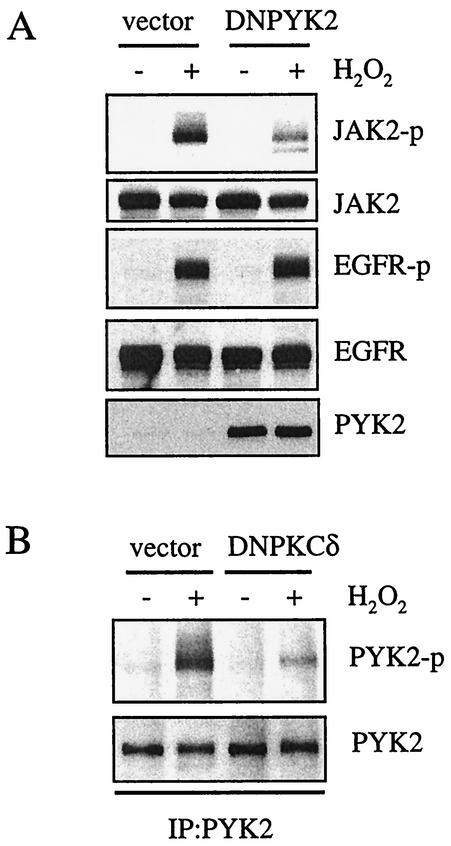
PYK2/CAKβ is a ROS-sensitive tyrosine kinase (25), and our group has recently demonstrated that this kinase is required for AngII-induced JAK2 activation in VSMCs (26). PYK2 is also implicated in EGF receptor transactivation by GPCRs (3). Therefore, we determined whether PYK2 plays a role in H2O2-induced activation of the EGF receptor and JAK2. VSMCs were transfected with adenovirus encoding a kinase-deficient PYK2 mutant, K457A (36). This mutant acts as a dominant negative PYK2 in VSMCs (24). As shown in Fig. 6A, kinase-deficient PYK2 mutant transfection markedly inhibited H2O2-induced JAK2 activation but not EGF receptor activation. Our group has previously shown that the treatment of VSMCs with H2O2 enhances PYK2 kinase activity (25). Thus, we further determined whether H2O2 directly activated PYK2 by measuring in vitro PYK2 kinase activity. However, H2O2 did not activate PYK2 kinase activity in vitro (data not shown), indicating that PYK2 is not a direct target of H2O2.
FIG. 6.
Involvement of PYK2 in H2O2-induced JAK2 activation. (A) Cells were transfected with adenovirus (MOI, 10) encoding dominant negative PYK2 (DNPYK2) for 48 h and stimulated by H2O2 (20 μM) for 10 min. The cell lysates were immunoblotted by antibodies toward phospho-JAK2 (JAK2-p), JAK2, phospho-EGF receptor (EGFR-p), EGF receptor (EGFR), and PYK2. (B) Cells were transfected with adenovirus (MOI, 100) encoding dominant negative PKC-δ (DNPKCδ) for 48 h and stimulated by H2O2 (20 μM) for 10 min. The cell lysates were immunoprecipitated with anti-PYK2 antibody and immunoblotted by antibodies toward phospho-PYK2 (PYK2-p) and PYK2. IP, immunoprecipitate.
PYK2 Tyr402 is a major autophosphorylation site of PYK2. Our group has previously shown that H2O2 induces PYK2 Tyr402 phosphorylation by using a phospho-specific antibody (25). To determine whether PYK2 activation by H2O2 is regulated by PKC-δ, we examined the effect of PKC-δ inhibitors on PYK2 Tyr402 phosphorylation. As shown in Fig. 6B, dominant negative PKC-δ transfection markedly inhibited the phosphorylation of PYK2 induced by H2O2. We also observed similar inhibition by rottlerin (data not shown). These data suggest that a PKC-δ-sensitive tyrosine kinase, PYK2, is required for the activation of JAK2 by H2O2 but not for EGF receptor activation in VSMCs.
DISCUSSION
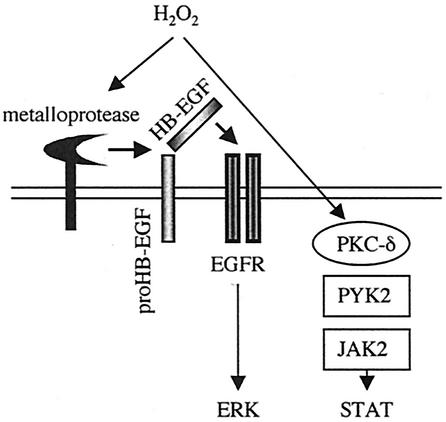
The significant finding reported in the present study is that ROS utilize distinct signaling mechanisms to mediate the activation of receptor and nonreceptor tyrosine kinases. Specifically, a metalloprotease-dependent cleavage of HB-EGF is required for H2O2-induced EGF receptor transactivation but not for JAK2 activation whereas PKC-δ is required for H2O2-induced PYK2/JAK2 activation and not for EGF receptor transactivation, as illustrated in Fig. 7. We believe that this is the first example showing that ROS activate tyrosine kinases through distinct mechanisms in the same cell culture system.
FIG. 7.
Scheme illustrating proposed two distinct activation mechanisms of protein tyrosine kinases by H2O2 in VSMCs. EGFR, EGF receptor.
Here, we report that H2O2 stimulates EGF receptor transactivation via metalloprotease-dependent HB-EGF cleavage. This observation uncovers a previously unknown mechanism by which ROS activate a receptor tyrosine kinase. In support of this notion, our group has shown that both metalloprotease and ROS are required for EGF receptor transactivation induced by a GPCR agonist, AngII (14, 23). Although the metalloprotease responsible for HB-EGF generation induced by ROS has not been identified, both matrix metalloproteases (62, 69) and ADAM family metalloproteases (4, 37, 42) are implicated in the ectodomain shedding of HB-EGF that is stimulated by various agonists. A thiol group from a cysteine residue in the inhibitory prodomains of these metalloproteases interacts with zinc in their catalytic domains. ROS may oxidize electrophilic thiol groups and disrupt the cysteine-zinc bond, leading to the activation of the metalloproteases. In fact, H2O2 was shown to enhance ADAM17 activity and ADAM17-mediated ectodomain shedding (70). Therefore, it is interesting to test whether ROS activate metalloprotease directly. However, to the best of our knowledge, no reports have been published of studies that have directly measured the shedding activity toward proHB-EGF by using isolated membranes. Thus, to examine the direct activation of metalloprotease by ROS, further information is required regarding the identification of the metalloprotease responsible for HB-EGF generation and/or establishment of the assay to measure metalloprotease activity toward proHB-EGF in isolated membranes.
Alternatively, ROS may modulate intracellular signals such as c-Src and ERK, which may indirectly activate the metalloprotease responsible for HB-EGF generation. The contribution of the ERK cascade to HB-EGF and transforming growth factor-α generation has been reported previously (20, 27, 65). However, the ERK cascade is unlikely to mediate HB-EGF generation by H2O2 in VSMCs. This is because the ERK cascade exists downstream of EGF receptor transactivation in H2O2-stimulated VSMCs (23). c-Src is involved in EGF receptor transactivation by GPCRs (3, 46, 66), and c-Src appears to exist upstream of HB-EGF release (51). Moreover, H2O2-induced EGF receptor transactivation was inhibited by a selective Src inhibitor, PP2, in endothelial cells (7). The role of c-Src in mediating HB-EGF-dependent EGF receptor transactivation by H2O2 is under current investigation.
Our findings presented here strongly suggest the requirement of PKC-δ for JAK2 activation by H2O2. In the present study, we have used rottlerin as a PKC-δ inhibitor because it is commonly used as a selective inhibitor of this PKC isoform. In fact, our group has shown that this inhibitor blocks the translocation of PKC-δ toward the membrane stimulated by AngII in VSMCs and that it also inhibits the autophosphorylation of human recombinant PKC-δ in vitro (26). In contrast, two recent publications reported a failure of PKC-δ inhibition by rottlerin in a kinase assay using a synthetic substrate and one of the publications reported that rottlerin showed additional inhibitory effects besides PKC-δ inhibition (10, 60). To further evaluate the involvement of PKC-δ in JAK2 activation by H2O2, we utilized kinase-inactive PKC mutants and showed that only the PKC-δ mutant inhibited JAK2 activation. This is in line with the recent finding that PKC-δ is required for JAK2 activation induced by AngII (26), a well-established ROS inducer (30), in VSMCs. In addition, several reports indicate that H2O2 stimulates PKC-δ activity in various cell types (40, 49). In this study, we also found that H2O2 could stimulate PKC-δ activity in VSMCs. In addition, PKC-δ was previously shown to be required for HB-EGF production, possibly through the activation of ADAM9 (37). However, our present findings rather eliminate the role of PKC-δ in H2O2-induced EGF receptor activation. This is in good agreement with previous findings by our group that PKC does not mediate EGF receptor transactivation induced by AngII (19).
VSMCs normally express PYK2 that is activated by ROS or AngII through ROS production (16, 25). It has been shown that PYK2 function is indispensable for several AngII-induced signaling pathways and subsequent hypertrophy in VSMCs (24, 54). Specifically, PYK2 is constitutively associated with JAK2 and is required for JAK2 activation by AngII (26). Although PYK2 is implicated in EGF receptor transactivation in fibroblasts (3), this may not be the case for EGF receptor transactivation in VSMCs (66). Here, we found that JAK2 activation but not EGF receptor activation by H2O2 requires PYK2, which appears to be downstream of PKC-δ. Interestingly, it was demonstrated that H2O2 stimulates PKC-δ and c-Abl association, where c-Abl is activated by a PKC-δ-dependent mechanism (61). Thus, the possibility that JAK2 or PYK2 is a substrate with which PKC-δ is capable of associating should be considered. PYK2 and its related tyrosine kinase FAK share a common structure with conserved important motifs (43, 55). Recently, FAK was shown to be involved in AngII-induced growth-promoting responses in cultured VSMCs (28). Although our present findings together with previous findings showing an interaction between PYK2 and JAK2 in VSMCs strongly suggest a critical role for PYK2 in mediating ROS-dependent JAK2 activation, it is possible that dominant negative PYK2 may interfere with the FAK function together with PYK2 function. Therefore, further studies are needed to examine the role of FAK in JAK2 activation.
In the present study, we recognized that most approaches that interfered with H2O2-dependent activation did not result in complete inhibition. Thus, both EGF receptor activation and JAK2 activation by ROS could involve additional pathways independent from the pathways identified in this study. In this regard, Src family kinase-dependent pathways have been proposed to mediate JAK2 activation (1) or EGF receptor activation by ROS (7, 66). Also, ROS are believed to stimulate tyrosine phosphorylation by the inhibition of tyrosine phosphatases via the cysteine residues in the active site regions of these enzymes (21). Knebel et al. (39) in fact demonstrated that H2O2 could inhibit the dephosphorylation of the EGF receptor through the inhibition of tyrosine phosphatases. Thus, future research should be conducted to determine whether a tyrosine phosphatase or Src kinase is involved in one or both mechanisms of tyrosine kinase activation by ROS in VSMCs.
In conclusion, we have shown that ROS utilize distinct signal transduction mechanisms to activate nonreceptor and receptor protein tyrosine kinases in VSMCs. This important finding may lead to the selective inhibition of various distinct ROS functions that may sufficiently prevent or attenuate several cardiovascular-related diseases.
Acknowledgments
We thank Kunie Eguchi and Trinita Fitzgerald for their excellent technical assistance.
This work was supported by National Institute of Health training grant HL07323 and a United Negro College Fund/Merck postdoctoral science research fellowship (G. Frank), by an American Heart Association Scientist Development Grant and a Vanderbilt University Diabetes Center Pilot & Feasibility Proposal (S. Eguchi), and in part by the research grants HL58205, DK20593, and CA68485 from the National Institute of Health (T. Inagami).
REFERENCES
- 1.Abe, J., and B. C. Berk. 1999. Fyn and JAK2 mediate Ras activation by reactive oxygen species. J. Biol. Chem. 274:21003-21010. [DOI] [PubMed] [Google Scholar]
- 2.Abe, J., and B. C. Berk. 1998. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends Cardiovasc. Med. 8:59-64. [DOI] [PubMed] [Google Scholar]
- 3.Andreev, J., M. L. Galisteo, O. Kranenburg, S. K. Logan, E. S. Chiu, M. Okigaki, L. A. Cary, W. H. Moolenaar, and J. Schlessinger. 2001. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J. Biol. Chem. 276:20130-20135. [DOI] [PubMed] [Google Scholar]
- 4.Asakura, M., M. Kitakaze, S. Takashima, Y. Liao, F. Ishikura, T. Yoshinaka, H. Ohmoto, K. Node, K. Yoshino, H. Ishiguro, H. Asanuma, S. Sanada, Y. Matsumura, H. Takeda, S. Beppu, M. Tada, M. Hori, and S. Higashiyama. 2002. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 8:35-40. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C., K. Meise, G. Plowman, R. Coffey, and P. Dempsey. 1998. Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. J. Biol. Chem. 273:17258-17268. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter, G. 1999. Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J. Cell Biol. 146:697-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, K., J. A. Vita, B. C. Berk, and J. F. Keaney. 2001. c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves SRC-dependent epidermal growth factor receptor transactivation. J. Biol. Chem. 276:16045-16050. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, P. 1996. Receptor ligand-facilitated gene transfer: enhancement of liposome-mediated gene transfer and expression by transferrin. Hum. Gene Ther. 7:275-282. [DOI] [PubMed] [Google Scholar]
- 9.Cunnick, J. M., J. F. Dorsey, T. Standley, J. Turkson, A. J. Kraker, D. W. Fry, R. Jove, and J. Wu. 1998. Role of tyrosine kinase activity of epidermal growth factor receptor in the lysophosphatidic acid-stimulated mitogen-activated protein kinase pathway. J. Biol. Chem. 273:14468-14475. [DOI] [PubMed] [Google Scholar]
- 10.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devary, Y., R. A. Gottlieb, T. Smeal, and M. Karin. 1992. The mammalian ultraviolet response is triggered by activation of src tyrosine kinases. Cell 71:1081-1091. [DOI] [PubMed] [Google Scholar]
- 12.Dong, J., L. K. Opresko, P. J. Dempsey, D. A. Lauffenburger, R. J. Coffey, and H. S. Wiley. 1999. Metalloprotease-mediated ligand release regulates autocrine signaling through the epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA 96:6235-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong, J., and H. S. Wiley. 2000. Trafficking and proteolytic release of epidermal growth factor receptor ligands are modulated by their membrane-anchoring domains. J. Biol. Chem. 275:557-564. [DOI] [PubMed] [Google Scholar]
- 14.Eguchi, S., P. J. Dempsey, G. D. Frank, E. D. Motley, and T. Inagami. 2001. Activation of MAP kinases by angiotensin II in vascular smooth muscle cells: metalloprotease-dependent EGF receptor activation is required for ERK and p38 MAP kinase, but not for JNK. J. Biol. Chem. 276:7957-7962. [DOI] [PubMed] [Google Scholar]
- 15.Eguchi, S., H. Iwasaki, Y. Hirata, J. D. Frank, E. D. Motley, T. Yamakawa, K. Numaguchi, and T. Inagami. 1999. Epidermal growth factor receptor is indispensable for c-Fos expression and protein synthesis by angiotensin II. Eur. J. Pharmacol. 376:203-206. [DOI] [PubMed] [Google Scholar]
- 16.Eguchi, S., H. Iwasaki, T. Inagami, K. Numaguchi, T. Yamakawa, E. D. Motley, K. M. Owada, F. Marumo, and Y. Hirata. 1999. Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension 33:201-206. [DOI] [PubMed] [Google Scholar]
- 17.Eguchi, S., H. Iwasaki, E. D. Motley, G. D. Frank, H. Ueno, K. Eguchi, F. Marumo, Y. Hirata, and T. Inagami. 1999. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser411 in vascular smooth muscle cells: possible requirement of EGF receptor, RAS, ERK, and AKT. J. Biol. Chem. 274:36843-36852. [DOI] [PubMed] [Google Scholar]
- 18.Eguchi, S., T. Matsumoto, E. D. Motley, H. Utsunomiya, and T. Inagami. 1996. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. Possible requirement of Gq-mediated p21ras activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J. Biol. Chem. 271:14169-14175. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi, S., K. Numaguchi, H. Iwasaki, T. Matsumoto, T. Yamakawa, H. Utsunomiya, E. D. Motley, H. Kawakatsu, K. M. Owada, Y. Hirata, F. Marumo, and T. Inagami. 1998. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J. Biol. Chem. 273:8890-8896. [DOI] [PubMed] [Google Scholar]
- 20.Fan, H., and R. Derynck. 1999. Ectodomain shedding of TGF-alpha and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 18:6962-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkel, T. 1998. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 10:248-253. [DOI] [PubMed] [Google Scholar]
- 22.Finkel, T., and N. J. Holbrook. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408:239-247. [DOI] [PubMed] [Google Scholar]
- 23.Frank, G. D., S. Eguchi, T. Inagami, and E. D. Motley. 2001. N-acetylcysteine inhibits angiotensin II-mediated activation of extracellular signal-regulated kinase and epidermal growth factor receptor. Biochem. Biophys. Res. Commun. 280:1116-1119. [DOI] [PubMed] [Google Scholar]
- 24.Frank, G. D., S. Eguchi, E. D. Motley, T. Sasaki, and T. Inagami. 2001. Unique regulation of c-Jun N-terminal kinase by PYK2/CAK-beta in angiotensin II-stimulated vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 286:692-696. [DOI] [PubMed] [Google Scholar]
- 25.Frank, G. D., E. D. Motley, T. Inagami, and S. Eguchi. 2000. PYK2/CAKbeta represents a redox-sensitive tyrosine kinase in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 270:761-765. [DOI] [PubMed] [Google Scholar]
- 26.Frank, G. D., S. Saito, E. D. Motley, T. Sasaki, M. Ohba, and T. Inagami. 2002. Requirement of Ca2+ and PKCdelta for Janus kinase 2 activation by angiotensin II: involvement of PYK2. Mol. Endocrinol. 16:367-377. [DOI] [PubMed] [Google Scholar]
- 27.Gechtman, Z., J. L. Alonso, G. Raab, D. E. Ingber, and M. Klagsbrun. 1999. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J. Biol. Chem. 274:28828-28835. [DOI] [PubMed] [Google Scholar]
- 28.Govindarajan, G., D. M. Eble, P. A. Lucchesi, and A. M. Samarel. 2000. Focal adhesion kinase is involved in angiotensin II-mediated protein synthesis in cultured vascular smooth muscle cells. Circ. Res. 87:710-716. [DOI] [PubMed] [Google Scholar]
- 29.Griendling, K. K., D. Sorescu, and M. Ushio-Fukai. 2000. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 86:494-501. [DOI] [PubMed] [Google Scholar]
- 30.Griendling, K. K., F. M. Ushio, B. Lassegue, and R. W. Alexander. 1997. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension 29:366-373. [DOI] [PubMed] [Google Scholar]
- 31.Gschwendt, M., H. J. Muller, K. Kielbassa, R. Zang, W. Kittstein, G. Rincke, and F. Marks. 1994. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 199:93-98. [DOI] [PubMed] [Google Scholar]
- 32.Gschwind, A., E. Zwick, N. Prenzel, M. Leserer, and A. Ullrich. 2001. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20:1594-1600. [DOI] [PubMed] [Google Scholar]
- 33.Hansen, L. L., Y. Ikeda, G. S. Olsen, A. K. Busch, and L. Mosthaf. 1999. Insulin signaling is inhibited by micromolar concentrations of H2O2. J. Biol. Chem. 274:25078-25084. [DOI] [PubMed] [Google Scholar]
- 34.Heeneman, S., J. Haendeler, Y. Saito, M. Ishida, and B. C. Berk. 2000. Angiotensin II induces transactivation of the platelet-derived growth factor beta receptor. J. Biol. Chem. 275:15926-15932. [DOI] [PubMed] [Google Scholar]
- 35.Irani, K. 2000. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res. 87:179-183. [DOI] [PubMed] [Google Scholar]
- 36.Ishino, M., H. Aoto, H. Sasaski, R. Suzuki, and T. Sasaki. 2000. Phosphorylation of Hic-5 at tyrosine 60 by CAKbeta and Fyn. FEBS Lett. 474:179-183. [DOI] [PubMed] [Google Scholar]
- 37.Izumi, Y., M. Hirata, H. Hasuwa, R. Iwamoto, T. Umata, K. Miyado, Y. Tamai, T. Kurisaki, A. Sehara-Fujisawa, S. Ohno, and E. Mekada. 1998. A metalloprotease-disintegrin, MDC9/meltrin-g/ADAM9 and PKCd are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 17:7260-7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalmes, A., B. R. Vesti, G. Daum, J. A. Abraham, and A. W. Clowes. 2000. Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ. Res. 87:92-98. [DOI] [PubMed] [Google Scholar]
- 39.Knebel, A., H. J. Rahmsdorf, A. Ullrich, and P. Herrlich. 1996. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 15:5314-5325. [PMC free article] [PubMed] [Google Scholar]
- 40.Konishi, H., M. Tanaka, Y. Takemura, H. Matsuzaki, Y. Ono, U. Kikkawa, and Y. Nishizuka. 1997. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA 94:11233-11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lander, H. M. 1997. An essential role for free radicals and derived species in signal transduction. FASEB J. 11:118-124. [PubMed] [Google Scholar]
- 42.Lemjabbar, H., and C. Basbaum. 2002. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8:41-46. [DOI] [PubMed] [Google Scholar]
- 43.Lev, S., H. Moreno, R. Martinez, P. Canoll, E. Peles, J. M. Musacchio, G. D. Plowman, B. Rudy, and J. Schlessinger. 1995. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature 376:737-745. [DOI] [PubMed] [Google Scholar]
- 44.Levitzki, A., and A. Gazit. 1995. Tyrosine kinase inhibition: an approach to drug development. Science 267:1782-1788. [DOI] [PubMed] [Google Scholar]
- 45.Luttrell, L. M., Y. Daaka, and R. J. Lefkowitz. 1999. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell Biol. 11:177-183. [DOI] [PubMed] [Google Scholar]
- 46.Luttrell, L. M., R. G. Della, T. van Biesen, D. K. Luttrell, and R. J. Lefkowitz. 1997. Gbetagamma subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J. Biol. Chem. 272:4637-4644. [DOI] [PubMed] [Google Scholar]
- 47.Madamanchi, N. R., S. Li, C. Patterson, and M. S. Runge. 2001. Thrombin regulates vascular smooth muscle cell growth and heat shock proteins via the JAK-STAT pathway. J. Biol. Chem. 276:18915-18924. [DOI] [PubMed] [Google Scholar]
- 48.Ohba, M., K. Ishino, M. Kashiwagi, S. Kawabe, K. Chida, N. Huh, and T. Kuroki. 1998. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the η and δ isoforms of protein kinase C. Mol. Cell. Biol. 18:5199-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohmori, S., Y. Shirai, N. Sakai, M. Fujii, H. Konishi, U. Kikkawa, and N. Saito. 1998. Three distinct mechanisms for translocation and activation of the delta subspecies of protein kinase C. Mol. Cell. Biol. 18:5263-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pai, R., B. Soreghan, I. L. Szabo, M. Pavelka, D. Baatar, and A. S. Tarnawski. 2002. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 8:289-293. [DOI] [PubMed] [Google Scholar]
- 51.Pierce, K. L., A. Tohgo, S. Ahn, M. E. Field, L. M. Luttrell, and R. J. Lefkowitz. 2001. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. J. Biol. Chem. 276:23155-23160. [DOI] [PubMed] [Google Scholar]
- 52.Prenzel, N., E. Zwick, H. Daub, M. Leserer, R. Abraham, C. Wallasch, and A. Ullrich. 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884-888. [DOI] [PubMed] [Google Scholar]
- 53.Rao, G. N. 1996. Hydrogen peroxide induces complex formation of SHC-Grb2-SOS with receptor tyrosine kinase and activates Ras and extracellular signal-regulated protein kinases group of mitogen-activated protein kinases. Oncogene 13:713-719. [PubMed] [Google Scholar]
- 54.Rocic, P., and P. A. Lucchesi. 2001. Down-regulation by antisense oligonucleotides establishes a role for the proline-rich tyrosine kinase PYK2 in angiotensin II-induced signaling in vascular smooth muscle. J. Biol. Chem. 276:21902-21906. [DOI] [PubMed] [Google Scholar]
- 55.Sasaki, H., K. Nagura, M. Ishino, H. Tobioka, K. Kotani, and T. Sasaki. 1995. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J. Biol. Chem. 270:21206-21219. [DOI] [PubMed] [Google Scholar]
- 56.Schieffer, B., M. Luchtefeld, S. Braun, A. Hilfiker, D. Hilfiker-Kleiner, and H. Drexler. 2000. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ. Res. 87:1195-1201. [DOI] [PubMed] [Google Scholar]
- 57.Schindler, C., and J. E. Darnell, Jr. 1995. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 64:621-651. [DOI] [PubMed] [Google Scholar]
- 58.Shichiri, M., M. Yokokura, F. Marumo, and Y. Hirata. 2000. Endothelin-1 inhibits apoptosis of vascular smooth muscle cells induced by nitric oxide and serum deprivation via MAP kinase pathway. Arterioscler. Thromb. Vasc. Biol. 20:989-997. [DOI] [PubMed] [Google Scholar]
- 59.Simon, A. R., U. Rai, B. L. Fanburg, and B. H. Cochran. 1998. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 275:C1640-C1652. [DOI] [PubMed] [Google Scholar]
- 60.Soltoff, S. P. 2001. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J. Biol. Chem. 276:37986-37992. [DOI] [PubMed] [Google Scholar]
- 61.Sun, X., W. Frank, R. Datta, S. Kharbanda, and D. Kufe. 2000. Interaction between protein kinase C delta and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J. Biol. Chem. 275:7470-7473. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki, M., G. Raab, M. A. Moses, C. A. Fernandez, and M. Klagsbrun. 1997. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J. Biol. Chem. 272:31730-31737. [DOI] [PubMed] [Google Scholar]
- 63.Tokumaru, S., S. Higashiyama, T. Endo, T. Nakagawa, J. I. Miyagawa, K. Yamamori, Y. Hanakawa, H. Ohmoto, K. Yoshino, Y. Shirakata, Y. Matsuzawa, K. Hashimoto, and N. Taniguchi. 2000. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J. Cell Biol. 151:209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Touyz, R. M., and E. L. Schiffrin. 1999. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 34:976-982. [DOI] [PubMed] [Google Scholar]
- 65.Umata, T., M. Hirata, T. Takahashi, F. Ryu, S. Shida, Y. Takahashi, M. Tsuneoka, Y. Miura, M. Masuda, Y. Horiguchi, and E. Mekada. 2001. A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J. Biol. Chem. 276:30475-30482. [DOI] [PubMed] [Google Scholar]
- 66.Ushio-Fukai, M., K. K. Griendling, P. L. Becker, L. Hilenski, S. Halleran, and R. W. Alexander. 2001. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 21:489-495. [DOI] [PubMed] [Google Scholar]
- 67.Wang, D., X. Yu, R. A. Cohen, and P. Brecher. 2000. Distinct effects of N-acetylcysteine and nitric oxide on angiotensin II-induced epidermal growth factor receptor phosphorylation and intracellular Ca2+ levels. J. Biol. Chem. 275:12223-12230. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto, T., H. Matsuzaki, H. Konishi, Y. Ono, and U. Kikkawa. 2000. H2O2-induced tyrosine phosphorylation of protein kinase cdelta by a mechanism independent of inhibition of protein-tyrosine phosphatase in CHO and COS-7 cells. Biochem. Biophys. Res. Commun. 273:960-966. [DOI] [PubMed] [Google Scholar]
- 69.Yu, W. H., J. F. Woessner, Jr., J. D. McNeish, and I. Stamenkovic. 2002. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 16:307-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, Z., P. Oliver, J. J. Lancaster, P. O. Schwarzenberger, M. S. Joshi, J. Cork, and J. K. Kolls. 2001. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 15:303-305. [DOI] [PubMed] [Google Scholar]