Abstract
Transcript-selective translational control of eukaryotic gene expression is often directed by a structural element in the 3′ untranslated region (3′-UTR) of the mRNA. In the case of ceruloplasmin (Cp), induced synthesis of the protein by gamma interferon (IFN-γ) in U937 monocytic cells is halted by a delayed translational silencing mechanism requiring the binding of a cytosolic inhibitor to the Cp 3′-UTR. Silencing requires the essential elements of mRNA circularization, i.e., eukaryotic initiation factor 4G, poly(A)-binding protein, and poly(A) tail. We here determined the minimal silencing element in the Cp 3′-UTR by progressive deletions from both termini. A minimal, 29-nucleotide (nt) element was determined by gel shift assay to be sufficient for maximal binding of the IFN-γ-activated inhibitor of translation (GAIT), an as-yet-unidentified protein or complex. The interaction was shown to be functional by an in vitro translation assay in which the GAIT element was used as a decoy to overcome translational silencing. Mutation analysis showed that the GAIT element contained a 5-nt terminal loop, a weak 3-bp helix, an asymmetric internal bulge, and a proximal 6-bp helical stem. Two invariant loop residues essential for binding activity were identified. Ligation of the GAIT element immediately downstream of a luciferase reporter conferred the translational silencing response to the heterologous transcript in vitro and in vivo; a construct containing a nonbinding, mutated GAIT element was ineffective. Translational silencing of Cp, and possibly other transcripts, mediated by the GAIT element may contribute to the resolution of the local inflammatory response following cytokine activation of macrophages.
Ceruloplasmin (Cp) is a multifunctional, copper-containing glycoprotein produced by the liver and secreted into the plasma. As an acute-phase protein, its plasma concentration increases up to twofold during multiple inflammatory conditions. Plasma Cp has been reported to be an independent risk factor for cardiovascular disease, including atherosclerosis, carotid restenosis after endarterectomy, and myocardial infarction (24, 36). Several laboratories have shown that Cp copper can cause oxidative modification of low density lipoprotein, and this activity may contribute to a direct role of Cp in the pathogenesis of atherosclerosis (7, 26, 39). Cp also has a ferroxidase activity thought to be necessary for optimal loading of iron into transferrin (27). The important physiological role for Cp in iron homeostasis has been convincingly demonstrated by finding iron overload in humans with hereditary Cp deficiency (40) and in mice with targeted Cp gene disruption (9).
In addition to its synthesis by hepatic cells, Cp is synthesized and secreted by activated monocyte/macrophages. Treatment of human monocytic U937 cells or peripheral blood monocytes with gamma interferon (IFN-γ) induces the expression of both Cp mRNA and protein (19). Our laboratory has shown that induced expression of Cp by IFN-γ is subject to a unique transcript-selective translational silencing mechanism in which synthesis is terminated after about 16 h, even in the presence of abundant Cp mRNA (18). Our results suggest the presence of a cytosolic inhibitor, since lysates from U937 cells treated with IFN-γ for 24 h (but not for 8 h) inhibited in vitro translation of Cp in a reticulocyte lysate. The inhibition was accompanied by the binding of a trans-acting factor to the 247-nucleotide (nt), Cp 3′ untranslated region (3′-UTR), as shown by an RNA electrophoretic mobility shift assay (EMSA) using radiolabeled Cp 3′-UTR as probe and by an in vitro translation assay in which unlabeled Cp 3′-UTR, added in excess as a decoy, overcame the inhibition by cytosolic extracts (18).
There are multiple examples of translational regulation directed by proteins interacting with the 3′-UTR (see references 32 and 34 for review). The mechanism by which 3′-UTR-binding proteins inhibit translation-initiation at the distant 5′-UTR is incompletely understood, but studies showing that mRNA may form a closed loop by 5′-to-3′ interactions may provide an important clue (10). This “circular” model has been helpful in understanding 3′-UTR-mediated translational silencing of Cp by IFN-γ (21). The mechanism of translational silencing was investigated by in vitro translation of a heterologous reporter transcript consisting of the luciferase (Luc) open reading frame (ORF) ahead of the full-length Cp 3′-UTR and a 30-nt poly(A) tail. Removal or inactivation of any of the components of transcript circularization, i.e., the poly(A) tail, eukaryotic initiation factor 4G, or poly(A)-binding protein (PABP), prevented the translational silencing activity (21). These results have led us to propose a mechanism of translational control in which interactions of the termini carry a 3′-interacting inhibitor protein (or complex) into the vicinity of the translation-initiation site where it can silence translation, possibly by binding to or interfering with an initiation factor or ribosomal protein (20, 21).
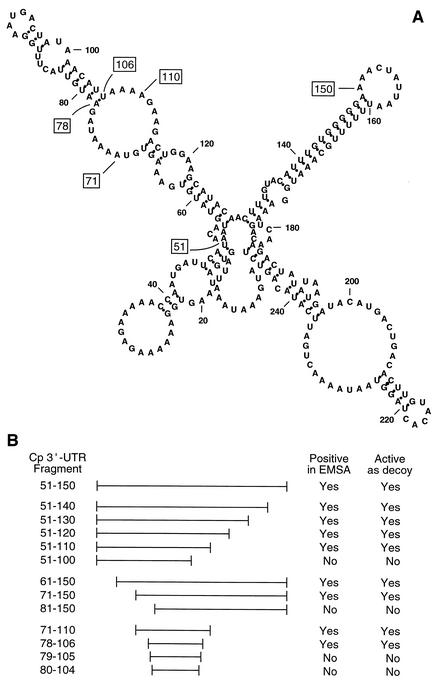
Transcript-selective translational control generally is directed by a specific cis element characterized by a requirement for specific structural features as well as for invariant sequences. In our previous study, a limited deletion analysis of the Cp 3′-UTR showed that the binding site of the translational inhibitor was present in overlapping UTR fragments 51-247 and 1-150 [where position 1 is the first nucleotide after the stop codon and 247 is the last nucleotide before the poly(A) tail in the short form of the Cp 3′-UTR] (18). This result suggested that the required element was contained in the UTR region 51-150 common to both fragments. Computational folding of the full-length Cp 3′-UTR indicates that the element is located within an elongated structure comprising multiple stems and loops (see Fig. 1A). We here describe a detailed analysis of the Cp 3′-UTR and provide evidence for a novel structural element that mediates the inhibition of Cp translation in IFN-γ-activated monocytic cells.
FIG. 1.
Location of the translational silencing element in the human Cp 3′-UTR. (A) Folding structure of the full-length Cp 3′-UTR as determined by the mfold algorithm. The terminal positions of key constructs are indicated by boxes. (B) A summary of the activities of synthetic Cp 3′-UTR cRNA fragments as measured in a competition EMSA and in an in vitro translation assay in which the fragment was added as a decoy.
MATERIALS AND METHODS
Reagents.
Rabbit reticulocyte lysate, methionine-minus amino acid mixture, and RNasin were purchased from Promega (Madison, Wis.). Human IFN-γ was from R & D Systems (Minneapolis, Minn.), and RNase H and Superscript were from Invitrogen (Gaithersburg, Md.). [35S]methionine (translation grade) was purchased from NEN-DuPont (Boston, Mass.) for in vitro translation. All other reagents were from Sigma (St. Louis, Mo.).
Preparation of cytosolic extracts from U937 cells.
Human U937 monocytic cells (CRL 1593.2; American Type Culture Collection, Rockville, Md.) were cultured in RPMI 1640 medium containing 10% fetal bovine serum. For preparation of cytosolic extracts, the cells (107 cells per 10 ml) were incubated for 1 h in medium containing 2% fetal bovine serum and then IFN-γ (500 U/ml) was added and the cells incubated for an additional 8 or 24 h. The cells were collected by centrifugation at low speed and then suspended in 0.5 ml of 50 mM Tris (pH 7.6), 50 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM dithiothreitol. The suspension was subjected to three freeze-thaw cycles, passed several times through a 26-gauge needle, and centrifuged at 100,000 × g for 30 min. The protein concentration of the supernatant was adjusted to 1 mg/ml, and 4 μg was used in the RNA gel shift assay and in the in vitro translation reaction, unless otherwise noted.
Cloning of Cp 3′-UTR(51-150).
Cloning of the full-length, 247-nt human Cp 3′-UTR and its insertion into pcDNA3 (pcDNA3/Cp 3′-UTR) was described previously (18). The 247-bp 3′-UTR insert was excised by digestion with BamHI and XhoI. The fragment was gel purified and used as template for PCR amplification with appropriate primers to give a fragment spanning nt 51 to 150. The product was digested with BamHI and XhoI and subcloned into pcDNA3 downstream of the T7 promoter [pcDNA3/Cp 3′-UTR(51-150)].
In vitro transcription of Cp 3′-UTR.
Radiolabeled, synthetic transcripts of the full-length human Cp 3′-UTR and Cp 3′-UTR(51-150) were prepared by in vitro transcription of XhoI-linearized pcDNA3/Cp 3′-UTR and pcDNA3/Cp 3′-UTR(51-150), respectively, using T7 RNA polymerase in the presence of [α-32P]UTP (MaxiScript kit; Ambion, Austin, Tex.). The transcripts were purified by electrophoresis on a 5% acrylamide gel containing 8 M urea followed by elution. Unlabeled, synthetic transcripts of Cp 3′-UTR and the 241-nt 15-lipoxygenase (15-LOX) 3′-UTR were prepared by in vitro transcription of pcDNA3/Cp 3′-UTR and pBS[SK(10R)], respectively, and were gel purified.
Generation of human Cp 3′-UTR RNA fragments.
For preparation of Cp 3′-UTR deletion products, Cp 3′-UTR(51-150) was used as a template for PCR amplification with appropriate primers. In each PCR the 5′ primer contained a T7 promoter sequence to generate a product containing the T7 promoter sequence upstream of the Cp 3′-UTR sequence. The PCR products were gel purified, sequence verified, and used as templates to generate the corresponding RNA segments by in vitro transcription with T7 RNA polymerase (MegaShortScript; Ambion). Transcripts were purified by electrophoresis on a 5% acrylamide, 8 M urea gel. Synthetic Cp 3′-UTR segments corresponding to nt 61 to 150, 71 to 150, 81 to 150, 51 to 140, 51 to 130, 51 to 120, 51 to 110, 51 to 100, and 71 to 110 were similarly prepared.
RNA transcripts smaller than 30 nt were prepared by oligonucleotide-directed transcription. Oligonucleotides complementary to the desired sequence were synthesized with a complementary T7 promoter sequence at the 3′ end and were annealed to an oligonucleotide containing the T7 promoter sequence in TES buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 0.1 M NaCl). Annealed, partially double-stranded templates were used for in vitro transcription using T7 RNA polymerase (MegaShortScript). Unincorporated nucleotides were removed using Micro Bio-spin P-30 columns (Bio-Rad). Transcripts were purified by phenol-chloroform extraction followed by ethanol precipitation.
RNA EMSA.
[α-32P]UTP-labeled Cp 3′-UTR (20 fmol) was incubated for 30 min at 4°C with U937 cell extract (4 μg of protein) in 20 μl of reaction buffer containing 12 mM HEPES (pH 8.0), 15 mM KCl, 0.25 mM dithiothreitol, 5 mM MgCl2, 0.1 mM PMSF, 200 mg of yeast tRNA/ml, 40 U of RNasin, and 10% glycerol. In competition experiments, unlabeled Cp 3′-UTR segments, at a 10-fold molar excess, were added to the extract 10 min before addition of radiolabeled probe. RNA-protein complexes were resolved by native gel electrophoresis (5% polyacrylamide in 0.5× Tris-buffered EDTA) at 4°C. The gel was dried, and the shifted probe was visualized by autoradiography.
Preparation of Luc-Cp 3′-UTR cRNA constructs.
A chimeric cRNA construct containing the Luc ORF (plus 48 nt of its proximal 3′-UTR and a 7-nt spacer) upstream of the full-length, 247-nt Cp 3′-UTR followed by a poly(A) tail [PSP64-Luc-Cp 3′-UTR-poly(A)] was prepared as described previously (21). Three related constructs with shorter fragments of the Cp 3′-UTR were prepared following excision of the Cp 3′-UTR by digestion with StuI and SacI. A synthetic, 29-nt fragment (nt 78 to 106) was inserted into the same restriction site to generate PSP64-Luc-Cp 3′-UTR(78-106)-poly(A). Likewise, a construct was prepared from the same 29-nt fragment containing a point mutation (U87C) to generate PSP64-Luc-Cp 3′-UTR(78-106;U87C)-poly(A). A third construct not containing any part of the Cp 3′-UTR was prepared by ligation of the two ends after Klenow fill-in without an insert [PSP64-Luc-poly(A)].
The PSP64 cRNA constructs were linearized using PvuII and subjected to in vitro transcription with Sp6 polymerase (MegaScript kit; Ambion). The cRNA transcripts were capped by the addition of the cap analog m7G(5′)ppp(5′)G and GTP in the ratio of 4:1 (Message Machine; Ambion). Capped T7 gene 10 cRNA was made by in vitro transcription of pGEMEX-2 with T7 RNA polymerase (Message Machine). Full-length cRNA transcripts were purified by electrophoresis on a 5% acrylamide gel containing 8 M urea.
In vitro translation of cRNA by reticulocyte lysate.
For in vitro translation, 200 ng of gel-purified transcript was added to 35 μl of rabbit reticulocyte lysate, 20 μM methionine-free amino acid mixture, 40 U of RNasin, 20 μCi of translation-grade [35S]methionine, and 4 μg of cell extract in a total volume of 50 μl for 1 h at 30°C. Oligonucleotides added as decoys were added at a 50-fold molar excess. A 5-μl aliquot of the reaction mixture was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide). The gel was fixed, treated with Amplify (Amersham), and dried, and radiolabeled bands were detected by autoradiography.
Northern blot analysis of Luc RNA.
RNA was extracted from in vitro translation reaction mixtures containing rabbit reticulocyte lysate and cytosolic extracts from U937 cells (but not [35S]methionine) with Trizol reagent. RNA was fractionated on 1% agarose-formaldehyde gel and transferred to nytran membranes. The blot was hybridized with a random primer-labeled Luc cDNA probe.
Preparation of chimeric CMV-Luc-Cp 3′-UTR constructs.
To facilitate transfection experiments, a mammalian expression vector was constructed to contain the cytomegalovirus (CMV) promoter driving Luc upstream of a polylinker site to facilitate insertion of 3′-UTR sequences and a bovine growth hormone polyadenylation signal. The Luc ORF was released from pGEM-Luc (Promega) by digestion with BamHI and XhoI. The resulting BamHI-Luc-StuI-XhoI fragment was gel purified and ligated to pcDNA3 that was digested with BamHI and XhoI. The modified pcDNA3-based vector (CMV-HindIII-KpnI-BamHI-Luc-StuI-XhoI-XbaI-ApaI) was verified by sequence analysis. For insertion of UTR sequences, DNA fragments corresponding to either the wild-type, 29-nt Cp 3′-UTR(78-106) or a UTR variant containing a U87C mutation were made by hybridizing the respective complementary sequence flanked by StuI and ApaI sites. The modified pcDNA3-based vector was digested with StuI and ApaI, gel purified, and ligated with the UTR segments. The resulting clones were verified by sequence analysis.
Transfection of U937 cells with chimeric Luc reporters.
To measure translation of chimeric Luc constructs, U937 cells (106 cells) were transiently transfected with 15 μg of chimeric CMV-firefly Luc-Cp 3′-UTR in 0.20 ml of RPMI 1640 medium containing 10 mM HEPES. To monitor transfection efficiency, cells were cotransfected with 0.75 μg of CMV-renilla Luc plasmid (CMV-ph RL; Promega). The cells were electroporated (Gene Pulser II; Bio-Rad) at 300 V and 950 μF in a 4-mm gap cuvette. Cells were allowed to recover for 24 h in RPMI 1640 medium containing 10% fetal calf serum and then treated with IFN-γ in medium containing 2% fetal calf serum. Treated cells were washed twice with phosphate-buffered saline, and firefly and renilla Luc activities in cell extracts were measured by chemiluminescence using the Dual Luciferase Reporter assay system (Promega). Firefly Luc activity was normalized to renilla Luc activity used as an internal standard.
RESULTS
Localization of the 3′-UTR element required for translational silencing of Cp.
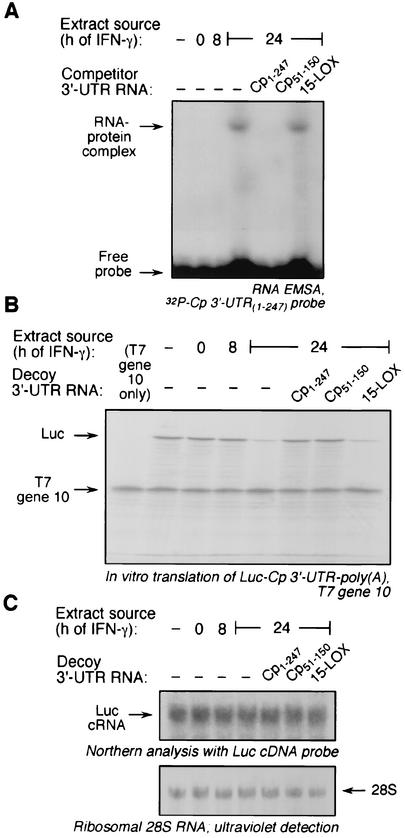
To determine the cis-acting element in the Cp 3′-UTR required for translational silencing by IFN-γ, we used a strategy in which successively finer deletions were made from both ends. As a starting point, we took advantage of our previous report in which 3′-UTR segments 1-150 and 51-247 were found to be as effective as the full-length UTR (18), suggesting that the required element was contained in Cp 3′-UTR(51-150) (Fig. 1A). A synthetic cRNA segment was made and its activity was tested by a competition RNA EMSA using [α-32P]UTP-labeled, full-length Cp 3′-UTR as probe. As shown previously, incubating the probe with a cytosolic extract from U937 cells treated with IFN-γ for 24 h caused the appearance of a major RNA-protein complex plus one or more minor complexes (Fig. 2A). Extracts from untreated cells or cells treated with IFN-γ for 8 h did not cause complex formation. Cp 3′-UTR(51-150) competed for binding as effectively as the full-length Cp 3′-UTR, thus confirming the presence of the cis-acting element (Fig. 2A). The specificity of the RNA-protein interaction was shown by the lack of competition by synthetic 15-LOX 3′-UTR, a cRNA that is nearly the same size as the full-length Cp 3′-UTR and is responsible for translational control of 15-LOX during reticulocyte maturation (28).
FIG. 2.
Protein binding to Cp 3′-UTR(51-150). U937 cells were incubated with IFN-γ (500 U/ml) for 0, 8, or 24 h, and cytosolic extracts were prepared. (A) Protein binding to Cp 3′-UTR(51-150) by competitive EMSA. Cell extracts were incubated with 32P-labeled, full-length Cp 3′-UTR. In competition experiments, the extracts were first incubated with a 10-fold molar excess of unlabeled, full-length Cp 3′-UTR (Cp1-247), Cp 3′-UTR(51-150) (Cp51-150), or the 15-LOX 3′-UTR (15-LOX) before addition of the labeled probe. RNA-protein complexes were resolved by electrophoresis on a nondenaturing, 5% polyacrylamide gel and detected by autoradiography. (B) Binding activity of Cp 3′-UTR(51-150) used as a decoy to overcome translational silencing. Capped, Luc-Cp 3′-UTR-poly(A) cRNA was subjected to in vitro translation in a rabbit reticulocyte lysate containing [35S]methionine and cytosolic extracts from U937 cells treated with IFN-γ (500 U/ml) for 8 or 24 h. A 50-fold molar excess of Cp1-247, Cp51-150, or 15-LOX 3′-UTR was added as decoy. Capped, T7 gene 10 mRNA was addedto each lysate as a loading and specificity control. Newly translated, 35S-labeled Luc and T7 gene 10 were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and detected by fluorography. (C) RNA from the reticulocyte lysates was isolated and subjected to Northern analysis using a radiolabeled, Luc cDNA probe (upper panel). rRNA from reticulocyte lysate was detected by UV and used as a loading control (lower panel, image inverted).
To show that the protein (or complex) that binds Cp 3′-UTR(51-150) is also responsible for translational silencing activity, the segment was tested for its ability to act as a decoy and restore translation of a reporter transcript (18). A capped, chimeric reporter transcript containing Luc upstream of the full-length Cp 3′-UTR and a 30-nt stretch of poly(A) tail [Luc-Cp 3′-UTR-poly(A)] was made. The reporter was subjected to in vitro translation in a rabbit reticulocyte lysate in the presence of extracts from IFN-γ-treated U937 cells. As reported previously (21), an extract from cells treated with IFN-γ for 24 h almost completely blocked translation of Luc-Cp 3′-UTR-poly(A), but extracts from untreated cells or cells treated with IFN-γ for 8 h were inactive (Fig. 2B). Both the full-length Cp 3′-UTR and Cp 3′-UTR(51-150) added as decoys to the reticulocyte lysate completely restored translation, confirming the presence of the cis-acting element responsible for silencing activity. As a control for specificity of the UTR, the 15-LOX 3′-UTR was shown to be ineffective as a decoy. As a control for target specificity, a cRNA encoding T7 gene 10 was added to each reaction mixture. The expression of the T7 gene 10 product was constant under all conditions, indicating that the inhibitory activity was specific for the Cp 3′-UTR-containing chimeric transcript. To test whether Luc cRNA destabilization contributed to decreased Luc protein expression, Luc-Cp 3′-UTR-poly(A) was incubated with cell extracts and a reticulocyte lysate as above, and RNA was extracted and subjected to Northern analysis using full-length Luc cDNA as probe. Equal Luc cRNA levels were observed under all experimental conditions, indicating that RNA destabilization is not significant here (Fig. 2C). We have reported previously a similar stability of Cp mRNA after incubation with a reticulocyte lysate (18). We conclude that the translational silencing element resides in the 3′-UTR region between nucleotide positions 51 and 150 (Fig. 1).
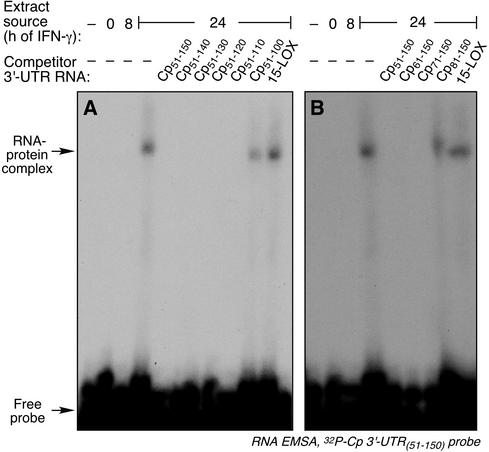
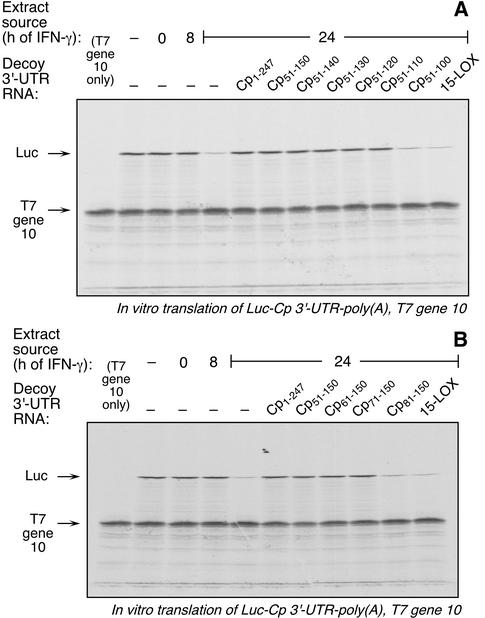
To more precisely localize the cis-acting inhibitor element in the Cp 3′-UTR, serial deletions were made in Cp 3′-UTR(51-150), successively removing 10 nt from either the 5′ or 3′ terminus. The activity of the synthetic cRNA fragments was tested by competition EMSA. Removal of up to 40 nt from the 3′ end did not decrease the binding activity of the fragment; however, removal of 50 nt markedly inactivated the resulting fragment (Fig. 3A). The smallest cRNA that retained activity was Cp 3′-UTR(51-110). Similarly, deletions from the 5′ end identified Cp 3′-UTR(71-150) as the smallest fragment retaining competitor activity (Fig. 3B). An identical pattern of activity was seen when these UTR fragments were used as decoys in the in vitro translation assay; Cp 3′-UTR(51-110) (Fig. 4A) and Cp 3′-UTR(71-150) (Fig. 4B) were the smallest active fragments of the 3′ and 5′ deletions, respectively.
FIG. 3.
RNA EMSA analysis of Cp 3′-UTR(51-150) fragments containing 5′ and 3′ deletions. Cytosolic extracts were prepared from U937 cells incubated with IFN-γ for 0, 8, or 24 h. The extracts were incubated with 32P-labeled, Cp 3′-UTR(51-150) as probe. The competition EMSA was as described in the legend for Fig. 2A. (A) Unlabeled competitors consisted of a 10-fold molar excess of the Cp 3′-UTR fragments Cp51-150, Cp51-140, Cp51-130, Cp51-120, Cp51-110, and Cp51-100, and 15-LOX 3′-UTR. (B) Unlabeled competitors were Cp 3′-UTR fragments Cp51-150, Cp61-150, Cp71-150, and Cp81-150, and also 15-LOX 3′-UTR.
FIG. 4.
Decoy activity of Cp 3′-UTR(51-150) fragments containing 5′ and 3′ deletions. Luc-Cp 3′-UTR-poly(A) and T7 gene 10 cRNA were subjected to in vitro translation in the presence of cytosolic extracts from U937 cells and a 50-fold molar excess of Cp 3′-UTR synthetic cRNAs transcripts as described in the legend for Fig. 2B. (A) The competitor cRNAs were the same as those described in the legend for Fig. 3A. (B) The competitor cRNAs were the same as those shown in Fig. 3B.
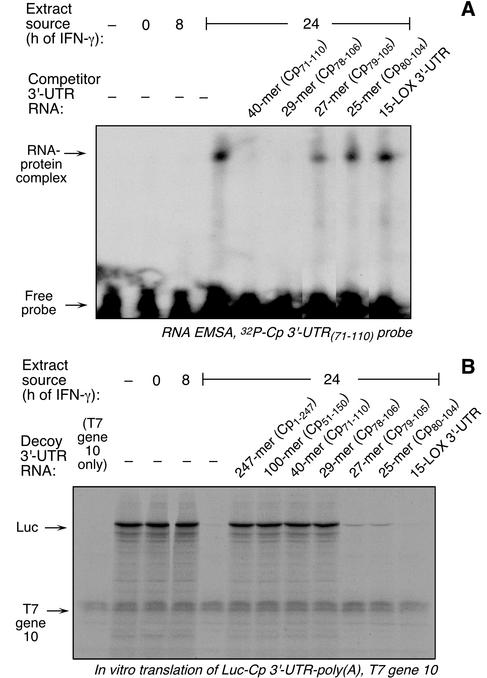
The activity of the UTR fragment containing both the 5′ and 3′ deletions, Cp 3′-UTR(71-110), was tested. The 40-nt segment bound a protein complex (Fig. 5A) and was active as a decoy in the in vitro translation assay (Fig. 5B). To further identify the minimal UTR fragment, deletions were made based on the secondary structure of the Cp 3′-UTR predicted by the mfold RNA folding algorithm (11). This analysis predicted 7-nt and 4-nt unpaired tails at the 5′ and 3′ ends of Cp 3′-UTR(71-110), respectively (Fig. 1A). A 29-nt cRNA fragment lacking these tails was made [Cp 3′-UTR(78-106)], as well as a 27-nt cRNA fragment lacking 1 nt from each end [Cp 3′-UTR(79-105)] and a 25-nt cRNA fragment lacking 2 nt from each end [Cp 3′-UTR(80-104)]. In a competition EMSA, the 29-nt fragment was an effective competitor to the radiolabeled 40-nt probe (Fig. 5A). The 27- and 25-nt fragments exhibited little competitor activity. The fragments gave similar results when used as decoys in the in vitro translation assay (Fig. 5B). We conclude that the 29-nt UTR fragment Cp 3′-UTR(78-106) is the minimal active sequence and thus represents the IFN-γ-activated inhibitor of translation (GAIT) element.
FIG. 5.
Cp 3′-UTR(78-106) is the minimum cRNA required for protein binding. (A) EMSA analysis was done as described in the legend for Fig. 2A, using Cp 3′-UTR(71-110) as radiolabeled probe. The unlabeled competitors were Cp 3′-UTR fragments Cp71-110, Cp78-106, Cp79-105, and Cp80-104, and also 15-LOX 3′-UTR. (B) Decoy assays were done as described in the legend for Fig. 2B. Unlabeled competitors were Cp 3′-UTR fragments Cp1-247, Cp51-150, Cp71-110, Cp78-106, Cp79-105, and Cp80-104, and 15-LOX 3′-UTR.
Analysis of the stem-loop structure of the GAIT element by nucleotide substitution.
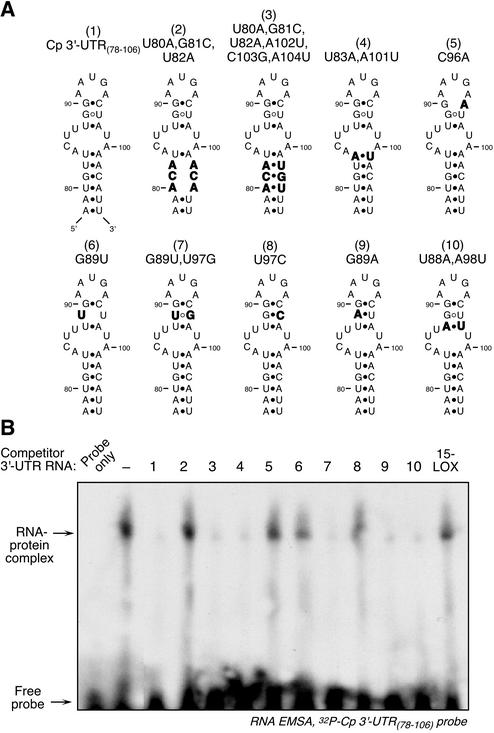
The mfold algorithm predicted that the GAIT element contained (starting from the termini) a 6-bp proximal stem, an asymmetric bulge with 2- and 4-nt loops, a 3-bp distal stem, and a 5-nt terminal loop (Fig. 6A, structure 1). A series of nucleotide substitutions were made to confirm and characterize both stems, and activity was tested by competition RNA EMSA. Disruption of the putative 6-bp stem by substitution of three consecutive nucleotides inactivated this fragment, indicating that the region is critical for activity (Fig. 6A, structure 2). Introduction of compensating substitutions on the other strand of the putative stem completely restored activity (Fig. 6A, structure 3). This sequence covariance provides strong experimental evidence for a stem structure. The structure was further tested by switching the two stem nucleotides closest to the asymmetric bulge (U83A and A101U); the covariant substitution maintained activity and further confirmed the stem structure (Fig. 6A, structure 4). The distal, 3-bp stem was similarly investigated. The most distal base pair was essential for activity, since a C96A substitution inactivated the fragment (Fig. 6A, structure 5). The central G·U wobble pair was also essential and was inactivated by a G89U substitution (Fig. 6A, structure 6). Covariant substitution that reformed a U·G wobble pair restored activity, providing evidence for a stem (Fig. 6A, structure 7). To test whether the wobble base pair was required for activity, the G·U pair was replaced with the Watson-Crick, G·C base pair. Surprisingly, this substitution completely inactivated the fragment despite the likely retention (predicted by mfold) of the stem structure (Fig. 6A, structure 8). When the G·U was replaced by A·U activity was maintained, suggesting that a weak interaction is required (Fig. 6A, structure 9). The most proximal base pair was substituted by a covariant replacement of A·U for U·A. This fragment retained activity, providing additional evidence for the 3-bp stem (Fig. 6A, structure 10). In summary, these data indicate that both the long, proximal and short, distal stems are required for GAIT element activity.
FIG. 6.
Analysis of the GAIT element to determine stem requirement. (A) Schematic diagrams of the nucleotide substitutions made in the GAIT element stems. Substituted nucleotides are indicated in boldface. (B) RNA EMSA using the 29-nt, 32P-labeled Cp 3′-UTR(78-106) fragment as probe. The probe was incubated with cytosolic extract made from cells treated with IFN-γ for 24 h. In the lanes showing competition, a 10-fold molar excess of unlabeled, modified Cp 3′-UTR fragments (and 15-LOX 3′-UTR) were incubated with the extract before the addition of the radiolabeled probe. RNA EMSA was done as described in the legend for Fig. 2A.
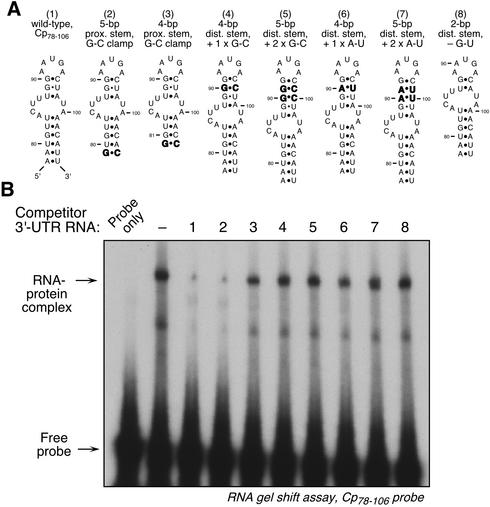
The lengths of the GAIT element stems required for binding activity were examined. We already showed that the removal of the terminal base pair from the 29-nt element generated an inactive 27-nt fragment, suggesting that a 6-bp proximal stem was required for activity (Fig. 5). We tested whether a shorter stem retained activity if it contained a terminal G·C clamp to stabilize the stem. Indeed, a 27-nt fragment terminated with a G·C-clamp was active (Fig. 7A, structure 2). When the terminus was shortened by 2 bp to generate a clamped, 4-bp proximal stem, all activity was lost (Fig. 7A, structure 3). To evaluate the maximal length of the distal stem, cRNAs containing 4- or 5-bp stems were tested. Insertion of one (Fig. 7A, structure 4) or two (Fig. 7A, structure 5) G·C base pairs into the distal stem almost completely inactivated the element. Similar inactivation was seen after insertions of one (Fig. 7A, structure 6) or two (Fig. 7A, structure 7) A·U pairs. To determine the minimal stem length required for activity, the G·U wobble base pair was deleted, leaving a 2-bp stem (which mfold predicts does not form a stem). This fragment was completely inactive in the competitor assay (Fig. 7A, structure 8). This analysis suggests that a proximal stem of at least 6 bp is required (or 5 bp if G·C clamped) and that the distal stem must be precisely 3 bp. The latter result is consistent with a requirement of a weak stem for GAIT-binding activity, as suggested by the substitution mutations described above.
FIG. 7.
Analysis of the GAIT element to determine optimal stem lengths required for activity. (A) Schematic diagrams of the nucleotide substitutions made in the GAIT element stems. Substituted nucleotides are indicated in boldface. (B) Competition RNA EMSA using the 29-nt, 32P-labeled Cp 3′-UTR(78-106) fragment as probe. The probe was incubated with cytosolic extract made from cells treated with IFN-γ for 24 h. In the lanes showing competition, a 10-fold molar excess of unlabeled, modified Cp 3′-UTR fragments were incubated with the extract before the addition of the radiolabeled probe. RNA EMSA was done as described in the legend for Fig. 2A.
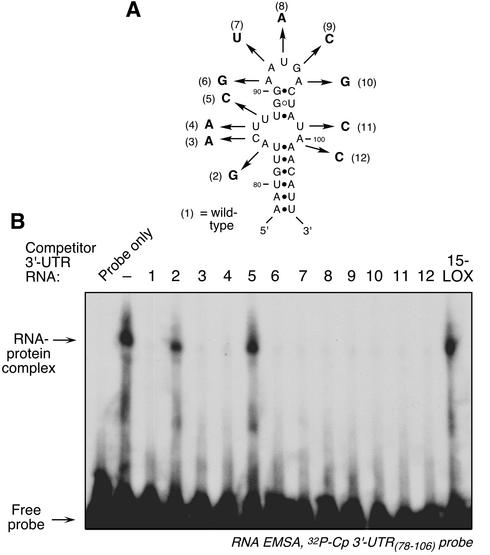
To determine which loop nucleotides were invariant, each of the loop nucleotides in the 29-nt GAIT element was modified. All substitutions were chosen so that the predicted secondary structure remained unchanged, and activity was tested by competition RNA EMSA. Two of the four nucleotides in the larger portion of the asymmetric bulge were invariant; A84G and U87C substitutions completely inactivated the element (Fig. 8, nos. 2 and 5). Substitution of the other nucleotides in this loop did not inhibit the activity of the element (Fig. 8, nos. 3 and 4). Likewise, replacement of both nucleotides in the smaller loop of the asymmetric bulge (Fig. 8, nos. 11 and 12), or any of the 5 nt in the terminal loop (Fig. 8, nos. 6 to 10), did not inhibit activity.
FIG. 8.
Analysis of the GAIT element loops. (A) Schematic diagrams of the nucleotide substitutions made in the GAIT element loops. (B) RNA EMSA using the 29-nt, 32P-labeled Cp 3′-UTR(78-106) fragment as probe. The probe was incubated with cytosolic extract made from cells treated with IFN-γ for 24 h. In the lanes showing competition, a 10-fold molar excess of unlabeled, modified Cp 3′-UTR fragments was incubated with the extract before the addition of the radiolabeled probe. RNA EMSA was done as described in the legend for Fig. 2A.
The 29-nt GAIT element confers the translational silencing response to a heterologous transcript in vitro and in vivo.
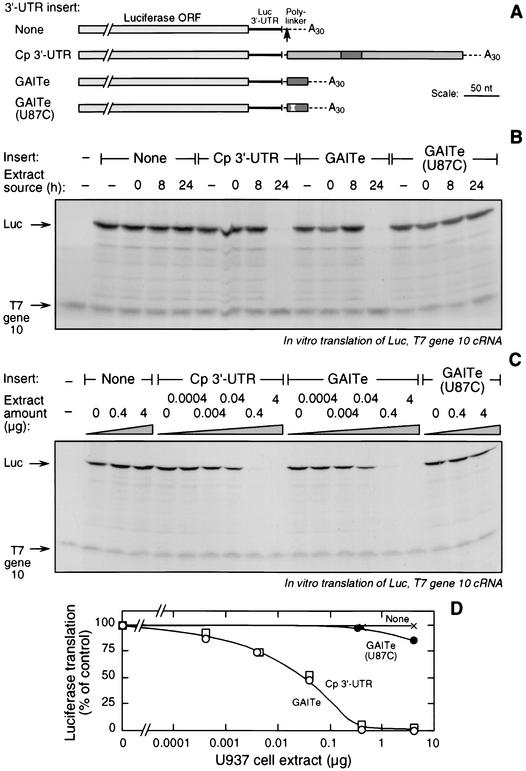
We tested whether the 29-nt GAIT element conferred a translational silencing response to a heterologous reporter. We engineered a capped, chimeric cRNA containing a Luc ORF (plus 55 nt of its 3′-UTR) upstream of the 29-nt GAIT element, followed by a 30-nt stretch of poly(A) [Luc-Cp 3′-UTR(78-106)-poly(A)]. Three other constructs were made in which the GAIT element was replaced by the full-length, 247-nt Cp 3′-UTR [Luc-Cp 3′-UTR-poly(A)], by the 29-nt GAIT element with the U87C mutation [Luc-Cp 3′-UTR(78-106;U87C)-poly(A)], or by a null insert [Luc-poly(A)] (Fig. 9A). The transcripts were subjected to in vitro translation by rabbit reticulocyte lysates in the presence of cytosolic extracts from IFN-γ-treated U937 cells. Basal translation (in the absence of extract) of all four constructs was essentially identical (Fig. 9B). Cytosolic extracts from cells treated with IFN-γ for 24 h completely inhibited translation of the reporter containing the wild-type, 29-nt GAIT element; however, extracts from untreated cells or cells treated with IFN-γ for 8 h were ineffective (Fig. 9B). Specificity was shown by the absence of inhibition of T7 gene 10 cRNA translation. Results for the transcript containing the full-length Cp 3′-UTR were essentially identical. The 24-h extract did not inhibit (or barely inhibited) translation of the reporter transcript containing the GAIT element with the U87C loop substitution (or the null reporter transcript). Thus, the GAIT element confers a strong translational silencing response to a heterologous transcript.
FIG. 9.
The 29-nt GAIT element is sufficient to confer a translational silencing response to a heterologous reporter cRNA. (A) Schematic diagram of chimeric Luc reporter cRNAs. Constructs contained the Luc ORF followed by 55 nt of the Luc 3′-UTR, a polylinker (dashed line), and a 30-nt poly(A) tail. Inserted into the polylinker site (arrow) was the full-length Cp 3′-UTR, the 29-nt GAIT element (GAITe; dark rectangle), or the GAIT element containing the U87C substitution (position indicated by x). A control, null reporter lacked an insertion at this site. (B) The chimeric Luc reporter constructs (and T7 gene 10 cRNA as specificity and loading control) were subjected to in vitro translation in the presence of cytosolic extracts made from U937 cells treated with IFN-γ (500 U/ml) for 0, 8, or 24 h, or with no extract. Translated protein was detected as described in the legend for Fig. 2B. (C) Chimeric Luc reporter constructs and T7 gene 10 cRNA were subjected to in vitro translation in the presence of cytosolic extracts (0 to 4 μg of protein) from U937 cells treated with IFN-γ for 24 h. (D) Translation of chimeric Luc constructs containing no insert (×), full-length Cp 3′-UTR (□), GAITe (○), and GAITe(U87C) (•) Cp were quantitated by densitometry and expressed as the percentage of translation of the construct in the absence of extract.
We considered the possibility that other elements in the Cp 3′-UTR, in addition to the GAIT element, contribute to the effectiveness of the inhibition. Such elements may become apparent only at submaximal levels of translation inhibitor. We titrated the inhibitory cytosolic extract from U937 cells treated with IFN-γ for 24 h. The susceptibilities of the 29-nt GAIT element and the full-length Cp 3′-UTR to translational silencing were essentially identical; half-maximal inhibition of both transcripts was observed at about 0.01 μg of extract protein (Fig. 9C and D). The transcript containing the GAIT element with the U87C loop substitution was inhibited by about 15% at the highest extract concentration; comparison to the wild-type GAIT element suggests that the mutated element is 3 to 4 orders of magnitude less sensitive to inhibition than the wild-type element. These results indicate that the GAIT element is sufficient by itself to convey maximal translational silencing by IFN-γ and that other regions of the Cp 3′-UTR are not involved.
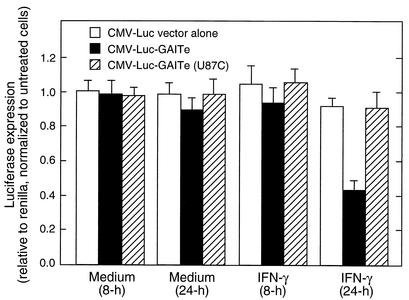
We tested the translational silencing activity of the GAIT element in vivo in transfected U937 cells. The 3-h half-life of Luc in mammalian cells permits its effective use as a reporter of protein synthesis rate in transfected cells (38). Chimeras were constructed to contain the CMV promoter driving Luc upstream of the 29-nt GAIT element or the U87C mutant (or a null insert). U937 cells were cotransfected by electroporation with chimeric CMV-Luc and with CMV-renilla to correct for transfection efficiency. After cell recovery, the U937 cells were treated with IFN-γ for 8 or 24 h. Incubation of cells with IFN-γ for 24 h (but not 8 h) inhibited expression of Luc containing the wild-type GAIT element by more than 50% compared to untreated control cells (Fig. 10). Expression of Luc containing the U87C mutant element, or no insert, was not inhibited by IFN-γ. The stability of firefly Luc mRNA in transfected U937 cells was tested by real-time PCR using primers for firefly Luc and glyceraldehyde 3-phosphate dehydrogenase (as a control transcript). Luc mRNA levels were similar in all treatments (data not shown). These findings recapitulate the in vitro results and are consistent with our earlier report of delayed translational silencing of Cp by IFN-γ in cultured U937 cells (18). The results clearly demonstrate that the 29-nt GAIT structural element is sufficient for translational silencing both in vitro and in vivo.
FIG. 10.
The 29-nt GAIT element confers translational silencing in U937 cells. U937 cells were transfected by electroporation with CMV renilla and constructs containing the CMV promoter driving firefly Luc upstream of the wild-type GAIT element (GAITe), or GAITe with a U87C mutation, or vector lacking a 3′-UTR insertion. All constructs contained a bovine growth hormone polyadenylation signal. After allowing cells to recover, they were treated with IFN-γ (500 U/ml), or with medium alone, for 8 or 24 h. Firefly and renilla Luc activity in extracts was measured by chemiluminescence. Firefly Luc expression was first normalized to renilla Luc expression and then expressed as a percentage compared to untreated control cells. The data shown represent the means and standard errors from five independent experiments.
DISCUSSION
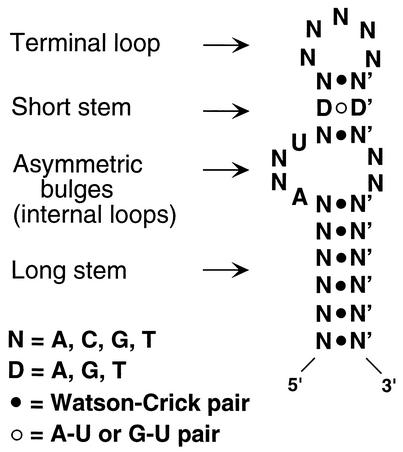
Fewer than two dozen functional elements in 5′- or 3′-UTRs have been described in all species (30). We have described a novel cis-acting element in the 3′-UTR involved in selective translational silencing of the Cp transcript. The 29-nt GAIT element is the minimal element required for formation of an RNA-protein complex in IFN-γ-treated U937 cells and for overcoming translational silencing when added as a decoy in an in vitro translation assay. Our results also show that the element by itself [with a poly(A) tail] is sufficient to convey a translational silencing response to a heterologous transcript, both in vitro and in vivo in U937 cells. Mutation analysis shows that the GAIT element exhibits specific structural as well as sequence requirements. Structurally, the element forms a 5-nt terminal loop separated from an asymmetric bulge by a short 3-nt stem. The entire structure is held together by a proximal 6-nt double-helical stem (or a 5-nt stem when G·C clamped). Although the secondary structures of only a few UTR elements have been reported, the bipartite stem-loop structure of the GAIT element resembles other elements involved in translational control. For example, the iron-responsive element (IRE) in the 5′-UTR of ferritin and 3′-UTR of transferrin also contains a distal loop and an asymmetric bulge separated by a double-helical stem (15). Likewise, the selenocysteine insertion sequence (SECIS) elements required for decoding UGA as selenocysteine also contain proximal and distal loops separated by a stem (8). Like the GAIT element, both IRE and SECIS elements by themselves convey translational control when ligated to heterologous transcripts. As a contrast, the histone 3′-UTR element involved in processing and translation of histone mRNAs is a single stem-loop structure which requires additional 3′-UTR elements for activity (3, 6).
An unusual structural feature of the element is the very short, 3-bp double-helical distal stem. A functional requirement for the stem was shown by inactivation after disruption of any one of the base pairs and by reactivation after substitutions that restored base pairing. Addition or removal of even a single base pair in the stem inactivated the GAIT element. A stem of this small size has not been described in other RNA control elements; helices generally range from 5 bp in the IRE to 13 bp in the SECIS element. The small size, and the presence of a single G·C base pair, suggest that the helix is thermodynamically unstable and may be susceptible to an “induced-fit” conformational change during interaction with regulatory proteins. The importance of a weak helical stem is further underscored by the total inactivation of the element upon substitution of the central G·U base pair with the strong Watson-Crick G·C pair. In addition to the structural features of the GAIT element, there is a sequence requirement for the interaction with GAIT. A mutagenesis scan in which every position in the three loop segments was altered showed that only two mutations, A84G and U87C, reduced binding activity. Both mutations are in the larger of the internal asymmetric bulges, and both are located in “shoulder” positions adjacent to double-helical stems. Single substitutions in the distal loop did not alter activity, suggesting that this region is not important in protein recognition. This distinguishes the GAIT element from the IRE, the histone 3′-UTR element, and the SECIS element, which all have invariant nucleotides in the distal loop. Our data indicate that the critical region of the element, possibly the site of binding of the GAIT protein or complex, is the region comprising the larger of the internal loops and the short stem.
Insertion of the 29-nt GAIT element [with a poly(A) tail] behind the Luc ORF is sufficient to confer translational silencing to the heterologous transcript (Fig. 9). This experiment also provides information on the positional specificity required for GAIT element activity. In the Luc construct containing the entire Cp 3′-UTR, the 5′ position of the element is 138 nt downstream of the termination codon, whereas in the construct containing the GAIT element by itself the element is 61 nt from the termination codon (and in Cp mRNA the element is 78 nt from the termination codon). Likewise, in the construct containing the entire Cp 3′-UTR the 3′ position of the element is 149 nt from the poly(A) tail, whereas in the construct containing the GAIT element by itself the element is only 26 nt from the poly(A) tail. The similar sensitivities of the constructs to translational silencing indicate the absence of a rigid positional requirement for the GAIT element with respect to either the termination codon or the poly(A) tail. The latter result is of interest given our previous report that a poly(A) tail is necessary for translational silencing of both endogenous Cp mRNA and also the heterologous Luc transcript (21). A second conclusion from these experiments is that only the GAIT element is required for optimal silencing, since removal of the remainder of the Cp 3′-UTR does not reduce susceptibility to translational inhibition.
A phylogenetic analysis of the species that can translationally silence Cp has not been addressed experimentally. Rat and mouse Cp 3′-UTR sequences have been reported, but GAIT elements are not detected by sequence, folding, or pattern analysis (see below). Using reverse transcription-PCR, we cloned and sequenced the Cp 3′-UTR from hamster liver, which also lacked the GAIT element. The only other species of Cp mRNA that has been described is from sheep (17). Sheep Cp 3′-UTR contained a sequence homologous to the GAIT element, and folding analysis indicated a similar secondary structure, but without the small 2-nt loop. This sequence was tested by competition EMSA and found to bind human GAIT, but with lower affinity than the human GAIT element (results not shown). We also cloned and sequenced the Cp 3′-UTR from baboon liver and found an element nearly identical to the human sequence. The only difference was a single nucleotide in the central position of the short stem where the G·U wobble base was replaced with an A·U base pair, an alteration that retains GAIT-binding activity (Fig. 6, no. 9). From the limited sequence information available, we conclude that the GAIT element is most likely not present in rodent Cp mRNA, but it is present in several other mammalian species, e.g., sheep, baboon, and human. The evolution of the GAIT element is not clear from these limited data; for example, it is not clear whether it evolved early and was lost in rodents, or is a later evolutionary adaptation.
The function of translational silencing of Cp is not known. One obstacle to our understanding is the multifunctional nature of Cp itself. It has important roles in iron metabolism and inflammation, and it may participate in copper transport, nitric oxide metabolism, and defense against oxidant stress (2, 33). We understand even less about the function of macrophage production of Cp. Its induction by IFN-γ (and by endotoxin [12]) suggests a role in host defense, a possibility consistent with previous observations (16, 35). Our own finding that Cp has a potent prooxidant activity is also consistent with a microbicidal function (25, 26). Identification of macrophage proteins that are coregulated with Cp may provide a clue to the function of GAIT-mediated translational silencing. Such proteins have not yet been observed, but candidates may be identified by the presence of GAIT elements in their 3′-UTR. The PatSearch pattern-matching algorithm (29) was used to search UTRdb (30), a database containing more than 36,000 human 3′-UTR entries. For input we gave a pattern consisting of the stems and loops in Fig. 11 (and an alternate structure with a G·C-clamped, 5-bp proximal stem). Almost 30 3′-UTRs contained this pattern, including death-associated protein kinase, a serine/threonine kinase induced by IFN-γ in U937 cells (5, 13), and Mox1, a recently identified NADPH oxidase related to gp91phox (37). Mox1 is particularly interesting since its superoxide product, like Cp, can cause oxidative damage. One speculative possibility is that translational silencing mediated by the GAIT element contributes to the resolution of inflammation, in particular the local inflammation due to cytokine activation of infiltrating or resident macrophages. The mechanisms of inflammation-termination are just now being clarified, and regulatory events involving translational control may be important (14, 22). For example, macrophage expression of tumor necrosis factor alpha is translationally silenced by interaction of TIA-1 to the AU-rich region in the transcript 3′-UTR (31). Relevant to our studies is the finding that IFN-γ mRNA autoregulates its translation by formation of an RNA pseudoknot (1). Those authors speculated that long-term activation by IFN-γ may have adverse consequences on the cellular environment and requires rapid down-regulation. Likewise, we suggest that Cp and other products of macrophage activation by IFN-γ may have injurious consequences when accumulated in excess and also require rapid down-regulation. Defects in this process may prolong or increase the inflammatory state, an idea consistent with recent evidence that disease-causing defects in noncoding regions of mRNA may not be uncommon and that the 3′-UTR in particular may be a pathological hot spot (4, 23).
FIG. 11.
Structure and sequence features of the GAIT element.
Acknowledgments
This work was supported by Public Health Service grants HL29582 and HL67725 from the National Heart, Lung and Blood Institute, National Institutes of Health (to P.L.F.), by an American Heart Association Predoctoral Fellowship, Ohio Valley Affiliate and a Cleveland State University doctoral dissertation research expense award (to P.S.), and by a Scientist Development Grant from the American Heart Association, National Affiliate (to B.M.).
We gratefully acknowledge Donna Driscoll of the Lerner Research Institute, Cleveland Clinic Foundation, for helpful discussions and Matthias Hentze of the European Molecular Biology Laboratory for the 15-LOX 3′-UTR construct.
REFERENCES
- 1.Ben-Asouli, Y., Y. Banai, Y. Pel-Or, A. Shir, and R. Kaempfer. 2002. Human interferon-γ mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell 108:221-232. [DOI] [PubMed] [Google Scholar]
- 2.Bielli, P., and L. Calabrese. 2002. Structure to function relationships in ceruloplasmin: a “moonlighting” protein. Cell. Mol. Life Sci. 59:1413-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho, D. C., E. C. Scharl, and J. A. Steitz. 1995. Decreasing the distance between the two conserved sequence elements of histone premessenger RNA interferes with 3′ processing in vitro. RNA 1:905-914. [PMC free article] [PubMed] [Google Scholar]
- 4.Conne, B., A. Stutz, and J. D. Vassalli. 2000. The 3′ untranslated region of messenger RNA: a molecular “hotspot” for pathology? Nat. Med. 6:637-641. [DOI] [PubMed] [Google Scholar]
- 5.Deiss, L. P., E. Feinstein, H. Berissi, O. Cohen, and A. Kimchi. 1995. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 9:15-30. [DOI] [PubMed] [Google Scholar]
- 6.Dominski, Z., and W. F. Marzluff. 1999. Formation of the 3′ end of histone mRNA. Gene 239:1-14. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenwald, E., G. M. Chisolm, and P. L. Fox. 1994. Intact human ceruloplasmin oxidatively modifies low density lipoprotein. J. Clin. Investig. 93:1493-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher, J. E., P. R. Copeland, D. M. Driscoll, and A. Krol. 2001. The selenocysteine incorporation machinery: interactions between the SECIS RNA and the SECIS-binding protein SBP2. RNA 7:1442-1453. [PMC free article] [PubMed] [Google Scholar]
- 9.Harris, Z. L., A. P. Durley, T. K. Man, and J. D. Gitlin. 1999. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Natl. Acad. Sci. USA 96:10812-10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imataka, H., A. Gradi, and N. Sonenberg. 1998. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 17:7480-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeger, J. A., D. H. Turner, and M. Zuker. 1989. Improved predictions of secondary structures for RNA. Proc. Natl. Acad. Sci. USA 86:7706-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalmovarin, N., W. E. Friedrichs, H. V. O'Brien, L. A. Linehan, B. H. Bowman, and F. Yang. 1991. Extrahepatic expression of plasma protein genes during inflammation. Inflammation 15:369-379. [DOI] [PubMed] [Google Scholar]
- 13.Katzenellenbogen, R. A., S. B. Baylin, and J. G. Herman. 1999. Hypermethylation of the DAP-kinase CpG island is a common alteration in B-cell malignancies. Blood 93:4347-4353. [PubMed] [Google Scholar]
- 14.Kirkpatrick, P. 2002. Putting the brake on inflammation. Nat. Rev. 1:99. [Google Scholar]
- 15.Klausner, R. D., T. A. Rouault, and J. B. Harford. 1993. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 72:19-28. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff, S. J. 1992. Bactericidal effect of Fe2+, ceruloplasmin, and phosphate. Arch. Biochem. Biophys. 295:302-308. [DOI] [PubMed] [Google Scholar]
- 17.Lockhart, P. J., and J. F. Mercer. 1999. Cloning and expression analysis of the sheep ceruloplasmin cDNA. Gene 236:251-257. [DOI] [PubMed] [Google Scholar]
- 18.Mazumder, B., and P. L. Fox. 1999. Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: role of the 3′ untranslated region. Mol. Cell. Biol. 19:6898-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazumder, B., C. K. Mukhopadhyay, A. Prok, M. K. Cathcart, and P. L. Fox. 1997. Induction of ceruloplasmin synthesis by IFN-γ in human monocytic cells. J. Immunol. 159:1938-1944. [PubMed] [Google Scholar]
- 20.Mazumder, B., V. Seshadri, and P. L. Fox. Translational control by the 3′-UTR: the ends specify the means. Trends Biochem. Sci., in press. [DOI] [PubMed]
- 21.Mazumder, B., V. Seshadri, H. Imataka, N. Sonenberg, and P. L. Fox. 2001. Translational silencing of ceruloplasmin requires the essential elements of mRNA circularization: poly(A) tail, poly(A)-binding protein, and eukaryotic translation initiation factor 4G. Mol. Cell. Biol. 21:6440-6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald, P. P., V. A. Fadok, D. Bratton, and P. M. Henson. 1999. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J. Immunol. 163:6164-6172. [PubMed] [Google Scholar]
- 23.Mendell, J. T., and H. C. Dietz. 2001. When the message goes awry: disease-producing mutations that influence mRNA content and performance. Cell 107:411-414. [DOI] [PubMed] [Google Scholar]
- 24.Mezzetti, A., M. D. Guglielmi, S. D. Pierdomenico, F. Costantini, F. Cipollone, D. De Cesare, T. Bucciarelli, S. Ucchino, F. Chiarelli, F. Cuccurullo, and F. Romano. 1999. Increased systemic oxidative stress after elective endarterectomy: relation to vascular healing and remodeling. Arterioscler. Thromb. Vasc. Biol. 19:2659-2665. [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhyay, C. K., and P. L. Fox. 1998. Ceruloplasmin copper induces oxidant damage by a redox process utilizing cell-derived superoxide as reductant. Biochemistry 37:14222-14229. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyay, C. K., B. Mazumder, P. F. Lindley, and P. L. Fox. 1997. Identification of the prooxidant site of human ceruloplasmin: a model for oxidative damage by copper bound to protein surfaces. Proc. Natl. Acad. Sci. USA 94:11546-11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osaki, S., D. A. Johnson, and E. Frieden. 1966. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J. Biol. Chem. 241:2746-2751. [PubMed] [Google Scholar]
- 28.Ostareck, D. H., A. Ostareck-Lederer, M. Wilm, B. J. Thiele, M. Mann, and M. W. Hentze. 1997. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3′ end. Cell 89:597-606. [DOI] [PubMed] [Google Scholar]
- 29.Pesole, G., S. Liuni, and M. D'Souza. 2000. PatSearch: a pattern matcher software that finds functional elements in nucleotide and protein sequences and assesses their statistical significance. Bioinformatics 16:439-450. [DOI] [PubMed] [Google Scholar]
- 30.Pesole, G., S. Liuni, G. Grillo, F. Licciulli, F. Mignone, C. Gissi, and C. Saccone. 2002. UTRdb and UTRsite: specialized databases of sequences and functional elements of 5′ and 3′ untranslated regions of eukaryotic mRNAs. Update 2002. Nucleic Acids Res. 30:335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piecyk, M., S. Wax, A. R. Beck, N. Kedersha, M. Gupta, B. Maritim, S. Chen, C. Gueydan, V. Kruys, M. Streuli, and P. Anderson. 2000. TIA-1 is a translational silencer that selectively regulates the expression of TNF-α. EMBO J. 19:4154-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preiss, T., and M. W. Hentze. 1999. From factor to mechanisms: translation and translational control in eukaryotes. Curr. Opin. Genet. Dev. 9:515-521. [DOI] [PubMed] [Google Scholar]
- 33.Rydén, L. 1984. Ceruloplasmin, p. 37-100. In R. Lontie (ed.), Copper proteins and copper enzymes, vol. III. CRC Press, Boca Raton, Fla.
- 34.Sachs, A. B., and G. Varani. 2000. Eukaryotic translation initiation: there are (at least) two sides to every story. Nat. Struct. Biol. 7:356-361. [DOI] [PubMed] [Google Scholar]
- 35.Saenko, E. L., O. V. Skorobogat'ko, P. Tarasenko, V. Romashko, L. Zhuravetz, L. Zadorozhnaya, O. F. Senjuk, and A. I. Yaropolov. 1994. Modulatory effects of ceruloplasmin on lymphocytes, neutrophils and monocytes of patients with altered immune status. Immunol. Investig. 23:99-114. [DOI] [PubMed] [Google Scholar]
- 36.Salonen, J. T., R. Salonen, K. Seppänen, M. Kantola, S. Suntioinen, and H. Korpela. 1991. Interactions of serum copper, selenium, and low density lipoprotein cholesterol in atherogenesis. Br. Med. J. 302:756-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suh, Y. A., R. S. Arnold, B. Lassegue, J. Shi, X. Xu, D. Sorescu, A. B. Chung, K. K. Griendling, and J. D. Lambeth. 1999. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401:79-82. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, J. F., L. S. Hayes, and D. B. Lloyd. 1991. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene 103:171-177. [DOI] [PubMed] [Google Scholar]
- 39.Van Lenten, B. J., S. Y. Hama, F. C. De Beer, D. M. Stafforini, T. M. McIntyre, S. M. Prescott, B. N. La Du, A. M. Fogelman, and M. Navab. 1995. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Investig. 96:2758-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida, K., K. Furihata, S. Takeda, A. Nakamura, K. Yamamoto, H. Morita, S. Hiyamuta, S. Ikeda, N. Shimizu, and N. Yanagisawa. 1995. A mutation in the ceruloplasmin gene is associated with systemic hemosiderosis in humans. Nat. Genet. 9:267-272. [DOI] [PubMed] [Google Scholar]