Abstract
Xenopus Aurora-A (also known as Eg2) is a member of the Aurora family of mitotic serine/threonine kinases. In Xenopus oocytes, Aurora-A phosphorylates and activates a cytoplasmic mRNA polyadenylation factor (CPEB) and therefore plays a pivotal role in MOS translation. However, hyperphosphorylation and activation of Aurora-A appear to be dependent on maturation-promoting factor (MPF) activation. To resolve this apparent paradox, we generated a constitutively activated Aurora-A by engineering a myristylation signal at its N terminus. Injection of Myr-Aurora-A mRNA induced germinal vesicle breakdown (GVBD) with the concomitant activation of MOS, mitogen-activated protein kinase, and MPF. Myr-Aurora-A-injected oocytes, however, appeared to arrest in meiosis I with high MPF activity and highly condensed, metaphase-like chromosomes but no organized microtubule spindles. No degradation of CPEB or cyclin B2 was observed following GVBD in Myr-Aurora-A-injected oocytes. In the presence of progesterone, the endogenous Aurora-A became hyperphosphorylated and activated at the time of MPF activation. Following GVBD, Aurora-A was gradually dephosphorylated and inactivated before it was hyperphosphorylated and activated again. This biphasic pattern of Aurora-A activation mirrored that of MPF activation and hence may explain meiosis I arrest by the constitutively activated Myr-Aurora-A.
The serine/threonine protein kinase Ipl1p was first identified in the budding yeast Saccharomyces cerevisiae in a screen for genes involved in chromosome separation during mitotic anaphase (12). A related kinase in Drosophila melanogaster, aurora, was identified in a similar fashion (19). In the last few years, a growing number of protein kinases highly related to Ipl1p and aurora have been identified in various organisms, including Caenorhabditis elegans, Xenopus laevis, and many mammalian species (6, 35). Although all members of this kinase family have a highly related C-terminal core catalytic kinase domain, their N-terminal noncatalytic domains vary considerably in both length and sequence (18). In all cases, the kinases are found associated with the mitotic apparatus, namely the centrosome, at the poles of the bipolar spindle or in the midbody. Consistent with this subcellular localization, functional studies have suggested that these protein kinases are involved in formation of the mitotic spindles, in proper chromosome separation, and/or in cytokinesis (12, 19, 45, 46).
Andresson et al. (1) isolated Eg2, hereafter referred to as Aurora-A according to Nigg (35), in a functional screen for proteins involved in progesterone signaling during Xenopus oocyte maturation. Aurora-A was phosphorylated and activated within 30 min after progesterone stimulation (1). Subsequent studies (30, 31) indicate that Aurora-A is involved in phosphorylating and activating a cytoplasmic polyadenylation factor, CPEB. CPEB plays a pivotal role in controlling cytoplasmic polyadenylation of various mRNA (e.g., that of MOS) whose subsequent translation is required for the resumption of meiosis. Therefore, these studies (1, 30, 31) would suggest a role for Aurora-A prior to the first meiotic metaphase during Xenopus oocyte maturation, contrasting with the roles of most Aurora kinases in regulating mitotic chromosomal events downstream of cdk1 (the mammalian homolog of the kinase component of maturation-promoting factor [MPF], Cdc2) (6, 35).
The timing and therefore the functional role of Aurora-A hyperphosphorylation and activation in Xenopus oocytes, however, have been disputed. Frank-Vaillant et al. (13) showed that progesterone-induced Aurora-A hyperphosphorylation and activation occur at about the same time as MPF activation. Moreover, Aurora-A hyperphosphorylation and activation are abolished if MPF activity is inhibited by injection of p21Cip1. Therefore, these authors argue that Aurora-A hyperphosphorylation and activation are events downstream of MPF, which suggests a similar role in meiotic chromosomal events (13).
To further investigate the functional role of Aurora-A in initiating resumption of meiosis upstream of MPF, we sought to create a constitutively active form of Aurora-A by engineering an N-terminal myristylation signal, based on a serendipitous finding that a similar construct was able to activate the Ras pathway in S. cerevisiae (C. Ma, D. Young, and X. J. Liu, unpublished data). We found that Myr-Aurora-A was capable of initiating hormone-independent resumption of meiosis, as indicated by activation of MOS, mitogen-activated protein (MAP) kinase, and MPF, and by germinal vesicle breakdown (GVBD). However, Myr-Aurora-A-injected oocytes did not proceed to metaphase II and, instead, appeared to be arrested at meiosis I, with highly condensed chromosomes but without any organized microtubule spindles. In agreement with the notion that a constitutively activated Aurora-A kinase was not compatible with the meiosis I-meiosis II transition, we demonstrated that endogenous Aurora-A exhibited a biphasic pattern of hyperphosphorylation and activation, coinciding with that of MPF activation during progesterone-induced oocyte maturation.
MATERIALS AND METHODS
Oocyte isolation and manipulations.
Adult female Xenopus laevis frogs were purchased from NASCO. Frogs were primed with pregnant mare serum gonadotropin (Sigma; 50 IU/frog) 3 to 10 days before oocyte retrieval. Oocytes were manually isolated from surgically removed ovarian tissues. Our standard oocyte medium was OR2 (83 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 5 mM HEPES, pH 7.8), and oocytes were incubated in a room maintained at 18 to 20°C. Oocyte injections were carried out in OR2 without CaCl2. Unless otherwise indicated, oocytes were injected with mRNA (10 nl, 2 mg/ml), delivered to the cytoplasm. We typically employed progesterone at 1 μM to induce GVBD (unless otherwise indicated).
For subcellular fractionation, 50 oocytes were homogenized (forced through a pipette tip) in 500 μl of ice-cold homogenization buffer (10 mM NaCl, 1 mM MgCl2, 10 mM HEPES [pH 7.9], 0.5 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml). The homogenate was clarified by two rounds of low-speed centrifugation (900 × g for 5 min). The clarified supernatant was then subjected to centrifugation at 10,000 × g for 10 min. The membrane pellet was enriched with oocyte plasma membrane as indicated by its marker, β-integrin (16). The supernatant was further centrifuged at 100,000 × g for 60 min. The pellet of the 100,000 × g spin was enriched for internal membrane (33) but also contained polymerized microtubules (8), whereas the supernatant represented the cytoplasm (33).
cDNA cloning and manipulations.
A C-terminal fragment of Aurora-A encoding amino acids 103 to 407 (1) was isolated in a Cyto-Trap yeast two-hybrid screen of an oocyte cDNA library made in the vector pMyr (3). The cDNA library construction and its screening with Xenopus progesterone receptor as the bait will be described in more detail elsewhere (Ma et al., unpublished). The Aurora-A insert was excised by digestion with EcoRI and XhoI. The excised fragment was treated with Klenow fragment and then ligated into pCS2+MT (51) previously digested with XbaI and treated with Klenow fragment. This plasmid, termed pCS2+MT-Aurora-A-C, encoded a functional kinase with an N-terminal Myc tag (Ma et al., unpublished).
To obtain an expression plasmid containing full-length Aurora-A, we amplified the missing N-terminal sequence from the same oocyte cDNA library by PCR. The sequence of the 5′ primer, derived from the published sequence (1), was 5′-TATCCATGGAGCGGGCTGTTAAGGAG-3′, and that of the 3′ primer, based on our partial Aurora-A clone, was 5′-ATGATCCATGGATGTTCGAGAA-3′, which included an internal NcoI site found in the Aurora-A sequence. The amplified fragment was digested with NcoI and ligated into pCS2+MT-Aurora-A-C previously digested with NcoI to remove the partial N-terminal Aurora-A sequence. The resultant plasmid, pCS2+MT-Aurora-A, encoded full-length Aurora-A with an N-terminal Myc tag.
To generate an untagged version of Aurora-A, we first inserted the PCR fragment, following NcoI digestion, into pSP64TM (36) previously digested with NcoI. We then replaced a StuI-BglII (Klenow-treated) fragment with a StuI-HindIII (Klenow-treated) fragment derived from pCS2+MT-Aurora-A. The StuI site was within the Aurora-A sequence, whereas the BglII and HindIII sites were 3′ to the Aurora-A sequence in the respective expression vectors. The resultant plasmid, pSP64TM-Aurora-A, encoded full-length Aurora-A without any sequence tags.
To generate Myr-Aurora-A, we first inserted the Aurora-A N-terminal PCR fragment, following NcoI digestion and Klenow treatment, into pMyr (Clontech) previously digested with EcoRI and treated with Klenow. An EcoRI fragment which encoded the N-terminal myristylation signal (2) was then excised from this plasmid, followed by the N terminus of Aurora-A. This fragment was then joined with the C terminus of Aurora-A following the removal of an internal EcoRI fragment from the original yeast two-hybrid clone, pMyr-Aurora-A-C. The entire coding region of Myr-Aurora-A was then excised with HindIII, treated with Klenow, and ligated into pCS2+ (51) previously digested with StuI. The resultant plasmid, pCS2-Myr-Aurora-A, encoded Aurora-A with an N-terminal myristylation signal. The kinase-deficient version, pCS2-Myr-Aurora-A-KA, was generated by the two-step PCR mutagenesis protocol (52) and confirmed by DNA sequencing.
Polyclonal antibodies against Xenopus Aurora-A.
A cDNA fragment encoding Aurora-A amino acids 1 to 123 was amplified by PCR and subcloned into pGEX-2T (22). The glutathione S-transferase (GST)-Aurora-A fusion protein was induced in Escherichia coli and purified via binding to glutathione-agarose beads. Immunization of rabbits with purified proteins was carried out according to standard protocols (24). Antiserum was used without further processing.
Immunoblotting.
We typically loaded extracts derived from one half of an oocyte on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels for subsequent immunoblotting except for anti-Xenopus MAP kinase blots, in which case as little as one fifth was sufficient. Therefore, extracts from individual oocytes were routinely and simultaneously analyzed for multiple kinases (Aurora-A and MAP kinase immunoblotting plus MPF assays; see below).
Protein kinase assays. (i) MPF assays.
We followed the protocol described by Nebreda and Hunt (34) for MPF assays. Oocytes were lysed in extraction buffer (20 mM HEPES [pH 7.3], 80 mM glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 1 mM dithiothreitol, 10 μM ATP, 150 μM NaF, 10 mg of leupeptin per ml, 200 μM phenylmethylsulfonyl fluoride, 25 μg of benzamidine per ml, 20 μl of oocytes). Following centrifugation at 15,000 rpm for 10 min, 8 μl of the clarified extract was added to 4 μl of the same extraction buffer containing 2 μg of histone H1, 3.2 μCi of [32P]ATP, and 100 μM ATP. Kinase reactions were carried out for 15 min at room temperature and stopped by the addition of 12 μl of 2× SDS sample buffer. To prepare MPF extracts used in microinjection experiments (Fig. 10), progesterone-treated metaphase II-arrested eggs were quickly rinsed in the same extraction buffer. Excess extraction buffer was removed, and oocytes were crushed by centrifugation at 15,000 rpm for 15 min. The clear middle layer was collected and frozen at −70 in aliquots. Five nanoliters of these extracts was injected into each oocyte.
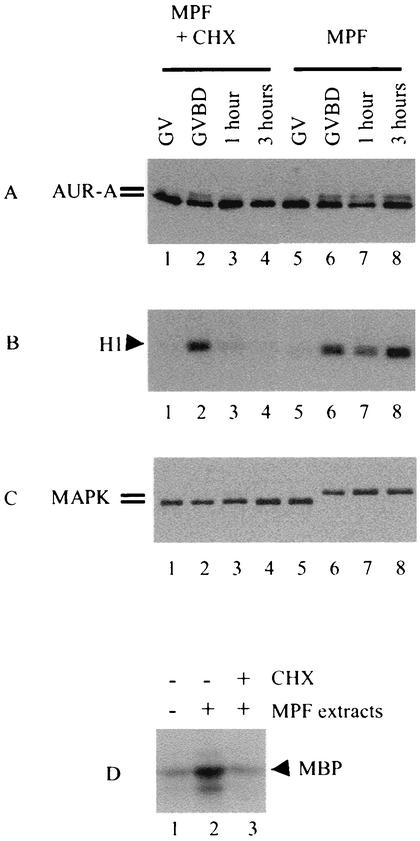
FIG. 10.
Injection of MPF extracts led to Aurora-A hyperphosphorylation and activation. Germinal vesicle oocytes were injected with MPF extracts (5 nl per oocyte) derived from metaphase II-arrested eggs and immediately placed in OR2 with or without cycloheximide (CHX). Individual oocytes were selected at the indicated times and analyzed for Aurora-A hyperphosphorylation (A), MPF activity (B), and MAP kinase phosphorylation (C). Shown is a representative of three independent experiments. (D) Oocytes injected with water (lane 1) or MPF extracts (lanes 2 and 3) were incubated in OR2 (lanes 1 and 2) or OR2 containing cycloheximide (lane 3). Oocytes in groups 2 and 3 similarly underwent GVBD (not shown). Three hours following GVBD, oocytes were individually lysed, and the resultant extracts from 30 oocytes in each group were combined and subjected to Aurora-A immune kinase assays. Shown is a representative of two independent experiments.
(ii) MAP kinase assays.
We lysed oocytes in the extraction buffer specified above. The clarified extract from 20 to 30 oocytes was immunoprecipitated by the addition of 5 μl of anti-Xenopus MAP kinase serum (39) and protein A-Sepharose beads, followed by incubation for 1 h at 4°C. The isolated immunocomplexes were washed twice with extraction buffer plus 0.15 M NaCl, once with kinase buffer (50 mM HEPES [pH 7.3], 10 mM MgCl2, 2 mM MnCl2, 1 mM dithiothreitol, 0.05% Triton X-100) and used immediately for kinase assays. Kinase reactions were started by the addition of 26 μg of myelin basic protein, 5 μCi of [γ-32P]ATP, and 20 μM ATP. Kinase reactions were carried out at room temperature for 10 min and stopped by the addition of an equal volume of SDS sample buffer.
(iii) Aurora-A kinase assays.
Similar immune kinase assays were performed for Aurora-A kinase with the exception that anti-Aurora-A serum was used. In addition, okadaic acid (1 μM) was included in the extraction buffer and in the kinase buffer.
In vitro phosphorylation of Aurora-A by p13SUC1 precipitates.
The procedure for determining in vitro phosphorylation of Aurora-A by p13SUC1 precipitates was adopted from Andresson et al. (1). pSP64TM-AUR-A mRNA was translated in vitro in rabbit reticulocyte lysates (Amersham) in the presence of [35S]methionine and okadaic acid (1 μM). Germinal vesicle oocytes or metaphase II-arrested eggs were homogenized in extraction buffer (10 μl per oocyte) followed by centrifugation at 15,000 rpm for 15 min. The supernatant from 20 to 30 oocytes was incubated with 10 μl of p13SUC1 beads (Calbiochem) for 1 h at 4°C. After washing three times with extraction buffer, the beads were resuspended in 5 μl of resuspension buffer (0.25 mM sucrose, 0.1 M NaCl, 2.5 mM MgCl2, 1 μM okadaic acid, 20 mM HEPES, pH 7.2) plus an ATP regeneration system (2 mM ATP, 2 mM MgCl2, 20 mM creatine phosphate, 0.1 mg of creatine kinase per ml). After the addition of 5 μl of in vitro-translated Aurora-A, samples were kept at room temperature for 1 h before the addition of SDS sample buffer.
Confocal microscopy.
For confocal microscopy, we followed the procedures described by Schwab et al. (47). Oocytes were fixed in methanol for 2 h at room temperature and transferred to dimethyl sulfoxide-methanol (20:80, by volume) overnight at −20°C. Oocytes were bleached for at least 24 h at room temperature in methanol with 10% H2O2. The bleached oocytes were rehydrated with increasing proportions of Tris-buffered saline (TBS; 10 mM Tris-Cl [pH 7.5], 150 mM NaCl). Oocytes were blocked for 3 h in 5% bovine serum albumin in TBST (TBS plus 0.1% Triton X-100). Oocytes were then incubated for at least 24 h at 4°C in TBST-5% bovine serum albumin-antitubulin-β (DM1B from ICN; used at a 1:50 dilution). Oocytes were washed five times with TBST over 8 h, followed by incubation with 2 μg of anti-mouse immunoglobulin G-Alexa 594 (Molecular Probes)/ml in TBST-5% bovine serum albumin. Following washing, oocytes were incubated with Sytox-green (Molecular Probes, 1:10000 in 0.5 × TBS). Following a brief wash with 0.5 × TBS, oocytes were dehydrated with methanol and mounted in benzylbenzoate-benzylalcohol (2:1).
Confocal microscopy was performed on a Bio-Rad model 1024 mounted to an Olympus model IX70 inverted microscope. In most cases (Fig. 7A through F) a 1.15 NA 40× water immersion objective was used, whereas a 1.4 NA 60× oil immersion objective was used for the images shown in Fig. 7G and H. Images are projections of Z-series of 30 to 100 optical sections 1 μm apart.
FIG. 7.
Chromosome morphology in Myr-Aurora-A-injected oocytes. Representative confocal images of oocytes injected with Myr-Aurora-A-KA mRNA (10 h following injection, B) showing partially condensed chromosomes similar to those found in control oocytes (A). In contrast, progressively more condensed chromosomes were observed in oocytes injected with Myr-Aurora-A mRNA (7 h, C; 8 h, E; and 10 h, G) or stimulated with progesterone (3 h, D; 4 h, F; and 6 h, H). Only oocytes shown in panels G and H had undergone GVBD (not shown). Bars, 10 μm.
Fluorescence resonance energy transfer (FRET) assays.
Raichu-Ras DNA (32) (a gift from Michyuki Matsuda, Osaka University, Osaka, Japan) was injected into the oocyte nucleus (1 ng per nucleus). After 36 h of incubation in OR2 (4), strongly fluorescing oocytes (indicating high levels of Raichu-Ras expression) were selected and randomly divided into four groups. The first group received no further injection (control), and the second group received nuclear injection of RasGRF/CDC25 DNA (27) (a gift of Ian Macara of the University of Virginia, 1 ng per oocyte). The third and fourth groups received cytoplasmic injections of mRNA (10 ng per oocyte) encoding Myr-Aurora-A or Myr-Aurora-A-KA, respectively.
After further incubation for 12 h, three oocytes from each group were lysed in 100 μl of phosphate-buffered saline lysis buffer (10 mM sodium phosphate [pH 7.5], 150 mM NaCl, 1% Triton X-100, protease inhibitor cocktail as in extraction buffer, plus 20 mM Mg2+). High concentrations of Mg2+ were used to stabilize Ras-RBD(Raf) (Ras-binding domain of Raf) interactions following cell lysis (53). The lysate was centrifuged at 15,000 rpm for 15 min, and the cleared supernatant was transferred to a black 96-well plate and read immediately. Emission intensities at 475 nm (donor green fluorescent protein [GFP] emission peak) and 527 nm (acceptor GFP emission peak) were measured simultaneously with an excitation wavelength of 433 nm, using the Fusion Universal microplate analyzer (BioSignal Packard).
The 527 nm/475 nm ratios of fluorescence were calculated after subtraction of background fluorescence measured in extracts derived from uninjected oocytes. A typical 527 nm/475 nm ratio for oocytes injected with Raichu-Ras alone was 1.2, very similar to that reported previously (32), which was arbitrarily set at 100%. Although Raichu-Ras was designed to be used in intact cells to measure Ras activation, we found it difficult to measure Raichu-Ras fluorescence in intact oocytes due to interference from the variable background fluorescence, depending on how individual oocytes sat on the measuring well. As the animal and vegetal hemispheres had significantly different levels of background fluorescence, excitation from the top generated increasing background fluorescence as more of the vegetal hemisphere became exposed (not shown). The method described here both reduced the background fluorescence (by partially removing the bulk of the yolk as a centrifugation pellet) and minimized variability associated with intact oocytes.
RESULTS
Isolation of Aurora-A.
We conducted a CytoTrap yeast two hybrid screen (3) for proteins interacting with the recently cloned Xenopus progesterone receptor (xPR) (4). The bait, pSOS-xPR, was cotransformed with a Xenopus oocyte cDNA library (cDNAs are engineered as fusion proteins with an N-terminal myristylation signal) in a yeast strain carrying a temperature-sensitive CDC25 (a membrane-bound yeast Ras guanine nucleotide exchange factor). Physical interaction between the Xenopus progesterone receptor and a library-derived protein will result in membrane targeting of SOS (a mammalian Ras-specific guanine nucleotide exchange factor) and therefore will rescue the CDC25-Ras survival pathway at the nonpermissive temperature (3).
One of the first clones that we isolated in such a screen encoded the C terminus (amino acids 103 to 407) of Aurora-A (1), including the complete kinase domain. However, further analyses in S. cerevisiae indicated that Aurora-A did not interact with the Xenopus progesterone receptor. Instead, the ability of membrane-bound Aurora-A but not the kinase-inactive version or one without the myristylation signal to rescue the temperature-sensitive CDC25 mutant was attributed to its ability to promote Ras activation independently of the original bait (pSOS-xPR) (Ma et al., unpublished). Consistent with the genetic analyses in S. cerevisiae, we failed to demonstrate direct interaction between the Xenopus progesterone receptor and Aurora-A either in vitro or in Xenopus oocytes following injection of the two mRNAs (not shown).
Myr-Aurora-A-induced oocyte GVBD.
Although the isolation of Aurora-A in such a screen may be considered a false-positive result, we were intrigued by the apparent coincidence given the recent studies suggesting a functional role for Aurora-A in early progesterone signaling pathways in Xenopus oocytes (1, 30). We therefore decided to explore the possibility that a membrane-bound Aurora-A kinase may be biologically active in inducing oocyte maturation.
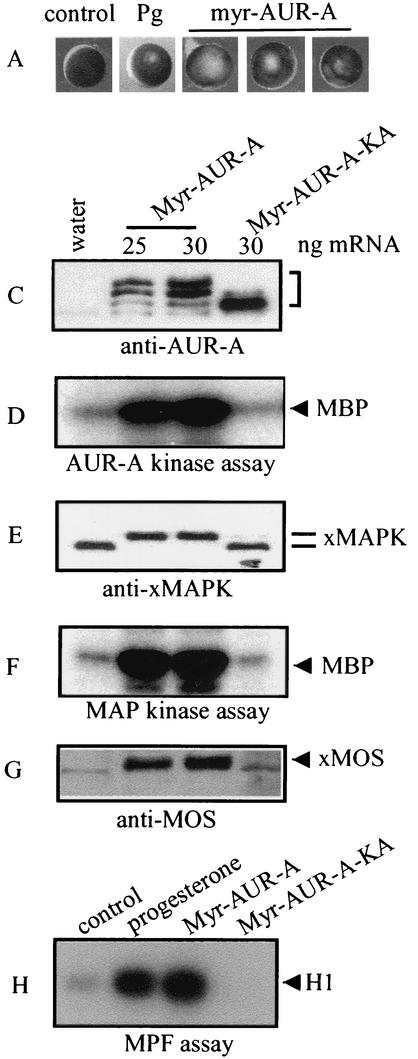
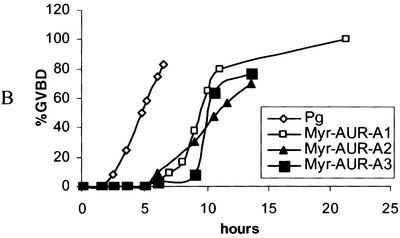
Injection of Myr-Aurora-A mRNA indeed resulted in germinal vesicle breakdown or GVBD (indicated by the absence of germinal vesicles following dissection; not shown), although the “maturation spot” was rarely typical (Fig. 1A). Myr-Aurora-A-induced GVBD typically lagged several hours behind progesterone-induced GVBD (Fig. 1B). To ascertain that oocytes had undergone resumption of meiosis, we analyzed the various biochemical markers typically associated with oocyte maturation. These include MOS biosynthesis (Fig. 1G), phosphorylation (Fig. 1E), and activation of MAP kinase (Fig. 1F) and MPF (Fig. 1H). As anticipated, Myr-Aurora-A was hyperphosphorylated (indicated by retardation in migration on SDS-PAGE) (Fig. 1C) and active (Fig. 1D).
FIG. 1.
Myr-Aurora-A induces GVBD. (A) Typical images of germinal vesicle stage oocytes (control), oocytes following overnight incubation with progesterone (Pg), or Myr-Aurora-A (AUR-A) mRNA-injected oocytes (15 to 24 h after injection). Upon dissection, the Myr-Aurora-A-injected oocytes were confirmed to have undergone GVBD (not shown). (B) Groups of 30 to 50 oocytes were either treated with progesterone (1 μM) or injected with Myr-Aurora-A mRNA. At the indicated time following the addition of progesterone or mRNA injection, oocytes were scored for GVBD and expressed as a percentage of total treated oocytes. Three experiments are shown for Myr-Aurora-A injection compared to one for progesterone treatment. (C through G) Groups of at least 20 oocytes were injected with water or mRNA encoding Myr-Aurora-A or Myr-Aurora-A-KA. The injected oocytes were incubated in OR2 for 20 h. Extracts were prepared and subjected to direct immunoblotting with anti-Aurora-A (C), anti-Xenopus MAP kinase (E), or anti-MOS (G). Assays for Aurora-A kinase (D) and MAP kinase (F) were performed following immunoprecipitation with the respective antibodies. Shown are representatives of three to five independent experiments. (H) Groups of 20 oocytes were either incubated with progesterone or injected with mRNA for Myr-Aurora-A or Myr-Aurora-A-KA. Following overnight incubation, extracts were prepared and subjected to MPF assays. MBP, myelin basic protein; xMAPK, Xenopus MAP kinase.
To determine whether the kinase activity of Aurora-A was required for its biological activity, we mutated the catalytically essential lysine-169 (1) to Ala (Myr-Aurora-A-KA). In contrast to Myr-Aurora-A, Myr-Aurora-A-KA was not hyperphosphorylated (Fig. 1C) or active (Fig. 1D) and did not cause GVBD (not shown, but see Fig. 7B) or activate any of the biochemical markers (Fig. 1E to H). In contrast to Myr-Aurora-A, injection of unmodified Aurora-A mRNA did not activate any of the kinases, nor did it cause GVBD (not shown), as reported previously (1, 13).
Myr-Aurora-A-induced GVBD was blocked by MEK inhibitor U0126.
We wished to confirm that Myr-Aurora-A was indeed targeted to the oocyte membranes. Five hours following the injection of Myr-Aurora-A mRNA, before oocytes underwent GVBD, extracts were made and subjected to differential centrifugation. The resultant fractions were analyzed by immunoblotting. Figure 2B shows that more than 50% of Myr-Aurora-A was recovered in the 10,000 × g pellet, which represented the plasma membrane (16). The remainder was partitioned between the 100,000 × g pellet (which contained low-density endosomal membranes [33] and polymerized microtubules [8]) and the supernatant (representing the cytosol).
FIG. 2.
Myr-Aurora-A enriched in oocyte plasma membrane fraction. (A) Control oocytes (without progesterone [Pg]) or oocytes following 10 min of progesterone stimulation (+Pg) were fractionated into plasma membrane (product of centrifugation at 10,000 × g [P-10K g], representing one oocyte), internal membrane, including polymerized microtubule (P-100K g; representing one oocyte), and cytosol (S-100K g; one-fifth of one oocyte). Samples were analyzed by immunoblotting with the indicated antibodies. Shown is a representative of five independent experiments. xMAPK, Xenopus MAP kinase. (B) Myr-Aurora-A (AUR-A)-injected oocytes were incubated for 5 h (before any sign of GVBD) and then subjected to subcellular fractionation followed by immunoblotting with the indicated antibodies. P-10K g and P-100K g represented one oocyte, and S-100K g represented two-fifths of one oocyte. Shown is a representative of three independent experiments.
The quality of the subcellular fractionation was ensured by monitoring various protein markers. The plasma membrane protein β-integrin and the cytosolic protein MAP kinase were recovered in the respective fractions only. β-Tubulin was mainly distributed between the 100,000 × g pellet (polymerized microtubules) and the cytosol (free tubulins). The significant association of Myr-Aurora-A with the 10,000 × g pellet was in contrast to endogenous Aurora-A, which was minimally associated with this fraction (Fig. 2A). Furthermore, incubation of oocytes with progesterone for 10 min (Fig. 2A, +Pg) or longer (up to 2 h, not shown) did not significantly change the subcellular distribution of the endogenous Aurora-A.
As Myr-Aurora-A was isolated by virtue of its ability to rescue a defect in the yeast Ras signaling pathway (Ma et al., unpublished), we wondered if Myr-Aurora-A induced GVBD by activating endogenous Xenopus Ras (7). Injection of v-Ras (5) and coinjection of c-Ras and a Ras guanine nucleotide exchange protein (36) are known to induce oocyte GVBD. To demonstrate Ras activation in Xenopus oocytes, we employed the recently developed FRET-based Raichu-Ras probe (32). Raichu-Ras contains, as a single fusion protein, a donor GFP (excitation peak/emission peak, 433/475 nm) and an acceptor GFP (480/527 nm) separated by the well-characterized Ras-RBD(Raf) binding partners. Activation of Ras within the fusion protein results in intramolecular binding of Ras.GTP to RBD(Raf) and consequently an increased fluorescence resonance between the donor and acceptor, as indicated by an increased FRET ratio (32).
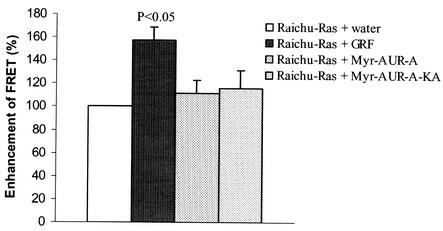
To test whether Raichu-Ras functioned properly in Xenopus oocytes, we injected the probe alone or in combination with a Ras-specific guanine nucleotide exchange factor, RasGRF/CDC25 (27, 48). We have previously demonstrated that RasGRF/CDC25 is capable of activating c-Ras and causing oocyte GVBD (36). As shown in Fig. 3, a significant FRET ratio was observed with the probe alone, a result that was anticipated given that the donor GFP and acceptor GFP were within the same fusion protein (32). Coinjection of RasGRF/CDC25 further increased the FRET ratio, indicating that RasGRF/CDC25 could indeed activate Ras within the fusion protein. In contrast, injection of Myr-Aurora-A or its kinase-deficient mutant (Myr-Aurora-A-KA) did not alter the FRET ratio. These results indicated that Myr-Aurora-A was not able to activate Ras within the Raichu-Ras probe and suggest that Myr-Aurora-A induced GVBD through a mechanism independent of endogenous Ras.
FIG. 3.
Myr-Aurora-A did not activate Ras in Xenopus oocytes. Oocytes were injected with Raichu-Ras DNA (directly into the germinal vesicle) and incubated for 36 h. Oocytes showing clear fluorescence were selected and divided into four groups, each of which received a second injection of water, RasGRF/CDC25 (germinal vesicle injection with DNA), Myr-Aurora-A (20 ng of mRNA per oocyte), or Myr-Aurora-A-KA (20 ng of mRNA per oocyte). Following overnight incubation, FRET ratios (527/475 nm) were measured and expressed as a percentage (means with standard deviations of three independent experiments) of the control (Raichu-Ras + water).
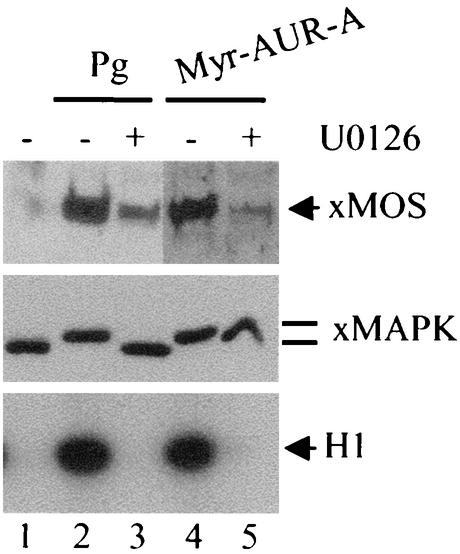
As Aurora-A has been implicated in mediating MOS mRNA polyadenylation and the activation of the MOS-MEK-MAP kinase pathway, we wished to determine whether a widely used MEK inhibitor, U1026, could inhibit Myr-Aurora-A-induced GVBD. Preincubation of oocytes with U0126 resulted in inhibition of progesterone-induced GVBD with corresponding inhibition of MAP kinase and MPF activation (Fig. 4). Following longer (overnight) incubation, the U0126-treated oocytes eventually underwent GVBD and MPF activation despite complete inhibition of MAP kinase (not shown). These results agreed with previous studies demonstrating that U0126 slows down but does not block progesterone-induced GVBD (21). U0126 completely inhibited Myr-Aurora-A-induced GVBD (not shown), MOS accumulation, MAP kinase activation, and MPF activation regardless of the length of incubation (Fig. 4, lane 5).
FIG. 4.
U0126 blocked Myr-Aurora-A-induced activation of MOS, MAP kinase and MPF. Oocytes were incubated with progesterone (Pg) in the absence (lane 2) or presence (lane 3) of U0126 (100 μM, added 1 h prior to the addition of progesterone). Another group of oocytes were injected with Myr-Aurora-A (AUR-A) mRNA and then immediately split into two groups. One group was incubated in OR2 (lane 4) and the other in OR2 containing U0126 (100 μM). Progesterone-treated oocytes (lanes 2 and 3) were lysed following 5 h of incubation, at which time the sample in lane 2 had reached 100% GVBD. Control oocytes (lane 1) and oocytes injected with Myr-Aurora-A (lanes 4 and 5) were lysed following overnight incubation. Extracts were subjected to immunoblotting with anti-Xenopus MOS (upper panel) or anti-Xenopus MAP kinase (xMAPK, middle panel) antibodies or MPF assays (bottom panel).
Myr-Aurora-A-injected oocytes failed to complete maturation.
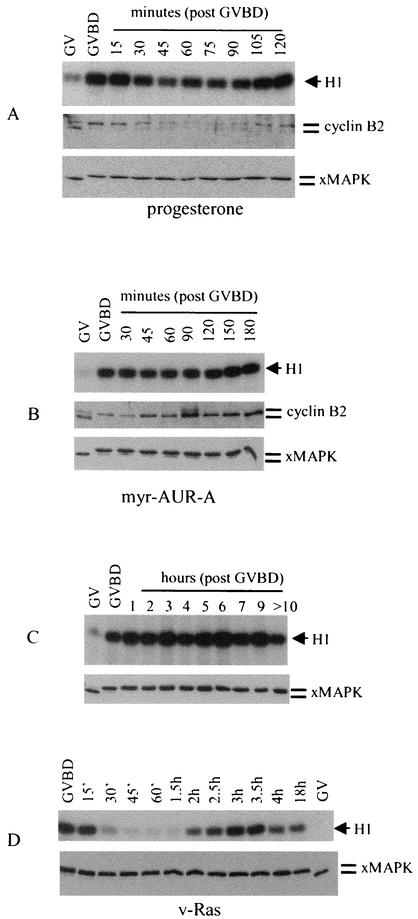
Since oocytes injected with Myr-Aurora-A exhibited atypical maturation spots (Fig. 1A), we wished to determine whether these oocytes had completed meiosis I and arrested at metaphase II, as do oocytes following progesterone stimulation. Figure 5A (upper panel) shows the typical biphasic pattern of MPF activation indicative of the meiosis I-meiosis II transition, as first observed by Gerhart et al. (17). Immunoblotting of the same samples showed the corresponding cyclin B2 phosphorylation (at GVBD) and partial destruction followed by reaccumulation and phosphorylation prior to metaphase II (2 h post-GVBD) (Fig. 5A, middle panel). MAP kinase, once activated at GVBD, remained fully activated throughout the meiosis I-meiosis II transition (Fig. 5A, lower panel). In contrast, in oocytes injected with Myr-Aurora-A, MPF was similarly activated at GVBD but remained activated for several hours following GVBD (Fig. 5B, upper panel). We monitored the Myr-Aurora-A-injected oocytes for longer than 10 h post-GVBD and observed no decrease in MPF activity (Fig. 5C, upper panel). Correspondingly, no cyclin B2 degradation was observed (Fig. 5B, middle panel). The MAP kinase activation pattern was similar to that in progesterone-stimulated oocytes (Fig. 5B, lower panel). Further evidence that Myr-Aurora-A-induced GVBD did not involve Ras activation was provided in Fig. 5D, which indicates that injection of v-ras oncogene mRNA caused the biphasic activation of MPF (Fig. 5D).
FIG. 5.
Myr-Aurora-A-injected oocytes contained sustained MPF activity following GVBD. (A) Oocytes were either left unstimulated (germinal vesicle [GV]) or stimulated with progesterone. Individual oocytes were lysed at the time of appearance of the maturation spot (GVBD) or at the indicated time following GVBD. Extracts were subjected to MPF assays (upper panel) or immunoblotting with antibodies against cyclin B2 (middle panel) or MAP kinase (lower panel). Shown is a representative of three independent experiments. xMAPK, Xenopus MAP kinase. (B) Oocytes were injected with water (germinal vesicle) or with Myr-Aurora-A mRNA. Individual oocytes were lysed and analyzed as in A. Shown is a representative of three independent experiments. (C) MPF activity was monitored in oocytes injected with Myr-Aurora-A mRNA exactly as described for B. (D) Oocytes were injected with water (germinal vesicle) or v-ras mRNA (36) (5 ng/oocyte). Individual oocytes were lysed at GVBD or the indicated time following GVBD. Extracts were analyzed for MPF activities (upper panel) or Xenopus MAP kinase phosphorylation (lower panel).
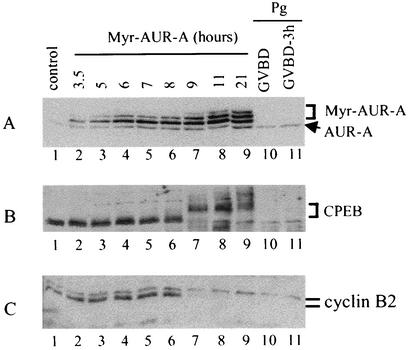
Aurora-A has been identified as a progesterone-activated kinase that phosphorylates and activates a key translation regulator, a cytoplasmic mRNA polyadenylation factor, CPEB (1, 30). We wished to determine whether Myr-Aurora-A injection led to CPEB phosphorylation. Figure 6A shows a typical time course experiment in which Myr-Aurora-A expression was analyzed following mRNA injection. Myr-Aurora-A first appeared as a doublet (Fig. 6A, lanes 2 to 6). Hyperphosphorylation of Myr-Aurora-A (the appearance of a third and slower migrating band) was first apparent at 9 h postinjection in this particular experiment (Fig. 6A, lane 7). This hyperphosphorylation was correlated with hyperphosphorylation of CPEB (Fig. 6B), phosphorylation of cyclin B2 (Fig. 6C), and activation of MPF (not shown). At this exposure, progesterone-induced hyperphosphorylation of the endogenous Aurora-A was not visible (Fig. 6A, lanes 10 and 11) but was clearly seen in a longer exposure (not shown). Interestingly, unlike in progesterone-treated oocytes, the majority of CPEB becomes hyperphosphorylated and degraded at GVBD (29) (Fig. 6B, lanes 10 and 11), Myr-Aurora-A-injected oocytes contained high levels of hyperphosphorylated CPEB more than 10 h following the first sign of GVBD (Fig. 6B, lanes 7 to 9).
FIG. 6.
CPEB was hyperphosphorylated but not degraded in Myr-Aurora-A-injected oocytes. Myr-Aurora-A-injected oocytes were withdrawn (10 oocytes per group) at the indicated times following mRNA injection. Extracts were prepared and immunoblotted with antibodies against Aurora-A (A), CPEB (B), or cyclin B2 (C); 20% of the injected oocytes had undergone GVBD at 9 h postinjection in this particular experiment. As a control, extracts from progesterone-treated oocytes at GVBD (lane 10) or 3 h post-GVBD (lane 11) were analyzed similarly. Shown is a representative of three independent experiments.
To further determine the cell cycle status of Myr-Aurora-A-injected oocytes, we examined their chromosomal morphology. In oocytes stimulated with progesterone, we observed chromosomal condensation prior to GVBD (Fig. 7D and 7F), and following GVBD, we observed the formation of microtubule spindles with the associated metaphase I chromosomes (Fig. 7H). Several hours after GVBD, we observed metaphase II spindles with a discernible polar body (not shown). In Myr-Aurora-A-injected oocytes, chromosomal condensation appeared normal prior to GVBD (Fig. 7C and 7E). However, following GVBD, the condensed chromosomes remained scat-tered, and no microtubule spindles were visible (Fig. 7G). We also noticed that, unlike oocytes following progesterone stimulation, in which the condensed chromosomes, with their associated microtubule spindles, were in the animal pole cortex, the condensed chromosomes in Myr-Aurora-A-injected oocytes remained deep in the cytoplasm (not shown).
Endogenous Aurora-A exhibits biphasic activation that coincides with MPF activation.
The fact that Myr-Aurora-A-injected oocytes did not proceed normally through maturation suggested that a constitutively active Aurora-A was incompatible with the transition from meiosis I to meiosis II. To examine hyperphosphorylation and activation of endogenous Aurora-A during physiological (progesterone-induced) oocyte maturation, we analyzed MPF activity in parallel with Aurora-A hyperphosphorylation. Figure 8A (upper panel) shows that hyperphosphorylation of endogenous Aurora-A followed a biphasic pattern. Hyperphosphorylation occurred at GVBD but then diminished within 1 h of GVBD. Hyperphosphorylation occurred again between 90 and 120 min post-GVBD, and Aurora-A remained hyperphosphorylated thereafter. This biphasic pattern of Aurora-A hyperphosphorylation mirrored MPF activity during the meiosis I-meiosis II transition (Fig. 8A, lower panel).
FIG. 8.
Endogenous Aurora-A exhibits biphasic pattern of hyperphosphorylation and activation during meiosis I-meiosis II transition. (A) Oocytes were either left unstimulated (germinal vesicle) or stimulated with progesterone. Individual oocytes were lysed at the time of appearance of the maturation spot (GVBD) or at the indicated times following GVBD. Extracts were subjected to immunoblotting with anti-Aurora-A antibodies (upper panel) or to MPF assays (lower panel). Shown is a representative of three independent experiments. (B) Oocytes were either left unstimulated (germinal vesicle) or stimulated with progesterone. At GVBD or 1 h or 3 h following GVBD, 30 oocytes were withdrawn and lysed. Extracts were subjected to immunoblotting with anti-Xenopus MAP kinase (xMAPK blot) or anti-Aurora-A (AUR-A blot) antibodies. MPF assays were carried out with extracts without prior fractionation, whereas Aurora-A kinase assays were carried out following immunoprecipitation (IP) with antibodies against Aurora-A. Shown is a representative of three independent experiments.
To directly demonstrate Aurora-A kinase activity during oocyte maturation, we performed immunoprecipitation followed by in vitro kinase assays. In contrast to hyperphosphorylation analyses, which were performed with a single oocyte per time point (Fig. 8A), 20 to 30 synchronized (within <5 min) oocytes were required per time point in immune kinase assays. For technical reasons, we selected four stages for these analyses: germinal vesicle, GVBD, and 1 and 3 h post-GVBD. Figure 8B shows that, indeed, initial activation of Aurora-A occurred at GVBD, corresponding to Aurora-A hyperphosphorylation. Aurora-A activity diminished at 1 h post-GVBD, corresponding to diminished hyperphosphorylation. Aurora-A hyperphosphorylation and activation occurred again 3 h post-GVBD. We also consistently observed higher Aurora-A kinase activities at metaphase II than at GVBD (Fig. 8B).
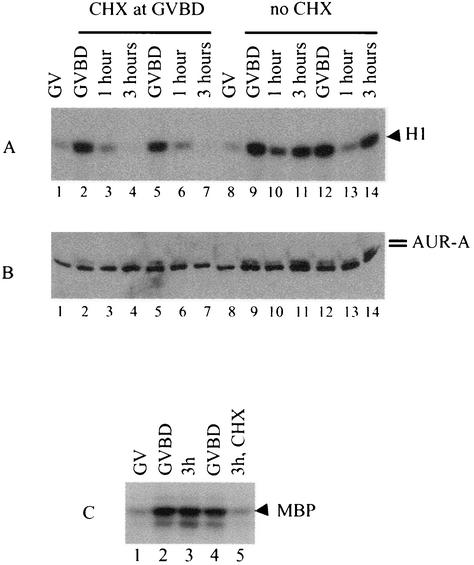
To further establish the coregulation of MPF and Aurora-A, we treated oocytes with cycloheximide at GVBD. Gerhart et al. previously demonstrated that such treatment prevents the reactivation of MPF and therefore failure of the meiosis I-meiosis II transition (17). As shown in Fig. 9A, the addition of cycloheximide at GVBD prevented reactivation of MPF (lanes 4 and 7 compared to lanes 11 and 14). Analyzing the same oocytes showed that this treatment also prevented reactivation of Aurora-A (Fig. 9B, corresponding lanes). Direct kinase as-says following anti-Aurora-A immunoprecipitation showed that the addition of cycloheximide prevented the reactivation of Aurora-A 3 h following GVBD (Fig. 9C, lane 5).
FIG. 9.
Inhibition of protein synthesis at GVBD similarly blocked MPF and Aurora-A reactivation. (A and B) Oocytes were stimulated with progesterone. Individual oocytes were lysed at germinal vesicle, GVBD, or 1 h or 3 h following GVBD. When cycloheximide (CHX, 100 μg/ml) was used, it was added at GVBD. Aliquots of the same extracts were used for MPF assays (A) and immunoblotting with antibodies against Aurora-A (B). Shown is a representative of three independent experiments. (C) Oocytes were either incubated in OR2 (germinal vesicle) or treated with progesterone. Following progesterone treatment, individual oocytes were selected at GVBD and grouped (synchronized within 5 min). Groups of 30 oocytes were either lysed immediately (GVBD, lanes 2 and 4) or further incubated for 3 h in the absence (lane 3) or presence (lane 5) of cycloheximide. Extracts were prepared and subjected to anti-Aurora-A immune kinase assays. Shown is a representative of two independent experiments. MBP, myelin basic protein.
Hyperphosphorylation of Aurora-A by a p13SUC1-precipitable protein kinase(s).
The fact that Myr-Aurora-A was able to initiate GVBD in the absence of progesterone clearly lends support to the notion that Aurora-A functions early in the progesterone signaling pathway (1). However, Frank-Vaillant et al. (13) have demonstrated that inhibition of MPF prevents Aurora-A phosphorylation and activation. We reasoned that the best way to reconcile these studies would be to show the existence of a positive feedback mechanism in which MPF contributes to the hyperphosphorylation and activation of Aurora-A.
We first determined whether injection of MPF extracts could also activate endogenous Aurora-A. Indeed, injection of MPF extracts caused hyperphosphorylation of Aurora-A (Fig. 10A, lane 6). Furthermore, Aurora-A hyperphosphorylation induced by MPF extract injection also followed a similar biphasic pattern. In the presence of cycloheximide, MPF extracts still caused Aurora-A hyperphosphorylation (Fig. 10A, lane 2) and GVBD (not shown), but the hyperphosphorylation was quickly lost and never reappeared (Fig. 10A, lanes 3 and 4). Similarly, autocatalytic activation of MPF occurred normally, but then MPF was quickly inactivated and never reactivated (Fig. 10B, lanes 1 to 4). As expected, no MAP kinase activation was observed in the presence of cycloheximide (Fig. 10C, lanes 1 to 4), indicating the need for de novo synthesis of MOS. Immune kinase assays confirmed that injection of MPF extracts had indeed caused activation of endogenous Aurora-A (Fig. 10D, lane 2). In the presence of cycloheximide, however, Aurora-A activities were eventually lost (Fig. 10D, lane 3, equivalent to lane 4 in Fig. 10A).
In an attempt to reconcile the apparent discrepancy in the timing of Aurora-A hyperphosphorylation during progesterone-induced oocyte maturation, we carried out in vitro phosphorylation experiments similar to those described by Andresson et al. (1). We incubated in vitro-translated Aurora-A with extracts derived from oocytes stimulated with progesterone for the indicated times. Whereas extracts from unstimulated G2 oocytes (0) did not cause alterations in the migration of Aurora-A on SDS-PAGE, extracts from oocytes retrieved at 30 min post-progesterone treatment caused significant alteration in Aurora-A migration (Fig. 11A, upper panel). This confirmed the observation of Andresson et al. (1).
FIG. 11.
Phosphorylation of Aurora-A in vitro by a p13SUC1-associated protein kinase. (A) [35S]methionine-labeled-Aurora-A was incubated with extracts derived from oocytes treated for the indicated times with progesterone (Pg, prepared according to Andresson and Ruderman [1]). Kinase reactions were carried out and analyzed (upper panel). The lower panel shows MPF activities of the corresponding extracts. Shown is a representative of two independent experiments. (B) [35S]methionine-labeled Aurora-A derived from in vitro translation (lane 4) was incubated with p13SUC1-agarose precipitates derived from extracts of germinal vesicle oocytes (lane 2) or metaphase II eggs (lane 3). The kinase reaction was carried out in the presence of an ATP regeneration system and stopped by the addition of SDS sample buffer. Aurora-A was visualized by SDS-PAGE and autoradiography. A control translation with no exogenous mRNA was included (lane 1). (C) Extracts from germinal vesicle oocytes (lane 1) or metaphase II eggs (lanes 2 and 3) were incubated with p13SUC1-agarose. Following washing, the beads were incubated for 10 min in the absence (lanes 1 and 2) or presence (lane 3) of roscovitine before being subjected to in vitro kinase assays with both histone H1 and myelin basic protein (MBP) as substrates. (D) Phosphorylation of in vitro-translated Aurora-A (lanes 2 through lane 4) or Aurora-A-T108A (lane 6) as described above (B) with the exception that p13SUC1 precipitates in lanes 3 and 4 had been treated for 10 min with the indicated concentrations of roscovitine as described for C. Lane 1 and lane 5 represent untreated translation products of Aurora-A and Aurora-A (T108A), respectively. (E) Aurora-A (lanes 1 and 2) and Aurora-A-KA (lanes 3 and 4) were translated in vitro with complete amino acid mixture (no [35S]methionine). The translated proteins were treated with p13SUC1 precipitates as described for B. Phosphorylation of Aurora-A was analyzed by SDS-PAGE followed by immunoblotting with anti-Aurora-A.
Interestingly, extracts from oocytes retrieved 2 h post-progesterone treatment caused further retardation in Aurora-A's migration on SDS-PAGE (Fig. 11A, upper panel). A parallel analysis of the oocyte extracts indicated that at 2 h post-progesterone treatment, MPF was fully activated (Fig. 11A, lower panel). These results again support the notion that MPF, or another protein kinase present in these extracts, was involved in a possible feedback regulation of Aurora-A. Although incubation of in vitro-translated Aurora-A with extracts derived at 30 min post-progesterone treatment reproducibly caused Aurora-A retardation on SDS-PAGE (Fig. 11A, upper panel), we did not observe similar changes in endogenous Aurora-A in intact oocytes following 30 min of treatment with progesterone (not shown). Instead, we observed the typical retardation of endogenous Aurora-A (Fig. 8 to 10) only when MPF activation had occurred, in agreement with Frank-Vaillant et al. (13).
To test directly whether MPF could cause Aurora-A hyperphosphorylation, we employed the same in vitro kinase assay (1) with the exception that purified MPF, via p13SUC1-agarose precipitation from extracts of germinal vesicle oocytes or metaphase II eggs, was used. 35S-labeled Aurora-A derived from in vitro translation was incubated with purified p13SUC1 precipitates in the presence of an ATP-regenerating system. As shown in Fig. 11B, p13SUC1 precipitates from metaphase II egg extracts phosphorylated Aurora-A efficiently, generating the characteristic shift on SDS-PAGE. As a control, we incubated Aurora-A with a p13SUC1-agarose precipitate derived from extracts of germinal vesicle oocytes, which contained no detectable MPF activity (Fig. 11C, lane 1). This treatment did not result in any significant alteration of Aurora-A migration on SDS-PAGE (Fig. 11B, lane 2 compared with lane 4).
To determine whether MPF was indeed responsible for the observed Aurora-A hyperphosphorylation, we incubated p13SUC1 precipitates with roscovitin (28), which greatly diminished the ability of MPF to phosphorylate either histone H1 or myelin basic protein (Fig. 11C, lane 3). However, treatment with roscovitin had little, if any, effect on the ability of the p13SUC1 precipitates to phosphorylate Aurora-A (Fig. 11D, lanes 3 and 4). Inspecting the Aurora-A sequence identified only one possible “proline-directed” serine/threonine phosphorylation site (Thr108) for cdk kinases (49). However, a mutant Aurora-A in which Thr108 was replaced with Ala was similarly “shifted” following incubation with p13SUC1-purified MPF (Fig. 11D, lane 6). Finally, like Aurora-A (Fig. 11E, lane 2), the kinase-dead mutant Aurora-A-K220A (Fig. 11E, lane 4) was phosphorylated similarly in vitro by p13SUC1 precipitates from metaphase II egg extracts. These results suggest that MPF is unlikely responsible for direct phosphorylation of Aurora-A. Instead, an MPF- or p13SUC1-associated metaphase-active protein kinase(s) may be responsible. We are currently carrying out experiments to identify this p13SUC1-precipitable kinase(s).
DISCUSSION
Although Myr-Aurora-A was isolated in a yeast two-hybrid screen (Cyto-Trap strategy [3]) with the Xenopus progesterone receptor as the bait, we do not believe that Aurora-A binds directly (or indirectly) to the Xenopus progesterone receptor in S. cerevisiae or in frog oocytes. The ability of Myr-Aurora-A to rescue the temperature-sensitive yeast CDC25 mutant was not dependent on coexpression of the bait (pSOS-xPR). However, Myr-Aurora-A did not rescue the STS8 ras mutant (ras1− ras2+) (40) (Ma et al., unpublished). These results suggest that Myr-Aurora-A either allows the mutant CDC25 to function at the nonpermissive temperature or activates yeast Ras proteins independently of CDC25.
Importantly, Myr-Aurora-A demonstrated constitutive (hormone-independent) kinase activity in frog oocytes and induced CPEB phosphorylation (Fig. 6B), MOS synthesis (Fig. 1G), MAP kinase activation, and MPF activation. It also caused GVBD. The action of Myr-Aurora-A in oocytes was not mediated through endogenous Ras protein, as might have been anticipated given the clear indication that it functioned upstream of yeast Ras proteins. However, inhibition of the MAP kinase pathway (by U0126) prevented Myr-Aurora-A from activating any of the protein kinases or GVBD. These results suggest that Myr-Aurora-A may signal through the MOS-MEK-MAP kinase pathway. This interpretation would agree with the earlier identification of Aurora-A as an upstream kinase and activator of CPEB (30, 31).
The most important finding of this study, however, is the demonstration that endogenous Aurora-A exhibited a biphasic pattern of activation that mirrored MPF activities during the meiosis I-meiosis II transition. To our knowledge, Aurora-A is the only protein kinase other than MPF that exhibits a biphasic pattern of activation during oocyte maturation. Further work will be required to determine the control mechanism for this biphasic pattern of Aurora-A activation and its precise role in regulating the meiosis I-meiosis II transition.
Biphasic activation of Aurora-A during meiosis I-meiosis II transition.
We have demonstrated that endogenous Aurora-A underwent a transient dephosphorylation and inactivation following GVBD, reaching the lowest level about 1 h post-GVBD. At first glance, this may appear to contradict an earlier time course experiment by Frank-Vaillant et al. (13), who did not observe this transient dephosphorylation/inactivation. However, these authors did not analyze post-GVBD oocytes in a synchronized fashion. In their time course experiments, Frank-Vaillant et al. also did not observe the transient inactivation of MPF following GVBD (13). To reveal the transient inactivation of Aurora-A (or MPF), it is necessary to analyze single oocytes based on the appearance of a maturation spot or groups of oocytes showing maturation spots within a very narrow time window (<5 min), as was done in this study and many others (17, 29).
That the transient dephosphorylation/inactivation of Aurora-A is important for the meiosis I-meiosis II transition is indicated by the failure of Myr-Aurora-A-injected oocytes to properly complete oocyte maturation. Myr-Aurora-A induced MOS protein accumulation, complete MAP kinase activation, and the activation of MPF. Myr-Aurora-A-injected oocytes also underwent GVBD and chromosome condensation. However, the highly condensed metaphase-like chromosomes found in Myr-Aurora-A-injected oocytes were scattered deep in the oocyte cytoplasm, without any associated microtubule spindle structure. Furthermore, Myr-Aurora-A-injected oocytes did not undergo partial cyclin B or CPEB degradation following GVBD, as do progesterone-treated oocytes. These observations suggest that Myr-Aurora-A did not properly enter metaphase I.
The lack of discernible spindle structures in Myr-Aurora-A-injected oocytes is reminiscent of previous studies in which Aurora-A function was inhibited by antisense depletion in C. elegans embryos (23, 45). In this regard, loss of Aurora-A function is phenotypically related to overexpression of membrane-targeted Aurora-A.
Although the precise subcellular localization of endogenous Aurora-A in oocytes has not been determined, Xenopus Aurora-A is a microtubule-binding protein that associates with centrosomes in cultured XL2 cells (43) and in embryos (20). The cofractionation of Aurora-A with polymerized microtubules (Fig. 2A) would agree with the notion that it may be associated with the centrosome-equivalent microtubule-organizing center in frog oocytes (15). Mislocalization of the overexpressed Myr-Aurora-A may compete with endogenous Aurora-A for binding partners important for spindle assembly. As such, Myr-Aurora-A may represent a valuable tool for further investigation of the regulation of spindle dynamics during oocyte maturation.
The Myr-Aurora-A-induced cell cycle arrest is in contrast to that observed by Mendez et al. (29) when they injected a degradation-resistant mutant CPEB (6A-CPEB). In 6A-CPEB oocytes treated with progesterone, MPF is activated normally prior to GVBD and inactivated following GVBD, but reactivation of MPF does not occur. In this regard, 6A-CPEB-treated oocytes treated with progesterone behave like oocytes treated with progesterone and then, at GVBD, treated with cycloheximide or injected with antisense MOS (14, 17). These oocytes prematurely abort meiosis and assume interphase-like characteristics (reformation of the nuclear envelope, decondensation of chromosomes, and replication of DNA) (14). Although the lack of CPEB degradation is common in both cases, the mechanisms are clearly different. CPEB is apparently subjected to two-step phosphorylation, first by Aurora-A and then by MPF. MPF-catalyzed phosphorylation is required for the subsequent partial destruction of CPEB by the proteasome following GVBD (29, 42). 6A-CPEB lacks MPF-catalyzed phosphorylation sites and therefore is resistant to this partial destruction. In Myr-Aurora-A-injected oocytes, the lack of degradation of endogenous CPEB is most likely due to a defect in the proteasome pathway, as cyclin B degradation is also inhibited in Myr-Aurora-A-injected oocytes.
In mitosis, perfectly formed metaphase chromosomes with the associated bipolar spindles are a prerequisite for the exit of metaphase and initiation of anaphase. Even a minor defect in spindle structure triggers the spindle checkpoint, which arrests cell cycle progression by inhibiting the anaphase-promoting complex/cyclosome protein degradation mechanism (35). Earlier studies, however, indicated the lack of a similar spindle checkpoint during the first few postfertilization mitotic cell cycles (these cell cycles consist of S and M phases only) or in meiosis in the frog (17). The lack of cyclin B2 degradation in Myr-Aurora-A-injected oocytes may be explained by sequestration of Cdc20 to the membranes. Aurora-A has been shown to bind Cdc20, an activator of the anaphase-promoting complex/cyclosome (9). This association presumably occurs at centrosomes (or the equivalent microtubule organizing center in frog oocytes), as both are centrosomal proteins (41, 43). Myr-Aurora-A may sequester Cdc20 in the membranes and therefore inhibit anaphase-promoting complex/cyclosome activity.
It must be pointed out that Myr-Aurora-A likely has multiple effects in oocytes instead of simply abrogating the normal biphasic pattern of Aurora-A activation. Therefore, more refined approaches will be required to further investigate the precise effect of abrogating the biphasic pattern of Aurora-A activation. In this regard, it is interesting that two excellent recent studies (37, 50) report the surprising finding that frog oocytes undergo normal meiosis I-meiosis II transition (measured by chromosome/spindle morphology) despite inhibition of anaphase-promoting complex/cyclosome activity and cyclin B degradation. Does Aurora-A, instead of MPF, represent the key kinase regulating the meiosis I-meiosis II transition?
Novel feedback mechanism: phosphorylation of Aurora-A by p13SUC1-associated protein kinase?
Functionally, injection of Myr-Aurora-A into oocytes induced full MPF activation (Fig. 1H and Fig. 4). Conversely, progesterone-induced activation of Aurora-A in vivo requires MPF activity (13). Furthermore, injection of MPF extracts caused activation of endogenous Aurora-A (Fig. 10A and D). The relationship between MPF and Aurora-A can therefore be best explained by a novel positive-feedback mechanism; many such mechanisms are already known to operate during progesterone-induced oocyte maturation (10, 44).
The identification of Aurora-A as a CPEB kinase (30, 31) would clearly place Aurora-A upstream of MOS synthesis and therefore upstream of MPF activation. This notion is supported by a recent study reporting that antisense depletion of Aurora-A cooperates with MOS-specific antisense in suppression of MOS synthesis (38). Our in vitro data, however, do not support a direct role for MPF in Aurora-A phosphorylation. First, incubation of p13SUC1 precipitates with the cyclin-dependent kinase inhibitor roscovitin, although almost eliminating MPF activities (Fig. 11C), did not alter hyperphosphorylation of Aurora-A (Fig. 11D). Second, mutating the only possible “proline-directed” cyclin-dependent kinase consensus phosphorylation site (Thr108) (49) to alanine did not affect hyperphosphorylation of Aurora-A by p13SUC1 precipitates (Fig. 11D). Therefore, a p13SUC1-associated (or MPF-associated) protein kinase may be responsible for the observed hyperphosphorylation. Nonetheless, the ability of p13SUC1 to bind this kinase, as demonstrated here, may provide a means for its molecular identification.
Regardless of the identity of the p13SUC1-associated kinase, the feedback mechanism proposed here would explain the paradox raised by two previous studies (1, 13). It appeared that endogenous Aurora-A underwent initial phosphorylation and activation shortly after progesterone stimulation (1). However, this phosphorylation did not consistently correspond to either gel retardation or activation, as measured in vitro by using artificial substrates (13) (data not shown). Aurora-A underwent hyperphosphorylation in a fashion that was dependent on the activation of MPF, and this phosphorylation caused gel retardation and correlated with increased kinase activity, as measured in vitro (13). The proposed two-step phosphorylation was demonstrated here (Fig. 11A) using in vitro-translated Aurora-A. However, attempts to demonstrate similar two-step phosphorylation of endogenous Aurora-A by 32P metabolic labeling in intact oocytes were not successful due to high background labeling (not shown).
Several recent studies from the Richter laboratory (25, 30, 31) have clearly demonstrated that CPEB is a key physiological target of Aurora-A. Our data showing that Myr-Aurora-A induced hyperphosphorylation of CPEB (Fig. 6B) is consistent with this notion. It is interesting that CPEB also experiences differential phosphorylation at two stages. Early phosphorylation of CPEB on a single site (Ser-174) is carried out by Aurora-A, which does not cause an electrophoretic shift (30). Hyperphosphorylation on multiple sites, by MPF, caused an electrophoretic shift (29). Although Mendez et al. (29) have demonstrated that MPF-catalyzed CPEB phosphorylation targets the latter for partial destruction, it is possible that MPF-catalyzed CPEB phosphorylation may also contribute to enhanced polyadenylation of key maternal mRNA (e.g., MOS). This role, together with the proposed feedback activation of Aurora-A, would help explain the dramatic accumulation of MOS (increased translation and increased stability) at almost the same time as activation of MAP kinase and MPF (an all-or-none event) (11, 26).
Acknowledgments
We thank J. M. Baltz for discussions and J. D. Richter (anti-CPEB), J. L. Maller (anti-cyclin B2), and N. Sagata (anti-Xenopus MOS) for antibodies. We also thank M. Matsuda for Raichu-Ras cDNA.
This study was supported by operating grants (to X.J.L) from the National Cancer Institute of Canada (NCIC) and the Canadian Institute of Health Research (CIHR). X.J.L. is a recipient of a Premier's Research Excellence Award (Ontario).
REFERENCES
- 1.Andresson, T., and J. V. Ruderman. 1998. The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signalling pathway. EMBO J. 17:5627-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronheim, A., D. Engelberg, N. Li, N. Al-Alawi, J. Schlessinger, and M. Karin. 1994. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell 78:949-961. [DOI] [PubMed] [Google Scholar]
- 3.Aronheim, A., E. Zandi, H. Hennemann, S. J. Elledge, and M. Karin. 1997. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol. Cell. Biol. 17:3092-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayaa, M., R. A. Booth, Y. Sheng, and X. J. Liu. 2000. The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. Proc. Natl. Acad. Sci. USA 97:12607-12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birchmeier, C., D. Broek, and M. Wigler. 1985. Ras proteins can induce meiosis in Xenopus oocytes. Cell 43:615-621. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff, J. R., and G. D. Plowman. 1999. The aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol. 9:454-459. [DOI] [PubMed] [Google Scholar]
- 7.Davis, D., and S. E. Sadler. 1992. Analysis of the p21 ras system during the development of meiotic competence in Xenopus laevis oocytes. Dev. Biol. 149:1-7. [DOI] [PubMed] [Google Scholar]
- 8.Elinson, R. P. 1985. Changes in levels of polymeric tubulin associated with activation and dorsoventral polarization of the frog egg. Dev. Biol. 109:224-233. [DOI] [PubMed] [Google Scholar]
- 9.Farruggio, D. C., F. M. Townsley, and J. V. Ruderman. 1999. Cdc20 associates with the kinase aurora2/Aik. Proc. Natl. Acad. Sci. USA 96:7306-7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrell, J. E. 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14:140-148. [DOI] [PubMed] [Google Scholar]
- 11.Ferrell, J. E. Jr., and E. M. Machleder. 1998. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science 280:895-898. [DOI] [PubMed] [Google Scholar]
- 12.Francisco, L., W. Wang, and C. S. M. Chan. 1994. Type 1 protein phosphatase acts in opposition to Ipl1 protein kinase in regulating yeast chromosome segregation. Mol. Cell. Biol. 14:4731-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank-Vaillant, M., O. Haccard, C. Thibier, R. Ozon, Y. Arlot-Bonnemains, C. Prigent, and C. Jessus. 2000. Progesterone regulates the accumulation and the activation of Eg2 kinase in Xenopus oocytes. J. Cell Sci. 113:1127-1138. [DOI] [PubMed] [Google Scholar]
- 14.Furuno, N., M. Nishizawa, K. Okazaki, H. Tanaka, J. Iwashita, N. Nakajo, Y. Ogawa, and N. Sagata. 1994. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 13:2399-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gard, D. L. 1992. Microtubule organization during maturation of Xenopus oocytes: assembly and rotation of the meiotic spindle. Dev. Biol. 151:516-530. [DOI] [PubMed] [Google Scholar]
- 16.Gawantka, V., H. Ellinger-Ziegelbauer, and P. Hausen. 1992. 1-integrin is a material that is inserted into all newly formed plasma membrane during early Xenopus embryogenesis. Development 115:595-605. [DOI] [PubMed] [Google Scholar]
- 17.Gerhart, J., M. Wu, and M. Kirschner. 1984. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol. 98:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giet, R., and C. Prigent. 1999. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine/threonine kinases. J. Cell Sci. 112:3591-3601. [DOI] [PubMed] [Google Scholar]
- 19.Glover, D. M., M. H. Leibowitz, D. A. McLean, and H. Parry. 1995. Mutations in aurora prevent centrosome separation leading the formation of monopolar spindles. Cell 81:95-105. [DOI] [PubMed] [Google Scholar]
- 20.Groisman, I., Y. S. Huang, R. Mendez, Q. Cao, W. Theurkauf, and J. D. Richter. 2000. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell 103:435-447. [DOI] [PubMed] [Google Scholar]
- 21.Gross, S. D., M. S. Schwab, F. E. Taieb, A. L. Lewellyn, Y. W. Qian, and J. L. Maller. 2000. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90Rsk. Curr. Biol. 10:430-438. [DOI] [PubMed] [Google Scholar]
- 22.Guan, K., and J. E. Dixon. 1991. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262-267. [DOI] [PubMed] [Google Scholar]
- 23.Hannak, E., M. Kirkham, A. A. Hyman, and K. Oegema. 2001. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155:1109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Hodgman, R., J. Tay, R. Mendez, and J. D. Richter. 2001. CPEB phosphorylation and cytoplasmic polyadenylation are catalyzed by the kinase IAK1/Eg2 in maturing mouse oocytes. Development 128:2815-2822. [DOI] [PubMed] [Google Scholar]
- 26.Huang, C. Y., and J. E. Ferrell, Jr. 1996. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 93:10078-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattingly, R. R., and I. Macara. 1996. Phosphorylation-dependent activation of the Ras-GRF/CDC25Mm exchange factor by muscarinic receptor and G-protein βgamma subunits. Nature 382:268-272. [DOI] [PubMed] [Google Scholar]
- 28.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 29.Mendez, R., D. Barnard, and J. D. Richter. 2002. Differential mRNA translation and meiotic progression require Cdc2-mediated CPEB destruction. EMBO J. 21:1833-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez, R., L. E. Hake, T. Andresson, L. E. Littlepage, J. V. Ruderman, and J. D. Richter. 2000. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404:302-307. [DOI] [PubMed] [Google Scholar]
- 31.Mendez, R., K. G. K. Murthy, K. Ryan, J. L. Manley, and J. D. Richter. 2000. Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol. Cell 6:1253-1259. [DOI] [PubMed] [Google Scholar]
- 32.Mochizuki, N., S. Yamashita, K. Kurokawa, Y. Ohba, T. Nagai, A. Miyawaki, and M. Matsuda. 2001. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411:1065-1068. [DOI] [PubMed] [Google Scholar]
- 33.Mora, S., P. Kaliman, J. Chillaron, X. Testar, M. Palacin, and A. Zorzano. 1995. Insulin and insulin-like growth factor 1 (IGF-1) stimulate GLUT4 glucose transporter translocation in Xenopus oocytes. Biochem. J. 311:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nebreda, A. R., and T. Hunt. 1993. The c-mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 12:1979-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nigg, E. A. 2001. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell. Biol. 2:21-32. [DOI] [PubMed] [Google Scholar]
- 36.Ohan, N., Y. Agazie, C. Cummings, R. Booth, M. Bayaa, and X. J. Liu. 1999. Rho-associated protein kinase α potentiates insulin-induced MAP kinase activation in Xenopus oocytes. J. Cell Sci. 112:2177-2184. [DOI] [PubMed] [Google Scholar]
- 37.Peter, M., A. Castro, T. Lorca, C. Le Peuch, L. Magnaghi-Jaulin, M. Doree, and J. C. Labbe. 2001. The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat. Cell Biol. 3:83-87. [DOI] [PubMed] [Google Scholar]
- 38.Peter, M., J. C. Labbe, M. Doree, and E. Mandart. 2002. A new role for Mos in Xenopus oocyte maturation: targeting Myt1 independently of MAPK. Development 129:2129-2139. [DOI] [PubMed] [Google Scholar]
- 39.Posada, J., and J. A. Cooper. 1992. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science 255:212-215. [DOI] [PubMed] [Google Scholar]
- 40.Powers, S., K. O'Neill, and M. Wigler. 1989. Dominant yeast and mammalian RAS mutations that interfere with the CDC25-dependent activation of wild-type RAS in Saccharomyces cerevisiae. Mol. Cell. Biol. 9:390-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raff, J. W., K. Jeffers, and J. Y. Huang. 2002. The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time. J. Cell Biol. 157:1139-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reverte, C. G., M. D. Ahearn, and L. E. Hake. 2001. CPEB degradation during Xenopus oocyte maturation requires a PEST domain and the 26S proteasome. Dev. Biol. 231:447-458. [DOI] [PubMed] [Google Scholar]
- 43.Roghi, C., R. Giet, R. Uzbekov, N. Morin, I. Chartrain, R. Le Guellec, A. Couturier, M. Doree, M. Philippe, and C. Prigent. 1998. The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 111:557-572. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt, A., and A. R. Nebreda. 2002. Signalling pathways in oocyte meiotic maturation. J. Cell Sci. 115:2457-2459. [DOI] [PubMed] [Google Scholar]
- 45.Schumacher, J. M., N. Ashcroft, P. J. Donovan, and A. Golden. 1998. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development 125:4391-4402. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher, J. M., A. Golden, and P. J. Donovan. 1998. AIR-2: an aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 143:1635-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwab, M. S., B. T. Roberts, S. D. Gross, B. J. Tunguist, F. E. Taieb, A. L. Lewellyn, and J. L. Maller. 2001. Bubi is activated by the protein kinase p90Rsk during Xenopus oocyte maturation. Curr. Biol. 11:141-150. [DOI] [PubMed] [Google Scholar]
- 48.Shou, C., C. L. Farnsworth, B. G. Neel, and L. A. Feig. 1992. Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for Ras p21. Nature 358:351-354. [DOI] [PubMed] [Google Scholar]
- 49.Songyang, Z., K. P. Lu, Y. T. Kwon, L.-H. Tsai, O. Filhol, C. Cochet, D. A. Brickey, T. R. Soderling, C. Bartleson, D. J. Graves, A. J. DeMaggio, M. F. Hoekstra, J. Blenis, T. Hunter, and L. C. Cantley. 1996. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinase I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol. Cell. Biol. 16:6486-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taieb, F. E., S. D. Gross, A. L. Lewellyn, and J. L. Maller. 2001. Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from Meiosis I to II in Xenopus oocytes. Curr. Biol. 11:508-513. [DOI] [PubMed] [Google Scholar]
- 51.Turner, D. L., and H. Weintraub. 1994. Expression of achaete-scute homology 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 8:1434-1447. [DOI] [PubMed] [Google Scholar]
- 52.Vallette, F., E. Mege, A. Reiss, and M. Adesnik. 1989. Construction of mutant and chimeric genes using the polymerase chain reaction. Nucleic Acids Res. 17:723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vojtek, A. B., S. M. Hollenberg, and J. A. Cooper. 1993. Mammalian Ras interacts directly with serine/threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]