Abstract
mof6-1 was originally isolated as a recessive mutation in Saccharomyces cerevisiae which promoted increased efficiencies of programmed −1 ribosomal frameshifting and rendered cells unable to maintain the killer virus. Here, we demonstrate that mof6-1 is a unique allele of the histone deacetylase RPD3, that the deacetylase function of Rpd3p is required for controlling wild-type levels of frameshifting and virus maintenance, and that the closest human homolog can fully complement these defects. Loss of the Rpd3p-associated histone deacetylase function, either by mutants of rpd3 or loss of the associated gene product Sin3p or Sap30p, results in a delay in rRNA processing rather than in an rRNA transcriptional defect. This results in production of ribosomes having lower affinities for aminoacyl-tRNA and diminished peptidyltransferase activities. We hypothesize that decreased rates of peptidyl transfer allow ribosomes with both A and P sites occupied by tRNAs to pause for longer periods of time at −1 frameshift signals, promoting increased programmed −1 ribosomal frameshifting efficiencies and subsequent loss of the killer virus. The frameshifting defect is accentuated when the demand for ribosomes is highest, suggesting that rRNA posttranscriptional modification is the bottleneck in ribosome biogenesis.
A growing yeast cell must produce ∼2,000 ribosomes per min, each of which must be able to translate mRNAs with extremely high accuracy (reviewed in reference 80). In the model organism Saccharomyces cerevisiae, the coordinate expression of 137 ribosomal protein (RP) genes and ∼150 rRNA genes is required to synthesize the appropriate levels of the 78 proteins and four species of rRNAs that are used to form mature small (40S) and large (60S) ribosomal subunits. Although a premium has been placed on fidelity of the translational apparatus, a number of examples have been described where translating ribosomes are directed to shift translational reading frames in response to specific cis-acting mRNA signals. As exceptions to the general rule, such programmed ribosomal frameshifting (PRF) events provide a convenient means to probe the molecular mechanisms underlying ribosome structure and function relationships as well as a unique window into the translation elongation cycle. In addition, PRF has been shown to play a critical role in the morphogenesis of RNA viruses (reviewed in reference 18). Given that human immunodeficiency virus and many other animal and plant pathogens utilize PRF, an understanding of the molecular mechanisms that govern PRF can also provide insight into rational antiviral therapeutic strategies.
The most common programmed ribosomal frameshifts involve slippage of the translational complex by 1 nucleotide in the 5′ (−1) or 3′ (+1) direction. A typical −1 PRF signal is composed of two cis-acting elements, a heptameric “slippery site” followed by a strong secondary mRNA structure such as a pseudoknot (reviewed in references 4, 24, and 28). The mRNA structure induces an elongating ribosome to pause, positioning the A and P site tRNAs over the slippery site (47, 65, 72). At a given frequency (depending on the viral system), the ribosome is directed to slip back a single base in the 5′ direction. After the slip, the nonwobble bases of the ribosome-bound tRNAs can base pair with the −1 frame codons, and translation continues in the −1 frame (37, 38). We have made extensive use of the yeast killer virus system to probe the genetics, molecular biology, and biochemistry of programmed −1 ribosomal frameshifting (reviewed in references 12 and 18). The 4.6-kb double-stranded RNA (dsRNA) genome of the L-A virus contains two open reading frames (ORFs), which are organized 5′ gag and 3′ pol with respect to the translation start codon. The gag gene encodes the major viral structural protein, and pol encodes a multifunctional enzyme that has RNA-dependent RNA polymerase (Pol) activity as well as a viral RNA packaging domain (27, 35). Host cell ribosomes translate the L-A mRNA, producing the 0 frame-encoded Gag and −1 PRF-encoded Gag-Pol fusion proteins (15, 74). The 1.8- to 1.6-kb dsRNA genome of the M1 satellite virus of L-A is both replicated and encapsidated in L-A-encoded viral particles and encodes a secreted toxin and an immunity factor that allows virus-infected cells to kill uninfected cells (reviewed in references 5 and 81). Generally, changing the frequency or efficiency of −1 PRF at the L-A frameshift signal promotes loss of the M1 virus (reviewed in references 12 and 18).
There are a number of parameters that can influence the ability of ribosomes to maintain translational reading frame (reviewed in reference 31). These include changes in the residence time of ribosomes at a particular PRF signal and the precise step of the elongation cycle when such a kinetic change might occur, changes in the stabilities of ribosome-bound tRNAs due to alterations in intrinsic ribosomal components such as RPs and rRNAs, and defects in the abilities of ribosomes to recognize and correct errors. In combination with the current high-resolution structural understanding of ribosomes, studies of PRF are leading to new insights into ribosome structure-function relationships and also have utility for the identification of new targets for antiviral agents.
In the course of this study, alleles of several yeast chromosomal genes that specifically increase −1 PRF efficiency were characterized. Nine chromosomal mof (maintenance of frame) mutants have been described previously (19-21), and alleles of other translation-associated genes with Mof− phenotypes have also been identified (3, 16, 50, 56, 59, 64). Concomitant loss of the killer virus occurs in strains harboring the mof1-1, mof2-1, mof4-1, mof5-1, and mof6-1 alleles (20). MOF2 and MOF4 are allelic to SUI1 (11) and UPF1 (12), respectively. This work focuses on the cloning and characterization of MOF6. We show that mof6-1 is an allele of RPD3, the well-characterized histone deacetylase that is involved in transcriptional activation and silencing (67, 68). The defect is dependent on the histone deacetylase activity of the gene product and can be rescued by the human homolog, HDAC1. Expression of the mutant rpd3 alleles results in delayed exit from lag-phase growth and premature auxotrophic shift, suggestive of a defect in carbon source mobilization and utilization. The frameshifting defect is most pronounced in early log phase, when demand for newly synthesized ribosomes is greatest. The demonstration that deletion of either the SIN3 or the SAP30 gene (69) also promoted frameshifting and virus maintenance defects suggests a heterochromatin-associated defect. Processing of the 35S precursor rRNA was delayed in isogenic strains harboring mutant rpd3 alleles and in cells containing gene knockout alleles of SIN3 and SAP30. In actively growing cells, this delay in rRNA processing appears to be the primary event that results in a 60S ribosomal subunit biogenesis defect. The resulting unstable ribosomes have specific aminoacyl (aa)-tRNA binding defects that result in decreased peptidyltransferase activities. This in turn results in decreased rates of peptidyl transfer, allowing ribosomes stalled at the −1 PRF signal more time to slip. We suggest that the heterochromatin-associated Rpd3p/Sin3p/Sap30p complex (69) could be physically involved in helping to coordinate a very early and critical step in the ribosome biogenesis program. Alternatively, elimination of the function of this complex may result in repression of synthesis of the snoRNAs responsible for modification of bases in the large-subunit rRNA that are associated with the A site, resulting in the observed phenotypic defects.
MATERIALS AND METHODS
Strains, oligonucleotides, sequencing, media, enzymes, and genetic methods.
The S. cerevisiae strains used in this study are presented in Table 1. Oligonucleotides were synthesized and purchased from IDT and are listed in Table 2. DNA sequence analysis was performed by the Robert Wood Johnson Medical School DNA Synthesis and Sequencing Laboratory. Escherichia coli strains DH5α and MV1190 were used to amplify plasmids, and E. coli transformations were performed by using the standard calcium chloride method as described previously (60). Yeast were transformed by using the alkali cation method (36). YPAD, YPG, SD, synthetic complete medium (H−), and 4.7MB plates for testing the killer phenotype were used (82). Cytoduction of L-A and M1 viruses from strain JD758 into rho-o strains was done as previously described (20). Restriction enzymes were obtained from Promega, MBI Fermentas, and Roche. T4 DNA ligase and T4 DNA Pol were obtained from Roche, and precision Taq Pol was obtained from Qiagen. Radioactive nucleotides were obtained from NEN. Michael Hampsey generously provided the rpd3Δ, sin3Δ, and sap30Δ yeast strains, and the transcription derepression-defective ume6 strains were a gift from Andrew Vershon.
TABLE 1.
Yeast strains used in this study
| Strain | Description | Source |
|---|---|---|
| 5X47 | Standard diploid killer tester | J. D. Dinman |
| JD758 | 3164 MATakar1-1 arg1 [L-AHN M1] | J. D. Dinman |
| JD469-2D | MATα leu2-1::pJD85 ura3 his4 mof6-1 | J. D. Dinman |
| JD932D | MATaade2-1 trp1-1 ura3-1 leu2-3,112 his3-11,15 can1-100 [L-AHN M1] | J. D. Dinman |
| LNY95 | MATaura3-SK1 leu2-hisG trp1-hisG lys2-SK1 ho::LYS2 ade3-210S | L. Neigeborn |
| JD972A | MATaura3-SK1 leu2-hisG trp1-hisG lys2-SK1 ho::LYS2 ade3-210SPEX6::URA3 | This study |
| YMH171 | MATα ura3-52 leu2-3,112 his3 trp1Δ | M. Hampsey |
| YMH265 | MATα ura3-52 leu2-3,112 his3 trp1Δ sin3::LEU2 | M. Hampsey |
| YMH270 | MATα ura3-52 leu2-3,112 his3 trp1Δ rpd3::LEU2 | M. Hampsey |
| YMH277 | MATα ura3-52 leu2-3,112 his3 trp1Δsap30::LEU2 | M. Hampsey |
| AJ82 | MATα trp1 leu2 ura3 his4 UME6 | A. Vershon |
| AJ82 11-2 | MATα trp1 leu2 ura3 his4 ume6-11-2 | A. Vershon |
| AJ82 66-2 | MATα trp1 leu2 ura3 his4 ume6-66-1 | |
| AJ82 77-2 | MATα trp1 leu2 ura3 his4 ume6-72-2 |
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| Sequencing primers | |
| Reverse primer | 5′ TTCACACAGGAAACAG 3′ |
| Universal primer | 5′ GTAAAACGACGGCCAGT 3′ |
| RPD3 sequencing oligonucleotide 1 | 5′ GCCGCATAGAATAAGAATGG 3′ |
| RPD3 sequencing oligonucleotide 2 | 5′ GGTTCAAACACAGATCTATACG 3′ |
| RPD3 sequencing oligonucleotide 3 | 5′ GCTGTCGTGTTACAGTGTGG 3′ |
| PCR primersa | |
| 1 (forward primer XhoI) | 5′ CCCCCTCGAGTGTCCCATATTTTGCCTTG 3′ |
| 2 (reverse primer PstI) | 5′ CCCCCTGCAGTTGTCATGCTCAACATGTAGG 3′ |
| 3 (forward primer KpnI) | 5′ CCCCGGTACCTCATGTAGCCAATTGCTACAC 3′ |
| 4 (reverse primer SalI) | 5′ CCCCGTCGACTCAAAATTAGCTCTCACCGC 3′ |
| 5 (forward primer XhoI) | 5′ CCCCCTCGAGTCAAATAAGTTGCATTGTTCG 3′ |
| 6 (reverse primer PstI) | 5′ CCCCCTGCAGTCAAAAGCTATCCTGGCAGA 3′ |
| Oligonucleotide H151Ab | 5′ GCTTCCGATTTTTTTGCAGCATGCAAACCACCCGC 3′ |
Primers 1 and 2, 3 and 4, and 5 and 6 were used to clone rpd3, PEX6, and AAD14 alleles, respectively, from genomic DNA.
Mutagenized bases are underlined.
To monitor temperature sensitivity, cells were grown to saturation and equal numbers, spotted on selective medium, and grown at either the permissive temperature (30°C) or the restrictive temperature (37°C) for 3 days. Lack of growth or severely reduced growth was indicative of temperature sensitivity. Similarly, 10-fold serial dilutions of cells were spotted onto medium containing sparsomycin (5 μg/ml), anisomycin (5 μg/ml), or paromomycin (800 μg/ml) for monitoring of sensitivities to these drugs. To monitor sensitivity to cycloheximide, cells were grown to saturation at an optical density at 595 nm (OD595) of 0.1 and were spread on selective medium. A sterile filter disk containing 100 ng of cycloheximide was placed in the center of each plate. Cells were grown for 3 days, and zones of growth inhibition were measured.
The killer virus assay was done by replica plating S. cerevisiae colonies onto 4.7MB plates (82) with a freshly seeded lawn of strain 5X47 (0.5 ml of a suspension at 1 U of OD550 per ml per plate). After 2 to 3 days at 20°C, killer activity was observed as a zone of growth inhibition around the killer colonies. dsRNAs of L-A and M1 viruses were prepared as described previously (26, 46) and separated by electrophoresis through 1.0% nondenaturing agarose gels and visualized by ethidium bromide staining. RNA blotting was used as previously described (20) to monitor the abundance of L-A and M1 dsRNAs.
Plasmid and strain constructs, PRF, and transcriptional derepression assays.
A YCp50-based S. cerevisiae genomic library was purchased from the American Type Culture Collection (58). The genomic clone that complemented the mof6-1 ts− phenotype was given the name pJD155. Subclones of pJD155 were generated by partial digestion with EcoRI and self ligated by using a Roche rapid ligation kit. The pRS series of plasmids have been previously described (9, 63). Full-length RPD3, PEX6, and AAD14 genes were amplified from genomic DNA from JD932D by PCR using the oligonucleotide primers 1 and 2 (rpd3 alleles), 3 and 4 (PEX6), and 5 and 6 (AAD14) (Table 2) and cloned into pRS314 and pRS316 and were then designated pRPD3, pPEX6, and pAAD14, respectively. The mof6-1 allele was amplified from genomic DNA obtained from strain JD469-2C by using primers 1 and 2. PCRs using the oligonucleotide primers 1 and 2 were carried out under the following conditions: denaturation of dsDNA for 30 s at 95°C, annealing at 48°C for 45 s, and elongation for 4 min. PCR products were purified by using a Qiagen kit, digested with XhoI and PstI, and ligated into pRS314 or pRS316 (63). PCRs using oligonucleotide primers 3 and 4 (PEX6) or 5 and 6 (AAD14) were carried out under the following conditions: denaturation of dsDNA for 30 s at 95°C, annealing for 45 s at 48°C, and elongation for 6 min. PCR products were purified by using a Qiagen kit, digested with KpnI and SalI (PEX6) or XhoI and PstI (AAD14), and ligated into pRS314. To make pHDAC1, the 1.5-kb BamHI fragment containing the HDAC1 cDNA (a generous gift from S. L. Schreiber) was excised from pBJ5/HDAC1-F (70) and inserted into BamHI-digested pG-1 (61), thus placing the human gene under control of the constitutive yeast PGK1 promoter. The synthetic oligonucleotide H151A (Table 2) was used to create pH151A by use of standard methods (43). Subcloning PEX6 into pRS306 (63) generated plasmid pPEX6, which was used to integrate URA3 into the PEX6 locus of yeast strain LNY95. pRPD3, pSIN3, and pSAP30 were generous gifts from M. Hampsey.
Plasmids p−1 and p0, which were used to monitor PRF, have been described previously (73). Briefly, in these plasmids, transcription is driven from the yeast PGK1 promoter into an AUG translational start site. The E. coli lacZ gene serves as the enzymatic reporter, and transcription termination utilizes the yeast PGK1 transcriptional terminator. In the p0 plasmids, lacZ is in the 0 frame with respect to the translational start site, and measurement of β-galactosidase activity generated from cells transformed with these plasmids serves to represent the 0-frame controls. In the p−1 series, lacZ is in the −1 frame with respect to the translational start site and is 3′ of the L-A programmed −1 ribosomal frameshift signal such that β-galactosidase can only be produced as a consequence of a programmed −1 ribosomal frameshift. The efficiency of PRF was calculated by determining the ratio of β-galactosidase activity produced by cells harboring p−1 to the β-galactosidase activity produced by cells harboring p0 and multiplying this ratio by 100. All assays were performed in triplicate.
Plasmids pAV73 and pAV138 were used to monitor transcriptional derepression in cells harboring alleles of RPD3 (76). pAV73 is a URA3 2μm CYC-LacZ fusion reporter plasmid that is used as a control to establish a baseline. PAV138 contains a URS1 site from the HOP1 promoter cloned into the XhoI site of pAV73. It represses lacZ expression in a Ume6p/Sin3p/Rpd3p-dependent manner. The pAV73/pAV138 ratios of β-galactosidase activities were used to calculate the ability of the RPD3 variants to derepress lacZ reporter gene transcription as previously described (76). All assays were performed in triplicate.
In vivo [35S]methionine incorporation.
Labeled methionine incorporation assays were performed as previously described (7). Briefly, isogenic rpd3Δ strains containing wild-type pRPD3 or pmof6-1 were grown in 30 ml of medium lacking methionine and tryptophan at 30°C to an OD595 of 1.0. Unlabeled methionine was added to a concentration of 50 μM, and [35S]methionine and [35S]cysteine (Expre35S35S Label; NEN Life Science Products) were added to each culture to final specific activities of 1.1 μCi/ml. Samples were harvested at 0 min and at 15-min intervals for 60 min. Incorporation of the [35S] labels was monitored by cold trichloroacetic acid (TCA) precipitation. For each time point, 1.2-ml aliquots were harvested, from which 0.2 ml was used to determine the OD595 of the cultures. One milliliter of ice-cold 20% TCA was added to the remaining 1 ml of each aliquot, incubated on ice for 10 min, heated to 70°C for 20 min, and filtered through prewet Whatman GF/C filters. Filters were sequentially washed with 10 ml of ice-cold 5% TCA and 10 ml of 95% ethanol and dried, and the radioactivity of samples was determined by scintillation counter. All time points were taken in triplicate.
Pulse-chase labeling of rRNA.
Pulse-chase labeling with l-[methyl-3H]methionine was carried out on the isogenic wild-type, mof6-1, rpd3Δ, rpd3-H151A, sin3Δ, and sap30Δ strains as previously described (23, 45). Twenty thousand counts per minute per sample were resolved on a 1.2% formaldehyde-agarose gel. Labeled RNAs were transferred to a zeta probe membrane (Bio-Rad), sprayed with En3Hance (Dupont), and exposed to X-ray film.
Polysome and two-dimensional NEPHGE analyses.
For polysome analyses, cytoplasmic extracts, prepared as described by Baim et al. (2), were fractionated on 7 to 47% sucrose gradients buffered with 50 mM Tris-acetate (pH 7.4), 50 mM NH4Cl, 12 mM MgCl2, and 1 mM dithiothreitol (DTT). Gradients were centrifuged in an SW41 rotor at 40,000 rpm for 135 min at 4°C, fractionated, and analyzed by continuous monitoring of A254 (55). For nonequilibrium pH gradient gel electrophoresis (NEPHGE) analyses, 40S and 60S ribosomal subunits were separated by ultracentrifugation through a 7 to 27% sucrose gradient in the presence of 500 mM EDTA. Purified ribosomal subunits (≈195 μg/sample) were separated by NEPHGE (pH gradient of 3.5 to 11.5% in the first dimension and 12.5% polyacrylamide separating gel in the second dimension) and were visualized by silver staining by the Kendrick Laboratories (Madison, Wis.).
Preparation of tRNAs and of donor and acceptor fragments.
Yeast tRNAs were charged with [14C]phenylalanine as previously described (32, 50). Briefly, a 400-μl reaction mix composed of 200 μg of yeast tRNA (Sigma), 25 mM Tris-HCl (pH 7.5), 20 mM MgCl2, 10 mM ATP, 50 nmol of phenylalanine (313 mCi/mmol; NEN), and 50 μl of aa-tRNA synthetase (9 U/ml; Sigma) was incubated for 25 min at 37°C. After addition of 40 ml of 3 M sodium acetate (pH 5.0), the mixture was extracted twice with an equal volume of water-saturated phenol and once with chloroform. It was then precipitated with 2.5 volumes of ethanol at −20°C for 1 h. After centrifugation for 10 min, the pellet was resuspended in 50 ml of 2 mM potassium acetate (pH 5.0). [14C]Phe-tRNAPhe was separated from uncharged tRNAs by using DEAE Sephadex as previously described (57). Acetylation of charged tRNAs was performed as previously described (30). Briefly, [14C]Phe-tRNAPhe was resuspended in 200 μl of 0.2 M sodium acetate (pH 5.0), followed by addition of 2.5 μl of acetic anhydride. After a 1-h incubation on ice, another 2.5 μl of acetic anhydride was added, and incubation at 0°C was done for an additional hour. The tRNA was precipitated by addition of 2.5 volumes of ethanol. The [14C]Phe-tRNAPhe and acetyl-[14C]Phe-tRNAPhe were subsequently digested with 500 U of RNase T1 in 200 μl of 0.3 M sodium acetate (pH 5.0) for 1 h at 37°C, and the reaction mixtures were purified by using DEAE Sephadex as previously described (57). The resulting A site-specific [14C]Phe-CACCA (acceptor) and P site-specific acetyl-[14C]Phe-CACCA (donor) fragments were used as substrates for the tRNA fragment binding assays.
Purification of ribosomes and tRNA binding assays.
Salt-washed ribosomes were purified as previously described (48, 50). Briefly, yeast cells were grown in 0.5 liters of YPAD overnight, collected by centrifugation, and washed twice with buffer A (20 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 0.25 M sucrose). The cell pellet was suspended in 20 ml of buffer A, 30 g of glass beads (0.45-mm diameter) were added, and cells were disrupted by vortexing. The yeast lysate was centrifuged twice for 15 min at 15,000 rpm in a Sorvall S34 rotor, and the supernatant was pelleted at 100,000 × g for 3 h. The pellet was suspended in 6 ml of buffer B (20 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 0.25 M sucrose, 0.5 M KCl) and placed on a cushion of 3 ml of buffer C (20 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 1 M sucrose, 0.5 M KCl). After centrifugation at 50,000 rpm for 4 h (SW55 Ti), the pellet was dissolved in 1 ml of buffer A. After a clarifying spin for 1 min in a microcentrifuge, OD260 readings were taken (1 A260 unit = 19 pmol of ribosomes [1]). The protein content of ribosomes was also estimated by using protein assay reagent (Bio-Rad). Ribosomes were suspended in buffer A at a concentration of 4 pmol/μl and stored at −70°C.
The whole-tRNA and tRNA fragment binding assays were performed by following a modification of previously published protocols (32, 50). Briefly, ribosomes (400 pmol) were incubated with 800 pmol of whole tRNAs or donor or acceptor fragments in 500 μl of a buffer containing 70 mM Tris-acetate (pH 7.2), 40 mM magnesium acetate, 0.4 M potassium acetate, and 50 mM NH4Cl. Ethanol was added to a final concentration of 30%, and 20-μl aliquots were taken during the time course at 24°C. Samples were diluted to 1 ml with cold buffer (50 mM Tris-HCl [pH 7.2], 0.4 M KCl, 40 mM MgCl2, 30% ethanol), immediately precipitated onto a Millipore filter, washed with 1 ml of the dilution buffer, and counted in a scintillation counter. The reaction mix without ribosomes was used as the control. All assays were performed in triplicate.
Puromycin reaction with tRNA fragments.
Puromycin reactions were performed as previously described (14), with slight modifications. Ribosomes (20 pmol) were incubated with 5 pmol of CACCA[14C]AcPhe (682 dpm/pmol) in 300 μl of PR buffer (25 mM HEPES-KOH [pH 7.4], 135 mM NH4Cl, 250 mM KCl, 20 mM MgCl2, 33% EtOH) at 0°C for 10 min. Puromycin was added to final concentrations of 1 mM, and reaction mixes were incubated on ice. At indicated time points, 50-μl aliquots were taken, and reactions were stopped by the addition of equal volumes of a 0.3 M sodium acetate solution saturated with MgSO4. Puromycin was extracted with 1 ml of ethyl acetate, and the radioactivity was determined by liquid scintillation counting. In all of the experiments, controls were analyzed in the absence of puromycin to determine the nonspecific extraction of CACCA[14C]AcPhe. Control values (generally less than 2%) were subtracted from the values obtained in the presence of puromycin.
RESULTS
mof6-1 is an allele of RPD3.
The ts− phenotype of mof6-1 cells (20) provided a simple selective trait for the cloning of the wild-type gene. mof6-1 cells (JD469-2D) transformed with a YCp50-based genomic library (58) were replica plated to selective medium and subsequently shifted to nonpermissive temperature (37°C). Approximately 1.2 × 104 colonies were screened (4.8 genome equivalents), and three colonies that grew at the restrictive temperature were isolated. Positive plasmids were rescued from yeast into E. coli, and reintroduction into mof6-1 cells confirmed their abilities to confer growth at the nonpermissive temperature. The inserts in all of the genomic clones were approximately 13 kb in length, and sequence analysis mapped them all to the same region of chromosome XIV. One of the genomic clones, pJD155, was used for the subsequent characterization of MOF6. Meiotic linkage analysis was used to ascertain whether pJD155 harbored MOF6 as opposed to a second site suppressor. The URA3 gene was inserted into the PEX6 locus of a MOF6 ura3 strain (JD972A) (Fig. 1A), providing a scorable phenotype for genetic linkage analyses. Diploid cells (JD972A × JD469-2D) were sporulated, and the genotypes of 26 tetrads were determined. All of the tetrads scored as parental ditypes, i.e., 2:2 segregation of Ura+ ts+:Ura− ts−. The absence of crossover events demonstrates tight genetic linkage between MOF6 and the site of URA3 integration (PEX6), confirming that MOF6 was present in the yeast genomic DNA insert of pJD155.
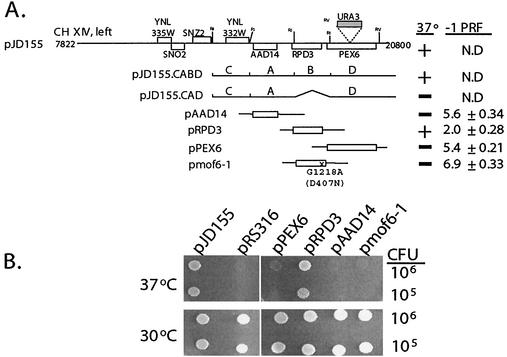
FIG. 1.
mof6-1 is an allele of RPD3. (A) Cloning of MOF6. At the top is the schematic representation of the ≈13-kb insert isolated from the YCp50 plasmid library (pJD155). Locations of all of the intact ORFs contained in this clone are represented as boxes. The URA3 gene is shown with a shaded box and the location of its insertion into PEX6 in strain JD972A is indicated. The subclones of the plasmid pJD155 and of PCR-generated clones of AAD14, RPD3, PEX6, and the mof6-1 allele of RPD3 are indicated at left, and their effects on growth and −1 ribosomal frameshifting efficiency (−1 PRF) are shown at right. The molecular lesion in the mof6-1 allele, a G-to-A transition at position 1218 of the coding sequence, is shown. This results in a change of aspartic acid to asparagine at amino acid 407. N.D, not determined; A, B, C, and D, the four EcoRI restriction fragments revealed by partial restriction analysis of pJD155 (see Results). (B) Complementation of the temperature-sensitive phenotype by RPD3. mof6-1 strains (JD469-2D) harboring the indicated clones were spotted onto selective medium and incubated at either the permissive (30°C) or the nonpermissive temperature (37°C) for 4 days.
Partial restriction analysis of pJD155 revealed four EcoRI restriction fragments, which were designated A, B, C, and D based on their relative electrophoretic mobilities (Fig. 1A). All subclones generated from partial EcoRI digestion which did not contain the B fragment were unable to complement the temperature-sensitive phenotype (e.g., pJD155.CAD [Fig. 1A]). Sequence analysis revealed that the B fragment contained RPD3. To determine whether MOF6 is RPD3 or whether the B fragment contained other genetic information required for transcription initiation or 3′-end formation of flanking genes, clones of the individual ORFs that were present on the genomic clone were generated by PCR as described in Materials and Methods (Fig. 1A). mof6-1 cells harboring either pJD155 or pRPD3 but not pPEX6 or pAAD14 were able to grow at restrictive temperatures (Fig. 1). Furthermore, an RPD3 clone generated from mof6-1 genomic DNA, pmof6-1, was not able to complement the ts− phenotype (Fig. 1), confirming that RPD3 is both necessary and sufficient to complement the mof6-1 ts− phenotype.
PRF assays were used to examine whether pRPD3 could complement the mof6-1 frameshifting defect. The frameshift test plasmids p−1 and the 0-frame control p0 were introduced into mof6-1 cells harboring pRPD3, pPEX6, pAAD14, or pmof6-1, and the effects on −1 PRF were assayed. Whereas introduction of the wild-type gene pRPD3 restored −1 PRF efficiencies to wild-type levels (approximately 2.0%), frameshifting efficiencies remained elevated in cells harboring the other clones (Fig. 1A). Sequence analysis of mof6-1 clones isolated from three independent PCRs revealed the presence of a single base transition, G1218A, corresponding to a change at the amino acid level of aspartic acid to asparagine (GAT → AAT) at position 407, approximately 30 residues from the C terminus of the protein (data not shown). A ClustalW analysis (71) revealed that that yeast has an acidic residue at this position while the RPD3 homologs from humans, mice, and Arabdopsis contain the basic arginine, suggesting that there may be a requirement for a charged residue in this environment. Unfortunately, the lack of structural information pertaining to this region of the protein precludes any further speculation on the functional role of this amino acid residue.
Two additional plasmid-borne rpd3 alleles were constructed for further study. Since the mof6-1 mutation did not occur in the putative deacetylation motif (41), we constructed an allele in this domain by changing the histidine at position 151 to an alanine residue (pH151A), which has previously been shown to nearly eliminate Rpd3p deacetylase activity (40). In addition, since HDAC1 is the most homologous to RPD3 among the at least seven different human histone deacetylases (70, 79), we used a clone in which transcription of the human HDAC1 cDNA was driven from the yeast PGK1 promoter (pHDAC1). In order to further characterize mof6-1 independently of strain-specific background effects, all subsequent experiments were performed with plasmid-borne alleles in the rpd3::LEU2 gene disruption strain YMH270. The resulting isogenic strains were subsequently transformed with the p−1 and p0 frameshift test vectors, and −1 PRF efficiencies were determined. Frameshifting efficiencies were significantly elevated in cells harboring pmof6-1, pH151A, and vector alone, while addition of the wild-type gene or the human homolog reduced −1 PRF efficiencies to wild-type levels (Fig. 2A).
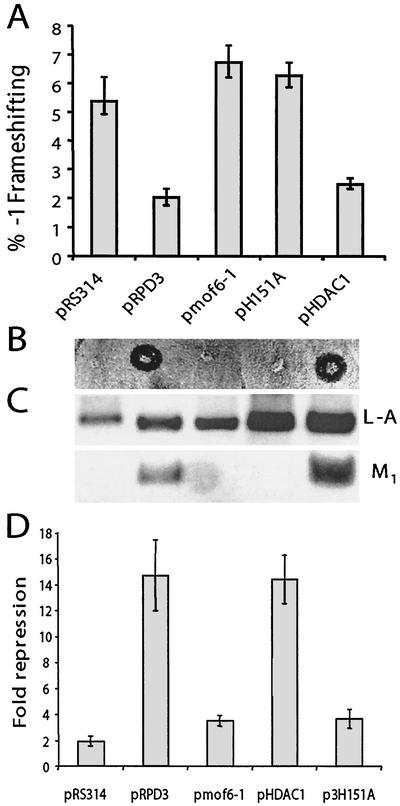
FIG. 2.
RPD3 allele-specific effects on programmed −1 ribosomal frameshifting, killer virus maintenance, transcriptional repression, and sensitivity to cycloheximide. Isogenic rpd3Δ cells (YMH270) were transformed with vector alone (pRS314), clones expressing variations of RPD3 (the wild-type, mof6-1, and H151A alleles of RPD3), or a clone expressing the human homolog, HDAC1. Colonies were selected on synthetic complete medium lacking tryptophan. (A) Functional Rpd3p is required to maintain appropriate levels of programmed −1 ribosomal frameshifting. pRS426-based versions of the frameshift test plasmids p0 and p−1 were introduced into cells, and transformants were selected on medium lacking tryptophan and uracil. Programmed −1 ribosomal frameshifting efficiencies were determined as described in Materials and Methods. All assays were performed in triplicate, and percent errors are indicated with error bars. (B and C) Functional Rpd3p is required to maintain the killer phenotype and the M1 satellite virus of L-A. The L-A and M1 viruses were introduced from JD758 into the isogenic strains harboring the various RPD3 variants by cytoplasmic mixing (20). The killer phenotypes are shown (B). Total nucleic acids were extracted from these cells, separated through a 1.0%nondenaturing agarose gel, denatured in the gel, transferred to a nylon membrane, and hybridized with L-A- and M1-specific (+) strand probes (C). Hybridizing bands were visualized by autoradiography. The positions of L-A and M1 are shown. (D) The transcriptional derepression phenotype of the mof6-1 allele is similar to those of the other loss-of-RPD3-function alleles. Derepression phenotypes of the RPD3 variants were determined as described in Materials and Methods.
To examine the effects of the different rpd3 alleles on killer virus maintenance, L-A and M1 viruses were first introduced by cytoplasmic mixing into the rpd3Δ strain harboring the wild-type RPD3 gene on a URA3-based CEN plasmid, and stable Killer+ colonies were identified. The resulting strain was then transformed with low-copy TRP1 vectors harboring the different RPD3 alleles or with a vector control. In parallel to the frameshifting results, increased −1 frameshifting efficiencies correlated with loss of the killer phenotype (Fig. 2B) and with loss of the M1 satellite virus (Fig. 2C). The results of assays for transcriptional repression (Fig. 2D) and cycloheximide hypersensitivity (data not shown) also demonstrated correlations between these classic rpd3-associated phenotypes and defects in programmed −1 ribosomal frameshifting. In addition, introduction of pmof6-1 into wild-type cells had no effect on −1 frameshifting efficiencies, demonstrating that this does not represent a gain-of-function allele (data not shown). Taken as a whole, these data define mof6-1, rpd3-H151A, and rpd3Δ as mof-specific alleles of RPD3.
Correlation of growth and frameshifting defects in the rpd3 mutants.
It was observed that the initial appearance and subsequent growth of colonies of rpd3Δ cells transformed with the various plasmid-borne mutant alleles of rpd3 was delayed relative to that of cells containing the wild-type gene. Measurement of growth rates revealed significant quantitative differences. In logarithmic growth, the doubling times of cells harboring either vector alone or the pH151A allele were approximately 1.4-fold longer than that of wild-type controls (doubling time of 3.5 h for cells harboring pRS314 or pH151A versus 2.5 h for cells harboring pRPD3). The growth defect was even greater in mof6-1 cells, where the doubling time was increased approximately 1.6-fold compared with that of isogenic wild-type cells (4.0 versus 2.5 h). Shifting of cells from stationary-phase growth to fresh medium and subsequent monitoring of cell growth rates revealed that the mutants also exhibited significantly different effects on the quality of growth compared with those of isogenic wild-type controls. Particularly striking was the observation that the mutants remained in lag-phase growth for approximately 2 h longer than did wild-type controls (Fig. 3A), suggestive of a defect in the ability of the biosynthetic apparatus to respond to the presence of a rich nutrient source. Similarly, the onset of diauxic shift occurred approximately 2 h earlier for cells harboring pmof6-1 and 1 h earlier for cells harboring pH151A and vector alone than for cells expressing the wild-type gene (Fig. 3A). This result suggests inefficient utilization of carbon source by the mutants.
FIG. 3.
Correlation of growth and frameshifting defects in the rpd3 mutants. (A) rpd3 mutants display delayed exit from lag phase and early entry into diauxic shift. Stationary-phase cultures of isogenic rpd3Δ strains harboring vector alone (pRS314) or the indicated plasmid-borne alleles of RPD3 were diluted into complete synthetic medium lacking tryptophan to 0.3 U of OD550/ml and grown at 30°. At 1-h time points thereafter, aliquots of cells were sampled and OD550 readings were determined. Data shown represent the means of each time point through three repetitions of the experiment. Standard deviations were <5%. Solid arrows indicate the approximate point of exit from lag phase, and dotted arrows indicate diauxic shift. (B) Decreased rates of protein synthesis in cells expressing the Mof6-1p form of Rpd3p.[35S]methionine and [35S]cysteine were added to mid-logarithmically growing isogenic rpd3Δ strains containing wild-type pRPD3 or pmof6-1, and samples were harvested at 0 min and at 15-min intervals for 60 min. Incorporation of the [35S] labels was monitored by cold TCA precipitation as described in Materials and Methods. The data were plotted by using the formula y = mx + B, where m is the slope, x is the sample, and B is the y intercept. Rates of protein synthesis correspond to m. All time points were taken in triplicate. (C) Frameshifting defects in the rpd3 mutants are maximized when demand for ribosomes is greatest. Programmed −1 ribosomal frameshifting efficiencies were determined in isogenic rpd3Δ strains harboring vector alone (pRS314) or the indicated plasmid-borne alleles of RPD3 during the three different phases of cell growth. La, lag-phase growth; Lo, log-phase growth; Dx, after diauxic shift. All assays were performed in triplicate. Error bars denote percent error.
Given the original translation-associated defect of mof6-1, we examined whether these cells exhibited any gross defect in protein synthesis. Rates of incorporation of [35S]-labeled methionine and cysteine into newly synthesized protein in mid-logarithmically growing cells were determined as described in Materials and Methods. The results (Fig. 3B) demonstrate that rates of protein synthesis in mof6-1 cells were approximately 75% of that in wild-type cells.
Previous experiments have demonstrated that programmed −1 ribosomal frameshifting efficiencies remain stable throughout the growth cycle in wild-type cells (15). In light of the effects of the mutants on cell growth, −1 PRF efficiencies were monitored during lag phase, log phase, and after diauxic shift in isogenic rpd3Δ cells harboring vector alone, pRPD3, pmof6-1, and pH151A. The results of these experiments show that −1 PRF defects were maximized in the mutants in lag phase, becoming less severe as cells progressed through the growth curve (Fig. 3C). The effect was most notable in mof6-1 cells. The results suggest that the frameshifting defects were maximized in the mutants when demand for newly synthesized ribosomes was the greatest and that −1 frameshifting efficiencies decreased in parallel with the demand for new ribosomes. That no such effect was observed in wild-type cells is in line with the bioeconomic model of regulation of ribosome biosynthesis (80) and suggests a defect in the regulation of ribosome biosynthesis (see below).
Deletion of other genes linked to heterochromatin-associated functions also results in the Mof− phenotype.
Deacetylation of histones by Rpd3p promotes local chromatin condensation, resulting in transcriptional repression of nearby RNA Pol II-transcribed genes (reviewed in reference 10). Although Rpd3p is able to deacetylate histones in vitro, in vivo deacetylation of histones by Rpd3p requires the cofactors. Mutations in any of the components of the Rpd3p/Sin3p/Ume6p repression complex lead to gene-specific derepression of RNA Pol II-regulated genes and concomitant transcriptional activation (33, 40). Conversely, mutations in any of the components of the Rpd3p/Sin3p/Sap30p repression complex lead to the opposite effect, i.e., enhanced transcriptional silencing of RNA Pol II-transcribed genes artificially inserted into heterochromatin contexts (69).
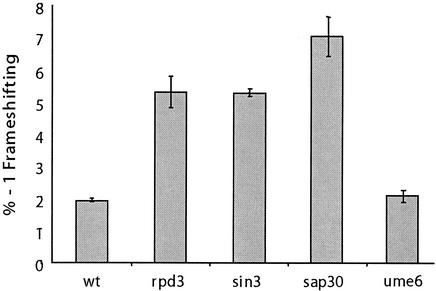
Given the model describing the histone deacetylase complex, if mof6-1 is acting through either of these complexes, then sin3 mutants would also exhibit Mof− phenotypes. Furthermore, if the effect is on a heterochromatin-associated function, e.g., transcription or maturation of rRNAs, then sap30 mutants should also promote increased −1 PRF efficiencies. Conversely, ume6 mutants should promote increased −1 PRF efficiencies if the effect is on genes found in euchromatin, e.g., RP genes. Figure 4 shows that deletion of either SIN3 or SAP30 resulted in increased −1 PRF efficiencies, while three separate ume6 alleles did not. The sin3Δ and sap30Δ strains also had significant killer virus maintenance defects, whereas ume6 strains were able to stably maintain the killer virus (data not shown). These results demonstrate that (i) the histone deacetylation apparatus is involved in a process that results in a specific translational fidelity defect and (ii) the effect is likely to involve a process in the heterochromatin environment.
FIG. 4.
Deletion of other genes linked to heterochromatin-associated functions also results in frameshifting defects. The frameshift test vectors p0 and p−1 were introduced into isogenic wild-type (YMH171), rpd3Δ (YMH270), sin3Δ (YMH265), and sap30Δ (YMH277) strains, along with four strains harboring different alleles of UME6 (AJ82, AJ82 11-2, AJ82 66-2, and AJ82 77-2), and programmed −1 ribosomal frameshift efficiencies were determined. All assays were performed in triplicate. Error bars denote percent error.
Mutation of genes involved in the histone deacetylation apparatus results in 60S ribosomal subunit biogenesis defects.
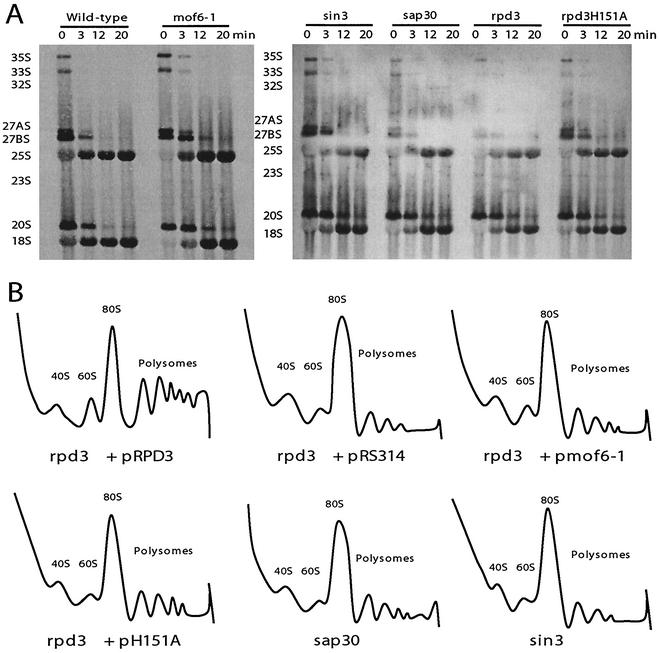
That a defect involving heterochromatin should result in a translational fidelity defect suggested a ribosome biogenesis defect involving rRNA transcription or processing. Given the involvement of the histone deacetylase complex in transcription-associated processes, the effects of these alleles on rRNA transcription and processing were examined by pulsing cells with [3H]methylmethionine, which specifically labels the methylated RNAs, the most abundant of which are those transcribed from the 35S operon. Though no differences were observed with regard to either the rates of 35S pre-rRNA synthesis or its eventual maturation to 18S and 25S rRNAs, the amount of time required for the initial processing step of the 35S pre-rRNA in the mutant cells was delayed by approximately 3 min compared with that for wild-type controls (Fig. 5A). A steady-state analysis revealed that there was no accumulation of any precursors in the mutant cells (data not shown). Polysome analyses of ribosomes isolated from isogenic wild-type, rpd3Δ, mof6-1, rpd3-H151A, sin3Δ, and sap30Δ strains were suggestive of biogenesis defects in the 60S ribosome subunits, as evidenced by decreased levels of 60S ribosomal subunits, increased areas under the 80S peaks, and decreased polysome peaks (Fig. 5B).
FIG. 5.
Mutations of genes involved in histone deacetylation result in ribosome biogenesis defects. (A) Delayed 35S rRNA processing. Pulse-chase labeling with l-[methyl-3H]methionine was carried out on the isogenic wild-type, mof6-1, rpd3Δ, rpd3-H151A, sin3Δ, and sap30Δ strains as previously described (23, 45). Twenty thousand counts per minute per sample were resolved on a 1.2% formaldehyde-agarose gel. Labeled RNAs were transferred to a zeta-probe membrane, sprayed with En3Hance, and exposed to X-ray film. (B) 60S ribosomal subunit biogenesis and polysome defects. Cytoplasmic extracts from isogenic strains were fractionated though sucrose gradients as described in Materials and Methods. Gradients were centrifuged in an SW41 rotor at 40,000 rpm for 135 min at 4°C, fractionated, and analyzed by continuous monitoring of A254 (55).
Ribosome biogenesis defects are not due to global defects in RP expression.
It has previously been found that defects in specific RPs result in increased PRF efficiencies (50, 56). Thus, one possible explanation for the observed effects could be that these alleles promote altered expression of RPs. To examine this possibility, approximately 200-μg samples of 60S and 40S ribosomal subunits isolated from isogenic wild-type and mutant cells were separated in two dimensions by NEPHGE, and RPs were visualized by silver staining. No gross differences in the staining patterns were observed between wild-type and mutant samples (data not shown). These results demonstrate that the effects of the rpd3 mutants on PRF and ribosome biogenesis are not due to defects in the synthesis of RPs.
Mutants result in aa-tRNA binding defects.
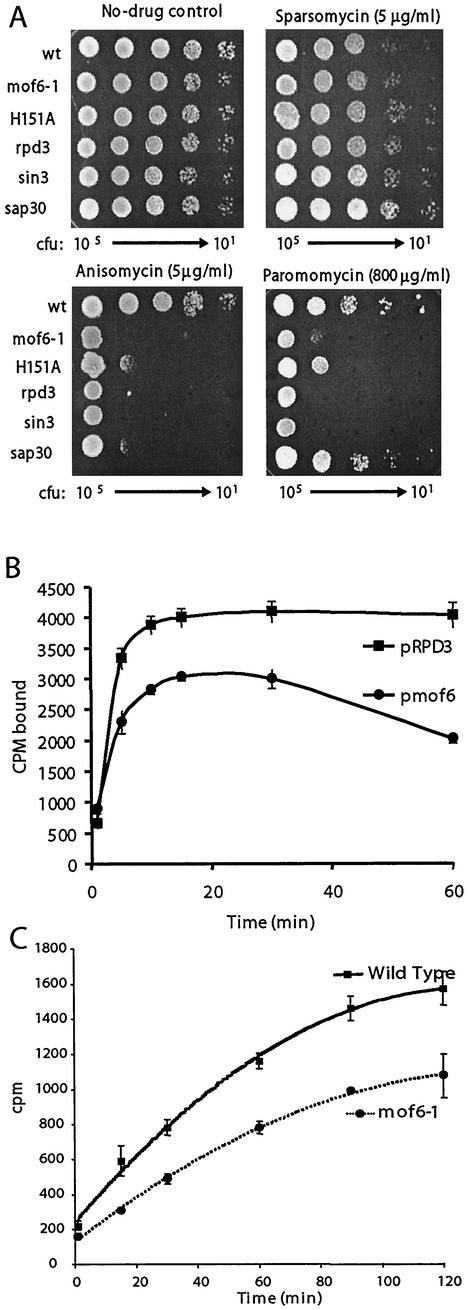
One previously unexplained phenotype of rpd3 mutants has been their sensitivity to cycloheximide, a translational inhibitor (78). In light of our data showing that these classes of mutants promote a ribosome biogenesis defect specific to 60S subunits, we employed a pharmacogenetic approach using well-characterized antibiotics to investigate the specificity of the defects. Sparsomycin, which increases the affinity of ribosomes for the 3′ (donor) end of peptidyl-tRNAs (34, 39, 51) was used as a P site-specific probe. Anisomycin, which decreases ribosomal affinities for the 3′ (acceptor) ends of aa-tRNAs (6, 29, 62), and paromomycin, which stabilizes binding of near-cognate tRNAs in the decoding center of the small-subunit rRNA (8, 54, 77) served as probes for A site-specific defects. Figure 6A shows that although sparsomycin had no effect relative to the wild type on cells harboring the various rpd3 alleles (rpd3Δ, mof6-1, and rpd3-H151A) or the sin3Δ and sap30Δ mutants, all of the mutants were hypersensitive to anisomycin and all but sap30Δ were hypersensitive to paromomycin. These data indicate that the translational defect caused by mutations in these genes is specific to the ribosomal A site. To further investigate the biochemical basis for these observations, tRNA binding experiments were performed comparing isogenic wild-type and mof6-1 ribosomes. Although no differences were observed in the binding of the 3′ ends of either donor or acceptor fragments (data not shown), the binding profiles for intact aa-tRNAs were significantly different (Fig. 6B). Specifically, mof6-1 ribosomes had decreased initial rates of aa-tRNA binding and had lower overall affinities for aa-tRNAs. In addition, precipitous drop-off in aa-tRNA binding at the 60-min time point suggests that mof6-1 ribosomes are less stable than their wild-type counterparts.
FIG. 6.
The mutants promote ribosomal A site-specific and peptidyl transfer defects. (A) Pharmacogenetic analyses show hypersensitivity to A site-specific drugs. Tenfold dilutions (from 105 to 101 CFU) of isogenic wild-type, rpd3Δ, mof6-1, rpd3-H151A, sin3Δ, and sap30Δ strains were spotted onto selective (H−Trp) medium containing the indicated concentrations of drugs or no-drug controls and incubated at 30°C for 3 days. (B) Ribosomes from mof6-1 cells have decreased binding for aa-tRNA. Ribosomes purified from isogenic wild-type and mof6-1 strains were incubated with molar excess amounts of purified [14C]Phe-tRNA, and salt-washed ribosomes were diluted at the indicated time points and quickly filtered through nitrocellulose filters. After drying, the filters were counted by liquid scintillation. Assays were performed in triplicate. Data points and error bars indicate means and standard deviations. (C) Ribosomes from mof6-1 cells have decreased peptidyltransferase activities. Time course of the formation of [14C]phenylalanine-puromycin product in assays using ribosomes isolated from isogenic rpd3Δ cells expressing wild-type or the mof6-1 forms of Rpd3p. Ethyl acetate soluble radioactivity was determined by liquid scintillation counting. Control studies were performed in the absence of puromycin to determine the nonspecific extraction of CACCA[14C]AcPhe. Control values (generally less than 2%) were subtracted from the values obtained in the presence of puromycin. All experiments were performed in triplicate. Data points and error bars indicate means and standard deviations.
Ribosomes from mof6-1 cells have decreased peptidyltransferase activities.
It has previously been demonstrated that peptidyl transfer defects can specifically promote increased −1 PRF efficiencies (17, 49). It is possible that a defect in binding of aa-tRNA could result in diminished peptidyltransferase activities. This in turn might enable elongating ribosomes to pause longer at the programmed −1 ribosomal frameshift signal, providing them with more time to shift. To test this hypothesis, we compared the peptidyltransferase activities of ribosomes isolated from isogenic wild-type and mof6-1 strains by using the puromycin reaction. Figure 6C shows that ribosomes purified from mof6-1 cells have significantly reduced peptidyltransferase activities compared with that of wild-type controls. These findings illuminate the biochemical basis for the Mof− phenotypes of these cells.
DISCUSSION
mof6-1 was originally isolated as a recessive mutation in S. cerevisiae that promoted increased efficiencies of programmed −1 ribosomal frameshifting and rendered cells unable to maintain the killer virus (20). In the present study, we show that MOF6 is a unique allele of RPD3, that it does not represent a gain-of-function allele, and that the deacetylase function of Rpd3p is required for maintenance of wild-type levels of frameshifting and maintenance of the yeast killer virus. The role of RPD3 in the specific aspect of translational fidelity described here explains the previously noted hypersensitivity to cycloheximide in other alleles of this gene. Furthermore, the ability of the closest human homolog to complement the translational fidelity and virus maintenance defect demonstrates that, in addition to the conservation of its function in the regulation of RNA Pol II transcription, its role in processes involved in the biogenesis of the protein translational machinery has also been preserved.
Specific interactions of the Rpd3p-Sin3p complex with other factors, e.g., Ume6p or Sap30p, have been genetically shown to have differential effects on RNA Pol II-transcribed genes in either euchromatin or heterochromatin environments (69). Our observation that mutants of RPD3, SIN3, and SAP30, but not of UME6, affect programmed −1 ribosomal frameshifting and virus maintenance suggests that these translation-associated defects are due to effects in the heterochromatin environment. Though previous work has shown that deletion of RPD3, SIN3, or SAP30 promotes repression of RNA Pol II-transcribed reporters that have been artificially inserted into the RDN1 locus, the fact that there was no quantitative effect on rates of 35S rRNA synthesis argues against the observed effects on ribosome biogenesis and function being a consequence of a simple rRNA transcriptional defect. Rather, we have shown that the defect is a delay at the earliest stage in the 35S pre-rRNA processing program. Since processing of the 35S pre-rRNA is cotranscriptional (25) and since the histone deacetylase complex is known to influence chromatin structure, one possible explanation for our observations could be that a change (or the lack thereof) in the heterochromatin topological environment is perturbing an early function of the apparatus, e.g., the 60S processosome (22), that is required for assembly of the large ribosomal subunit.
In light of the well-defined steps involved in rRNA maturation (for reviews, see references 42, 44, and 75), our data suggest the possibility that the defect may be at the level of rRNA base modification, e.g., 2′-O-ribose methylation and/or pseudouridylation. These types of base modification have been specifically shown to localize to functional regions of the ribosome (13). Of particular interest with regard to the ribosomal A site-specific defect observed here are the large numbers of modified bases clustered in regions of the large-subunit rRNA that are associated with the A site-aa-tRNA interactions and the peptidyltransferase center (reviewed in references 13 and 53). These include (i) the peptidyltransferase center core region; (ii) the region of helix 38 that forms an “A-minor motif” with 5S rRNA (52); (iii) helix 69, which appears to form an important bridge between the aa- and peptidyl-tRNAs (66); and (iv) the A loop at the end of helix 92 (though the lack of effect of mof6-1 ribosomes on acceptor fragment binding argues against the defect affecting this particular structure). Thus, an alternative to the altered heterochromatin topology hypothesis could be that a deficiency in the histone deacetylation machinery could result in repression of the RNA Pol II-transcribed box C+D and/or box H+ACA snoRNAs, which act as essential guides for base-specific rRNA modification (reviewed in references 13 and 53), resulting in the observed delay in 35S rRNA processing. Future studies will investigate this hypothesis directly, examining the base modification status of rRNAs in mof6-1 mutants, the abundances of these snoRNAs, and the frameshifting and virus maintenance phenotypes of mutants defective in these processes.
Whatever its origin, the early delay in rRNA maturation affects a series of downstream processes involved in the biogenesis and functionality of ribosomes. These effects are specific to the formation and/or the function of the A site. The resulting ribosomes are less accurate than their wild-type counterparts and have decreased peptidyltransferase activities. The presence of these specific, rather than global, effects on ribosome function also provides strong arguments against the hypothesis that the observed frameshifting defects may be due to global misregulation of RP biosynthesis or to derepression of the lacZ frameshift reporter mRNAs. We hypothesize that the decreased rates of peptidyl transfer allow ribosomes with both A and P sites occupied by tRNAs to pause for longer periods of time at −1 frameshift signals, promoting increased −1 PRF efficiencies. Alternatively, decreased affinities of the mutant ribosomes for aa-tRNAs may make this tRNA more inclined to slip. Either of these mechanisms can account for the specific effect of mof6-1 on programmed −1 ribosomal frameshifting and virus maintenance (20, 31).
Our observations also suggest that a functional histone deacetylase complex is essential for the proper timing and extent of downstream rRNA processing events and ribosome maturation. The delayed exit from lag-phase growth in the mutant cells suggests that limiting the availability of this complex is rate limiting with regard to these events. Corollary to this is that under such rate-limiting conditions, as the demand for new ribosomes outpaces the ability of the cell to supply them, the cell would tend to produce a greater fraction of defective ribosomes in an attempt to keep up with demand. That the frameshifting defect is accentuated when the requirement for ribosomes is highest supports this bioeconomic hypothesis. Furthermore, the early entry of the mutants into diauxic shift shows that these cells are depleting the growth medium of carbon source at an accelerated rate. We hypothesize that functionally compromised ribosomes produce a large amount of inaccurately translated, inactive protein products. These dead-end protein products would be shunted to the protein degradation pathway, imposing a nonproductive energetic load on the mutant cells. By such a scenario, the bioenergetic cost per functional protein product would be substantially increased, which would account for the observation that these cells deplete the growth medium of carbon source and enter diauxic shift more rapidly than their wild-type counterparts.
Acknowledgments
A.M. and J.L.B. contributed equally to this work.
We thank Andrew Vershon, Michael Hampsey, Lenore Neigeborn, and S. L. Shreiber for strains and clones and for helping to provide critical insights.
This work was supported by a grant to J.D.D. from the NIH (GM58859). J.L.B. was supported in part by a training grant from the NIH (T32 AI51967).
REFERENCES
- 1.Abraham, A. K., and A. Pihl. 1983. Effect of protein synthesis inhibitors on the fidelity of translation in eukaryotic systems. Biochim. Biophys. Acta 741:197-203. [DOI] [PubMed] [Google Scholar]
- 2.Baim, S. B., D. F. Pietras, D. C. Eustice, and F. Sherman. 1985. A mutation allowing an mRNA secondary structure diminishes translation of Saccharomyces cerevisiae iso-1-cytochrome c. Mol. Cell. Biol. 5:1839-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasundaram, D., J. D. Dinman, C. W. Tabor, and H. Tabor. 1994. Two essential genes in the biosynthesis of polyamines that modulate +1 ribosomal frameshifting in Saccharomyces cerevisiae. J. Bacteriol. 176:7126-7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brierley, I. 1995. Ribosomal frameshifting on viral RNAs. J. Gen. Virol. 76:1885-1892. [DOI] [PubMed] [Google Scholar]
- 5.Bussey, H. 1991. K1 killer toxin, a pore-forming protein from yeast. Mol. Microbiol. 5:2339-2343. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco, L., M. Barbacid, and D. Vazquez. 1973. The tricodermin group of antibiotics, inhibitors of peptide bond formation by eukaryotic ribosomes. Biochim. Biophys. Acta 312:368-376. [DOI] [PubMed] [Google Scholar]
- 7.Carr-Schmid, A., N. Durko, J. Cavallius, W. C. Merrick, and T. G. Kinzy. 1999. Mutations in a GTP-binding motif of eukaryotic elongation factor 1A reduce both translational fidelity and the requirement for nucleotide exchange. J. Biol. Chem. 274:30297-30302. [DOI] [PubMed] [Google Scholar]
- 8.Carter, A. P., W. M. Clemons, D. E. Brodersen, R. J. Morgan-Warren, B. T. Wimberly, and V. Ramakrishnan. 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407:340-348. [DOI] [PubMed] [Google Scholar]
- 9.Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Yeast 110:119-122. [DOI] [PubMed] [Google Scholar]
- 10.Courey, A. J., and S. Jia. 2001. Transcriptional repression: the long and the short of it. Genes Dev. 15:2786-2796. [DOI] [PubMed] [Google Scholar]
- 11.Cui, Y., J. D. Dinman, T. G. Kinzy, and S. W. Peltz. 1998. The Mof2/Sui1 protein is a general monitor of translational accuracy. Mol. Cell. Biol. 18:1506-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui, Y., J. D. Dinman, and S. W. Peltz. 1996. mof4-1 is an allele of the UPF1/IFS2 gene which affects both mRNA turnover and −1 ribosomal frameshifting efficiency. EMBO J. 15:5726-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decatur, W. A., and M. J. Fournier. 2002. rRNA modifications and ribosome function. Trends Biochem. Sci. 27:344-351. [DOI] [PubMed] [Google Scholar]
- 14.Diedrich, G., C. M. Spahn, U. Stelzl, M. A. Schafer, T. Wooten, D. E. Bochkariov, B. S. Cooperman, R. R. Traut, and K. H. Nierhaus. 2000. Ribosomal protein L2 is involved in the association of the ribosomal subunits, tRNA binding to A and P sites and peptidyl transfer. EMBO J. 19:5241-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinman, J. D., T. Icho, and R. B. Wickner. 1991. A −1 ribosomal frameshift in a double-stranded RNA virus forms a gag-pol fusion protein. Proc. Natl. Acad. Sci. USA 88:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinman, J. D., and T. G. Kinzy. 1997. Translational misreading: mutations in translation elongation factor 1α differentially affect programmed ribosomal frameshifting and drug sensitivity. RNA 3:870-881. [PMC free article] [PubMed] [Google Scholar]
- 17.Dinman, J. D., M. J. Ruiz-Echevarria, K. Czaplinski, and S. W. Peltz. 1997. Peptidyl transferase inhibitors have antiviral properties by altering programmed −1 ribosomal frameshifting efficiencies: development of model systems. Proc. Natl. Acad. Sci. USA 94:6606-6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinman, J. D., M. J. Ruiz-Echevarria, and S. W. Peltz. 1998. Translating old drugs into new treatments: identifying compounds that modulate programmed −1 ribosomal frameshifting and function as potential antiviral agents. Trends Biotechnol. 16:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinman, J. D., and R. B. Wickner. 1992. Ribosomal frameshifting efficiency and gag/gag-pol ratio are critical for yeast M1 double-stranded RNA virus propagation. J. Virol. 66:3669-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinman, J. D., and R. B. Wickner. 1994. Translational maintenance of frame: mutants of Saccharomyces cerevisiae with altered −1 ribosomal frameshifting efficiencies. Genetics 136:75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinman, J. D., and R. B. Wickner. 1995. 5S rRNA is involved in fidelity of translational reading frame. Genetics 141:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dragon, F., J. E. Gallagher, P. A. Compagnone-Post, B. M. Mitchell, K. A. Porwancher, K. A. Wehner, S. Wormsley, R. E. Settlage, J. Shabanowitz, Y. Osheim, A. L. Beyer, D. F. Hunt, and S. J. Baserga. 2002. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417:967-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunbar, D. A., S. Wormsley, T. M. Agentis, and S. J. Baserga. 1997. Mpp10p, a U3 small nucleolar ribonucleoprotein component required for pre-18S rRNA processing in yeast. Mol. Cell. Biol. 17:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farabaugh, P. J. 1996. Programmed translational frameshifting. Microbiol. Rev. 60:103-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fath, S., P. Milkereit, A. V. Podtelejnikov, N. Bischler, P. Schultz, M. Bier, M. Mann, and H. Tschochner. 2000. Association of yeast RNA polymerase I with a nucleolar substructure active in rRNA synthesis and processing. J. Cell Biol. 149:575-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried, H. M., and G. R. Fink. 1978. Electron microscopic heteroduplex analysis of “killer” double-stranded RNA species from yeast. Proc. Natl. Acad. Sci. USA 75:4224-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujimura, T., and R. B. Wickner. 1992. Interaction of two cis sites with the RNA replicase of the yeast L-A virus. J. Biol. Chem. 267:2708-2713. [PubMed] [Google Scholar]
- 28.Gesteland, R. F., and J. F. Atkins. 1996. Recoding: dynamic reprogramming of translation. Annu. Rev. Biochem. 65:741-768. [DOI] [PubMed] [Google Scholar]
- 29.Grollman, A. P. 1967. Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. J. Biol. Chem. 242:3226-3233. [PubMed] [Google Scholar]
- 30.Haenni, A. L., and F. Chapeville. 1966. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim. Biophys. Acta 114:135-148. [DOI] [PubMed] [Google Scholar]
- 31.Harger, J. W., A. Meskauskas, and J. D. Dinman. 2002. An “integrated model” of programmed ribosomal frameshifting and post-transcriptional surveillance. Trends Biochem. Sci. 27:448-454. [DOI] [PubMed] [Google Scholar]
- 32.Harris, R., and S. Pestka. 1973. Studies on the formation of transfer ribonucleic acid-ribosome complexes. XXIV. Effects of antibiotics on binding of aminoacyl-oligonucleotides to ribosomes. J. Biol. Chem. 248:1168-1174. [PubMed] [Google Scholar]
- 33.Hassig, C. A., T. C. Fleischer, A. N. Billin, S. L. Schreiber, and D. E. Ayer. 1997. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 89:341-347. [DOI] [PubMed] [Google Scholar]
- 34.Herner, A. E., I. H. Goldberg, and L. B. Cohen. 1969. Stabilization of N-acetylphenylalanyl transfer ribonucleic acid binding to ribosomes by sparsomycin. Biochemistry 8:1335-1344. [DOI] [PubMed] [Google Scholar]
- 35.Icho, T., and R. B. Wickner. 1989. The double-stranded RNA genome of yeast virus L-A encodes its own putative RNA polymerase by fusing two open reading frames. J. Biol. Chem. 264:6716-6723. [PubMed] [Google Scholar]
- 36.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacks, T., H. D. Madhani, F. R. Masiraz, and H. E. Varmus. 1988. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell 55:447-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacks, T., and H. E. Varmus. 1985. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science 230:1237-1242. [DOI] [PubMed] [Google Scholar]
- 39.Jayaraman, J., and I. H. Goldberg. 1968. Localization of sparsomycin action to the peptide-bond-forming step. Biochemistry 7:418-421. [DOI] [PubMed] [Google Scholar]
- 40.Kadosh, D., and K. Struhl. 1998. Histone deacetylase activity of Rpd3 is important for transcriptional repression in vivo. Genes Dev. 12:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasten, M. M., S. Dorland, and D. J. Stillman. 1997. A large protein complex containing the yeast Sin3p and Rpd3p transcriptional regulators. Mol. Cell. Biol. 17:4852-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kressler, D., P. Linder, and C. J. de La Cruz. 1999. Protein trans-acting factors involved in ribosome biogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:7897-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunkel, T. 1985. Rapid and efficient site-specific mutagenesis without phenotype selection. Proc. Natl. Acad. Sci. USA 82:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leary, D. J., and S. Huang. 2001. Regulation of ribosome biogenesis within the nucleolus. FEBS Lett. 509:145-150. [DOI] [PubMed] [Google Scholar]
- 45.Lee, S. J., and S. J. Baserga. 1999. Imp3p and Imp4p, two specific components of the U3 small nucleolar ribonucleoprotein that are essential for pre-18S rRNA processing. Mol. Cell. Biol. 19:5441-5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liermann, R. T., J. D. Dinman, L. A. Sylvers, and J. C. Jackson. 2000. Improved purification of the double-stranded RNA from killer strains of yeast. BioTechniques 28:64-65. [DOI] [PubMed] [Google Scholar]
- 47.Lopinski, J. D., J. D. Dinman, and J. A. Bruenn. 2000. Kinetics of ribosomal pausing during programmed −1 translational frameshifting. Mol. Cell. Biol. 20:1095-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merrick, W. C. 1979. Assays for eukaryotic protein synthesis. Methods Enzymol. 60:108-123. [DOI] [PubMed] [Google Scholar]
- 49.Meskauskas, A., and J. D. Dinman. Decreased peptidyltransferase activity correlates with increased programmed −1 ribosomal frameshifting and viral maintenance defects in the yeast Saccharomyces cerevisiae. RNA, in press. [DOI] [PMC free article] [PubMed]
- 50.Meskauskas, A., and J. D. Dinman. 2001. Ribosomal protein L5 helps anchor peptidyl-tRNA to the P-site in Saccharomyces cerevisiae. RNA 7:1084-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moazed, D., and H. F. Noller. 1991. Sites of interaction of the CCA end of peptidyl-tRNA with 23S rRNA. Proc. Natl. Acad. Sci. USA 88:3725-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nissen, P., J. A. Ippolito, N. Ban, P. B. Moore, and T. A. Steitz. 2001. RNA tertiary interactions in the large ribosomal subunit: the A-minor motif. Proc. Natl. Acad. Sci. USA 98:4899-4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ofengand, J. 2002. Ribosomal RNA pseudouridines and pseudouridine synthases. FEBS Lett. 514:17-25. [DOI] [PubMed] [Google Scholar]
- 54.Ogle, J. M., D. E. Brodersen, W. M. Clemons, Jr., M. J. Tarry, A. P. Carter, and V. Ramakrishnan. 2001. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292:897-902. [DOI] [PubMed] [Google Scholar]
- 55.Ohtake, Y., and R. B. Wickner. 1995. Yeast virus propagation depends critically on free 60S ribosomal subunit concentration. Mol. Cell. Biol. 15:2772-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peltz, S. W., A. B. Hammell, Y. Cui, J. Yasenchak, L. Puljanowski, and J. D. Dinman. 1999. Ribosomal protein L3 mutants alter translational fidelity and promote rapid loss of the yeast killer virus. Mol. Cell. Biol. 19:384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pestka, S., T. Hishizawa, and J. L. Lessard. 1970. Studies on the formation of transfer ribonucleic acid-ribosome complexes. 8. Aminoacyl oligonucleotide binding to ribosomes: characteristics and requirements. J. Biol. Chem. 245:6208-6219. [PubMed] [Google Scholar]
- 58.Rose, M. D., P. Novick, J. H. Thomas, D. Botstein, and G. R. Fink. 1987. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60:237-243. [DOI] [PubMed] [Google Scholar]
- 59.Ruiz-Echevarria, M. J., J. M. Yasenchak, X. Han, J. D. Dinman, and S. W. Peltz. 1998. The Upf3p protein is a component of the surveillance complex that monitors both translation and mRNA turnover and affects viral maintenance. Proc. Natl. Acad. Sci. USA 95:8721-8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 61.Schena, M., and K. Yamamoto. 1988. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science 241:965-967. [DOI] [PubMed] [Google Scholar]
- 62.Schindler, D. 1974. Two classes of inhibitors of peptidyl transferase activity in eukaryotes. Nature 249:38-41. [DOI] [PubMed] [Google Scholar]
- 63.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith, M. W., A. Meskauskas, P. Wang, P. V. Sergiev, and J. D. Dinman. 2001. Saturation mutagenesis of 5S rRNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:8264-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Somogyi, P., A. J. Jenner, I. A. Brierley, and S. C. Inglis. 1993. Ribosomal pausing during translation of an RNA pseudoknot. Mol. Cell. Biol. 13:6931-6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stark, H., M. V. Rodnina, H. J. Wieden, F. Zemlin, W. Wintermeyer, and M. van Heel. 2002. Ribosome interactions of aminoacyl-tRNA and elongation factor Tu in the codon-recognition complex. Nat. Struct. Biol. 9:849-854. [DOI] [PubMed] [Google Scholar]
- 67.Struhl, K. 1998. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12:599-606. [DOI] [PubMed] [Google Scholar]
- 68.Suka, N., A. A. Carmen, S. E. Rundlett, and M. Grunstein. 1998. The regulation of gene activity by histones and the histone deacetylase RPD3. Cold Spring Harbor Symp. Quant. Biol. 63:391-399. [DOI] [PubMed] [Google Scholar]
- 69.Sun, Z. W., and M. Hampsey. 1999. A general requirement for the Sin3-Rpd3 histone deacetylase complex in regulating silencing in Saccharomyces cerevisiae. Genetics 152:921-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taunton, J., C. A. Hassig, and S. L. Schreiber. 1996. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272:408-411. [DOI] [PubMed] [Google Scholar]
- 71.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tu, C., T.-H. Tzeng, and J. A. Bruenn. 1992. Ribosomal movement impeded at a pseudoknot required for ribosomal frameshifting. Proc. Natl. Acad. Sci. USA 89:8636-8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tumer, N. E., B. Parikh, P. Li, and J. D. Dinman. 1998. Pokeweed antiviral protein specifically inhibits Ty1 directed +1 ribosomal frameshifting and Ty1 retrotransposition in Saccharomyces cerevisiae. J. Virol. 72:1036-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tzeng, T.-H., C. L. Tu, and J. A. Bruenn. 1992. Ribosomal frameshifting requires a pseudoknot in the Saccharomyces cerevisiae double-stranded RNA virus. J. Virol. 66:999-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Venema, J., and D. Tollervey. 1999. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 33:261-311. [DOI] [PubMed] [Google Scholar]
- 76.Vershon, A. K., N. M. Hollingsworth, and A. D. Johnson. 1992. Meiotic induction of the yeast HOP1 gene is controlled by positive and negative regulatory sites. Mol. Cell. Biol. 12:3706-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vicens, Q., and E. Westhof. 2001. Crystal structure of paromomycin docked into the eubacterial ribosomal decoding A site. Structure 9:647-658. [DOI] [PubMed] [Google Scholar]
- 78.Vidal, M., and R. F. Gaber. 1991. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:6317-6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang, A. H., N. R. Bertos, M. Vezmar, N. Pelletier, M. Crosato, H. H. Heng, J. Th'ng, J. Han, and X. J. Yang. 1999. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol. Cell. Biol. 19:7816-7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warner, J. R. 1999. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24:437-440. [DOI] [PubMed] [Google Scholar]
- 81.Wickner, R. B. 1996. Double-stranded RNA viruses of Saccharomyces cerevisiae. Microbiol. Rev. 60:250-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wickner, R. B., and M. J. Leibowitz. 1976. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics 82:429-442. [DOI] [PMC free article] [PubMed] [Google Scholar]