Abstract
Optimizing pharmacokinetic/pharmacodynamic indices of antibiotics to obtain clinical and microbiological efficacy is essential, but dosing regimens must also be tailored to minimize the risk for emergence of resistance. The aim of the present study was to investigate whether certain concentrations of benzylpenicillin are critical for the selection of resistant subpopulations. A mixed culture of Streptococcus pneumoniae containing ca. 90% susceptible (MIC = 0.031 mg/liter), 9% intermediate (MIC = 0.25 mg/liter), and 1% resistant (MIC = 8 mg/liter) was studied in an in vitro kinetic model. The time that concentrations exceeded the MIC (T>MIC) for the three strains in the culture was varied by different initial concentrations of benzylpenicillin. Samples for viable counts were withdrawn at different times during 24 h and seeded on blood agar plates and on selective antibiotic-containing plates. The T>MIC varied from 46 to 100% for the susceptible strain, from 6 to 100% for the intermediate strain, and from 0 to 48% for the resistant strain. Our study, which may mimic the clinical situation with carriage of a mixed population of S. pneumoniae with different antibiotic susceptibilities, has shown that selection of resistant bacteria may easily occur if dosing regimens are only targeted toward fully susceptible strains.
Streptococcus pneumoniae is the most significant bacterial cause of community-acquired respiratory tract infections (23). During the last decade, the spread of penicillin nonsusceptible pneumococci with increased MICs has created clinically significant treatment problems, especially in infections against which therapeutic concentrations are difficult to achieve, such as meningitis (1, 15-18, 28). Clinical and animal studies indicate, however, that by optimizing the dosing regimen, it may still be possible in many cases to use penicillin for treatment of S. pneumoniae with decreased susceptibility (3-5, 8-10, 12-14, 25). Dosing regimens should, however, be optimized not only to obtain a maximal antibacterial effect but also to reduce the risk for the emergence of resistance (2, 19, 24). Little is known about the selective properties of different antibiotic regimens in a mixed culture of bacteria with different antibiotic susceptibilities. The normal nasopharyngeal flora of patients may be simultaneously colonized with different types of S. pneumoniae having a variable resistance pattern. A certain dosage regimen could select more resistant strains even if these at the time constitute a minority for the original population (11, 21, 26, 27). The aim of the present investigation was to use an in vitro kinetic model to study the selective effects of different concentrations of benzylpenicillin on a culture containing a mixture of 90% penicillin-susceptible, 9% penicillin-intermediate, and 1% penicillin-resistant S. pneumoniae strains.
(This study was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, on 17 to 20 September 2000.)
MATERIALS AND METHODS
Antibiotic.
Benzylpenicillin was provided by AstraZeneca, Södertälje, Sweden. The antibiotic was obtained as a reference powder with known potency and dissolved in distilled water prior to each experiment.
Bacterial strains.
Three clinical strains of S. pneumoniae belonging to serotype 6B obtained from the Department of Microbiology, University Hospital, Reykjavik, Iceland, were used in the study: S. pneumoniae A 2000 (PSP), S. pneumoniae 9506 (PIP), and S. pneumoniae BCC-67 (PRP).
Determination of MICs.
The MICs for the three strains of S. pneumoniae were determined in fluid media by a macrodilution technique in duplicate on different occasions according to the National Committee for Clinical Laboratory Standards. Twofold serial dilutions of benzylpenicillin were added to broth and inoculated with a final inoculum of 105 CFU of the test strain per ml and incubated at 37°C in 5% CO2 for 20 h. The MIC was defined as the lowest concentration of the antibiotic allowing no visible growth. The MICs of erythromycin were determined with E-test (Biodisk, Solna, Sweden).
The MICs of penicillin were 0.031, 0.25, and 8 mg/liter, respectively. The PSP strain was erythromycin resistant (MIC > 128 mg/liter), whereas the two other strains were susceptible to erythromycin (MIC < 0.03 mg/liter).
Concentrations of benzylpenicillin.
The concentrations of benzylpenicillin were determined with a microbiological agar diffusion method using Bacillus stearothermophilus ATCC 3032 as the test organism. A standardized inoculum of spore suspension was mixed with tryptone-glucose agar (pH 7.4), and the mixture was poured into plates. After the plates were dried, antibiotic standards and samples were placed in agar wells at a volume of 0.01 ml or 0.03 ml (for lower concentrations). The assays were made in triplicate, and the plates were incubated overnight at 56°C. The lower limit of detection was 0.03 mg/liter and the coefficient of variation was ca. 10%. The concentrations of benzylpenicillin were determined during all experiments.
In vitro kinetic model.
A previously described model (20, 22) was used in these experiments. It consists of a 100-ml spinner flask with a 0.45-μm-pore-size filter membrane and a prefilter fitted in between the upper and bottom parts. A magnetic stirrer ensures homogeneous mixing of the culture and prevents membrane pore blockage. In one of the sidearms of the culture vessel, a silicon membrane is inserted to enable repeated sampling. A thin plastic tubing to a vessel containing fresh medium is connected the other arm. The medium is removed from the culture flask, through the filter, in order to prevent bacterial dilution, at a constant rate with a pump (P-500; Pharmacia Biotech, Uppsala, Sweden). Fresh sterile medium is sucked into the flask at the same rate by the negative pressure built up inside the culture vessel. The antibiotic is added to the vessel and eliminated at a constant rate according to the first-order kinetics: C = C0 × e−kt, where C0 is the initial antibiotic level, C is the antibiotic level at time t, k is the rate of elimination, and t is the time elapsing since the addition of antibiotic. The apparatus is placed in a thermostatic room at 37°C during the experiments.
Pharmacodynamics of different dosage regimens in a mixed culture.
Before the experiments, the strains were grown in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) for 6 h at 37°C in 5% CO2, resulting in ca. 5 × 108 CFU/ml. A mixed culture of the PSP (90%), PIP (9%), and PRP (1%) was prepared. The initial bacterial concentrations of the cultures in the kinetic model were ca. 5 × 104 CFU/ml, 5 × 103 CFU/ml, and 5 × 102 CFU/ml, respectively. The medium used was Todd-Hewitt broth saturated with 5% CO2. In all experiments, a half-life of 4 h for benzylpenicillin was used. This half-life was chosen in order to be able to compare the results with data from an in vivo model with implanted tissue cages in rabbits (14). The time that concentrations exceeded the MIC (T>MIC) for the strains in the culture mixture was varied by five different initial concentrations of benzylpenicillin (0.33, 1.5, 2.9, 10.2, and 53.5 mg/liter). Samples for viable counts were withdrawn at 0, 3, 6, 9, 12, and 24 h and seeded onto blood agar plates containing 100,000 IE of penicillinase (Difco Laboratories)/liter for the measurement of the total numbers of bacteria. Plates containing 10 mg of erythromycin/liter were used to count the susceptible strain, and selective plates with 0.062 and 1 mg of penicillin/liter were used to detect the PIP and PRP strains, respectively. At least three dilutions, the lowest being 0.01 ml, were drawn from each sampling and seeded onto the different plates, respectively. The limit of detection of the viable counts was 5 × 101 CFU/ml.
Controls were grown simultaneously in a flask, incubated in 5% CO2, with the same volume of broth and bacterial mixture as for the in vitro kinetic model. All experiments were done in triplicate.
RESULTS
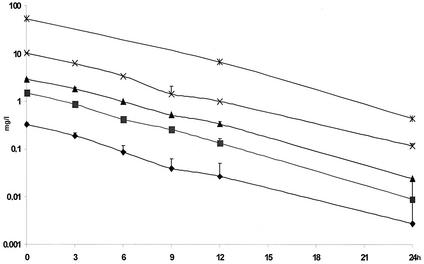
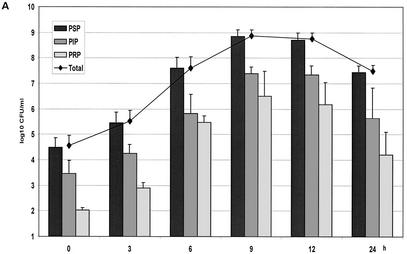
The different concentrations of benzylpenicillin are shown in Fig. 1. The concentrations of benzylpenicillin in the in vitro kinetic model were as expected, with little variation between the experiments. In the growth controls, maximal numbers of bacteria for all three strains were obtained at 9 h (Fig. 2A). The generation times, as calculated between 3 and 9 h, were 32, 36, and 34 min for PSP, PIP, and PRP, respectively. These differences yielded a minor shift in the proportions of the strains at 9 h. Between 12 and 24 h spontaneous autolysis occurred, which was more rapid for the nonsusceptible strains.
FIG. 1.
Concentrations of benzylpenicillin in the in vitro kinetic model. The mean ± the standard deviation is shown.
FIG. 2.
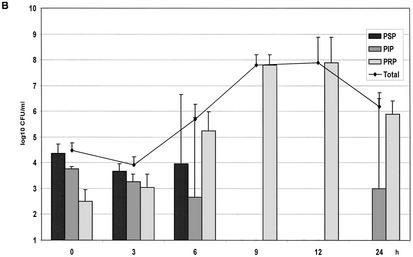
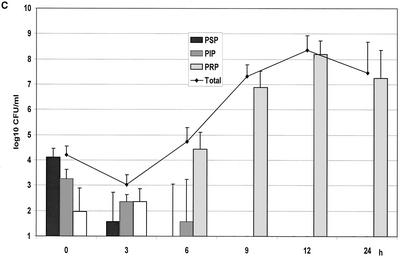
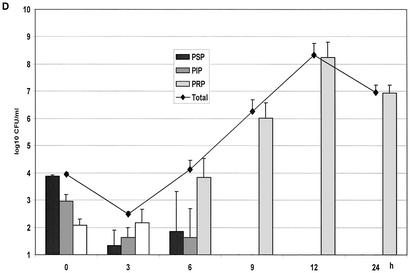
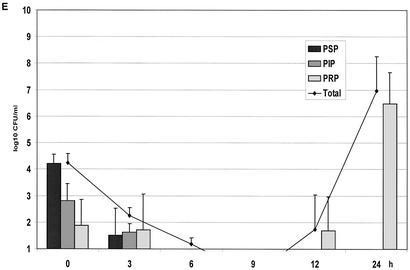
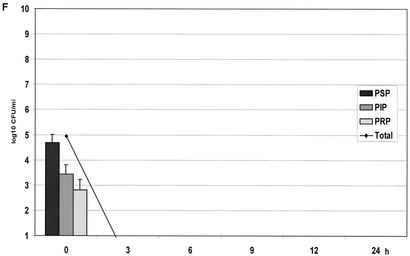
(A) Growth of the controls in the kinetic model. The mean ± the standard deviation is shown. (B) Effect of benzylpenicillin on PSP (T>MIC = 46%), PIP (T>MIC = 6%), and PRP (T>MIC = 0%). (C) Effect of benzylpenicillin on PSP (T>MIC = 75%), PIP (T>MIC = 38%), and PRP (T>MIC = 0%). (D) Effect of benzylpenicillin on PSP (T>MIC = 100%), PIP (T>MIC = 54%), and PRP (T>MIC = 0%). (E) Effect of benzylpenicillin on PSP (T>MIC = 100%), PIP (T>MIC = 83%), and PRP (T>MIC = 7%). (F) Effect of benzylpenicillin on PSP (T>MIC = 100%), PIP (T>MIC = 100%), and PRP (T>MIC = 48%).
The pharmacokinetic/pharmacodynamic (PK/PD) indices for the different strains in the vitro kinetic model are shown in Table 1.
TABLE 1.
PK/PD indices for the different strains of S. pneumoniaea
| Cmax |
Cmax/MIC
|
T>MIC24 (%)
|
AUC/MIC24
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| PSP | PIP | PRP | PSP | PIP | PRP | PSP | PIP | PRP | |
| 0.33 | 11 | 1.3 | 0 | 46 | 6 | 0 | 60 | 8 | 0.2 |
| 1.5 | 48 | 6 | 0 | 75 | 38 | 0 | 275 | 34 | 1.1 |
| 2.9 | 94 | 12 | 0 | 100 | 54 | 0 | 531 | 67 | 2 |
| 10.2 | 329 | 41 | 1.8 | 100 | 83 | 7 | 1,869 | 234 | 7.2 |
| 53.5 | 1,726 | 214 | 6.7 | 100 | 100 | 48 | 9,804 | 1,225 | 38 |
PSP, S. pneumoniae A 2000; PIP, S. pneumoniae 9506; PRP, S. pneumoniae BCC-67.
With initial concentrations of 0.33, 1.5, and 2.9 mg/liter, the concentrations were always below the MIC for the PRP strain. The lowest concentration yielded a T>MIC of 46% for PSP, and this strain became undetectable at 6 h and did not reappear. The PIP strain (T>MIC 6%) was below the detection limit at 9 h but reappeared at 24 h. The PRP strain became dominant from 6 h onward (Fig. 2B).
With an initial concentration of 1.5 mg/liter, yielding T>MIC values of 75 and 38% for the PSP and PIP strains, respectively, these strains were below the detection limit after 3 and 6 h, respectively. Also, at this concentration the resistant strain became dominant at 6 h (Fig. 2C).
The same pattern was seen at the concentration of 2.9 mg/liter with T>MIC values of 100 and 54% for PSP and PIP, respectively, whereas the PRP strain increased steadily during the 24 h (Fig. 2D).
With a concentration of 10.2 mg/liter, giving T>MIC values of 100 and 83% for the PSP and PIP strains, respectively, and of 7% for the PRP strain, all strains were below the detection limit at 6 h (Fig. 2E). However, regrowth of the PRP strain was noted at 12 and 24 h. At the highest dose with a T>MIC value of 100% for the PSP and PIP strains and a T>MIC value of 48% for the PRP strain, complete killing of all strains was achieved after 3 h (Fig. 2F).
DISCUSSION
The goal of antibiotic therapy should be both to optimize the eradication of the infecting pathogen and to minimize the risk for the emergence of resistant strains. The clinical and microbiological efficacy of β-lactam antibiotics seems to correlate with the time that free (non-protein-bound) concentrations exceed the MIC for the infecting pathogen (3, 5, 13). Reduced penicillin susceptibility in S. pneumoniae is caused by alterations of the bacterial penicillin-binding proteins, leading to a lower affinity for the drug. The design of rational optimal dosage regimens for the prevention of the selection of nonsusceptible strains requires that the PK/PD relationship for the elimination of such strains be determined. In animal studies, e.g., in neutropenic mice, a high survival rate has been noted if the concentrations of penicillins and cephalosporins in serum exceed the MIC for ca. 40 to 50% of the dosing interval both for penicillin-susceptible and -nonsusceptible strains (2, 12, 14). In a recent study using the same in vitro kinetic model, we showed that a T>MIC value of ca. 50% was required to obtain maximal efficacy of amoxicillin against a penicillin-susceptible and a penicillin-intermediate (MIC = 0.25 mg/liter) strain of S. pneumoniae. For a strain with an MIC of 2 mg/liter, a T>MIC of 60% was needed, but the Cmax was also important (20).
The importance of local resistance epidemiology and the pharmacodynamic properties of antibiotics for the determination of dosing strategies has also been demonstrated in clinical studies. High-dose intravenous therapy with penicillin is still considered to be effective in the management of pneumococcal pneumonia caused by strains with moderate reduced susceptibility (10, 25). Guillemot et al. (19) found a statistically significant increased risk for the carriage of penicillin-resistant S. pneumoniae in children treated with low doses of aminopenicillins and cephalosporins. Dagan et al. showed, in children with otitis media, a clear correlation between the degree of susceptibility to penicillin and the ability of oral cephalosporins to eradicate S. pneumoniae from middle-ear fluid. The eradication of bacteria correlated with the time that concentrations exceeded the MIC in serum (6, 7). Similar results were obtained in another study that included 248 children with otitis media receiving β-lactam antibiotics in which the overall success rate was 92% for penicillin-susceptible organisms but only 72% for those infected with penicillin-nonsusceptible strains (9).
Several mechanisms may be responsible for the association between recent antimicrobial therapy and nasopharyngeal carriage of resistant pneumococci. Unmasking of a minority subpopulation of resistant strains seems to be a plausible explanation for the recurrent infections with nonsusceptible pneumococci (11, 26). It has been demonstrated that the antibiotic treatment of otitis media could promote the growth of preexisting nonsusceptible organisms that may predispose to rapid superinfection of the middle ear (11). It is also known that several serotypes of S. pneumoniae can be carried simultaneously in the same individual. In such situations, one serotype is often predominant, and the minor population can only be detected if a large number of colonies are analyzed (21, 26). Negri et al. exposed a mixture of S. pneumoniae with different susceptibilities for penicillin to various constant concentrations of β-lactam antibiotics during 4 h and showed that certain antibiotic concentrations selected for low- and high-level resistant populations (24). However, in clinical practice, bacteria are exposed to fluctuating antibiotic concentrations. We have confirmed that nonsusceptible S. pneumoniae, although in low numbers in a mixed bacterial population, was easily selected by suboptimal dosing regimens of penicillin in an in vitro kinetic model. Although the design of our study did not allow us to determine the exact magnitude of the T>MIC needed to eliminate the different strains, no regrowth was noted when T>MIC was 48, 38, and 46% for the PSP, PIP, and PRP strains, respectively.
Resistance is an increasing public health problem that needs a multifaceted approach. Optimizing dosage regimens may be one factor of importance to minimize the emergence of resistant strains. Our study, which may mimic the clinical situation with a carriage of a mixed population of S. pneumoniae strains with different antibiotic susceptibilities, has shown that the selection of resistant bacteria may easily occur if dosing regimens are only targeted toward fully susceptible strains. The complex interaction between the pharmacological and microbiological factors involved in the selection of resistant bacteria at different infectious sites and in the commensal flora during antibiotic treatment needs to be explored in more detail.
Acknowledgments
This study was supported by the AFA Health Research Fund, Stockholm, Sweden, and by EU commission project QLRT-2000-00873.
We thank Anita Perols and Sara Olofsson for excellent technical assistance.
REFERENCES
- 1.Baquero, F. 1996. Epidemiology and management of penicillin-resistant pneumococci. Curr. Opin. Infect. Dis. 9:372-379. [Google Scholar]
- 2.Baquero, F., and M. C. Negri. 1997. Strategies to minimize the development of antibiotic resistance. J. Chemother. 9(Suppl. 3):29-37. [PubMed] [Google Scholar]
- 3.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: the rationale for antibacterial dosing in mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Choosing an antibiotic on the basis of pharmacodynamics. Ear Nose Throat J. 77:7-12. [PubMed] [Google Scholar]
- 5.Craig, W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 3):S233-S237. [DOI] [PubMed] [Google Scholar]
- 6.Dagan, R., O. Abramson, E. Leibovitz, R. Lang, S. Goshen, D. Greenberg, P. Yagupsky, A. Leiberman, and D. M. Fliss. 1996. Impaired bacteriologic response to oral cephalosporins in acute otitis media caused by pneumococci with intermediate resistance to penicillin. Pediatr. Infect. Dis. J. 15:980-985. [DOI] [PubMed] [Google Scholar]
- 7.Dagan, R., O. Abramson, E. Leibovitz, D. Greenberg, R. Lang, S. Goshen, P. Yagupsky, A. Leiberman, and D. M. Fliss. 1997. Bacteriologic response to oral cephalosporins: are established susceptibility breakpoints appropriate in the case of acute otitis media? J. Infect. Dis. 176:1253-1259. [DOI] [PubMed] [Google Scholar]
- 8.Dagan, R. 2000. Clinical significance of resistant organisms in otitis media. Pediatr. Infect. Dis. J. 19:378-382. [DOI] [PubMed] [Google Scholar]
- 9.Dagan, R., E. Leibovitz, A. Leiberman, and P. Yagupsky. 2000. Clinical significance of antibiotic resistance in acute otitis media and implication of antibiotic treatment on carriage and spread of resistant organisms. Pediatr. Infect. Dis. J. 19:S57-S65. [DOI] [PubMed] [Google Scholar]
- 10.Dagan, R., K. P. Klugman, W. A. Craig, and F. Baquero. 2001. Evidence to support the rationale that bacterial eradication in respiratory tract infection is an important aim of antimicrobial therapy. J. Antimicrob. Chemother. 47:129-140. [DOI] [PubMed] [Google Scholar]
- 11.Dagan, R., E. Leibovitz, G. Cheletz, A. Leiberman, and N. Porat. 2001. Antibiotic treatment in acute otitis media promotes superinfection with resistant Streptococcus pneumoniae carried before initiation of treatment. J. Infect. Dis. 183:880-886. [DOI] [PubMed] [Google Scholar]
- 12.Dahl Knudsen, J., N. Frimodt-Möller, and F. Espersen. 1995. Experimental Streptococcus pneumoniae infection in mice for studying correlation of in vitro and in vivo activities of penicillin against pneumococci with various susceptibilities to penicillin. Antimicrob. Agents Chemother. 39:1253-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drusano, G. L., and W. A. Craig. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J. Chemother. 9(Suppl. 3):38-44. [PubMed] [Google Scholar]
- 14.Erlendsdottir, H., J. Dahl-Knudsén, I. Odenholt, O. Cars, F. Espersen, N. Frimodt-Møller, K. Fuursted, K. G. Kristinsson, and S. Gudmunsson. 2001. Penicillin pharmacodynamics in four experimental pneumococcal infection models. Antimicrob. Agents Chemother. 45:1078-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedland, I. R., and K. P. Klugman. 1992. Failure of chloramphenicol therapy in penicillin-resistant pneumococcal meningitis. Lancet 339:405-408. [DOI] [PubMed] [Google Scholar]
- 16.Friedland, I. R., and K. P. Klugman. 1992. Antibiotic-resistant pneumococcal disease in South African children. Am. J. Dis. Child. 146:920-923. [DOI] [PubMed] [Google Scholar]
- 17.Friedland, I. R. 1995. Comparison of the response to antimicrobial therapy of penicillin-resistant and penicillin-susceptible pneumococcal disease. Pediatr. Infect. Dis. J. 14:885-890. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein, F. W., J. F. Acar, et al. 1996. Antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae: results of a 1992-93 Western Europe and USA collaborative study. J. Antimicrob. Chemother. 38(Suppl. A):71-84. [DOI] [PubMed] [Google Scholar]
- 19.Guillemot, D., C. Carbon, B. Balkau, P. Geslin, H. Lecoeur, F. Vauzelle-Kervroedan, G. Bouvenot, and E. Eschwege. 1998. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 279:365-370. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson, I., E. Löwdin, I. Odenholt, and O. Cars. 2001. Pharmacokinetic and pharmacodynamic parameters for antimicrobial effects of cefotaxime and amoxicillin in an in vitro kinetic model. Antimicrob. Agents Chemother. 45:2436-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huebner, R. E., R. Dagan, N. Porath, A. D. Wasas, and K. Klugman. 2000. Lack of utility of serotyping multiple colonies for detection of simultaneous nasopharyngeal carriage of different pneumococcal serotypes. Pediatr. Infect. Dis. J. 19:1017-1020. [DOI] [PubMed] [Google Scholar]
- 22.Löwdin, E., I. Odenholt, S. Bengtsson, and O. Cars. 1996. Pharmacodynamic effects of sub-MICs of benzylpenicillin against Streptococcus pyogenes in a newly developed in vitro kinetic model. Antimicrob. Agents Chemother. 40:2478-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mufson, M. A. 1990. Streptococcus pneumoniae, p. 1539-1551. In G. L. Mandell, R. G. Douglas, and J. E. Bennet (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, Inc., New York, N.Y.
- 24.Negri, M.-A., M. Morosini, E. Loza, and F. Baquero. 1994. In vitro selective antibiotic concentrations of β-lactams for penicillin-resistant Streptococcus pneumoniae populations. Antimicrob. Agents Chemother. 38:122-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pallares, R., J. Linares, M. Vadillo, C. Cabellos, F. Manresa, P. F. Viladrich, R. Martin, and F. Gudiol. 1995. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N. Engl. J. Med. 333:474-480. [DOI] [PubMed] [Google Scholar]
- 26.Schrag, S. J., B. Beall, and S. F. Dowell. 2000. Limiting the spread of resistant pneumococci: biological and epidemiologic evidence for the effectiveness of alternative interventions. Clin. Microbiol. Rev. 13:588-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinke, D., and P. Davey. 2001. Association between antibiotic resistance and community prescribing: a critical review of bias and confounding in published studies. Clin. Infect. Dis. 33(Suppl. 3):S193-S205. [DOI] [PubMed] [Google Scholar]
- 28.Tomasz, A. 1997. Antibiotic resistance in Streptococcus pneumoniae. Clin. Infect. Dis. 24(Suppl. 1):85-88. [DOI] [PubMed] [Google Scholar]