Abstract
A comparative in vivo pharmacokinetic study of florfenicol was conducted in 18 crossbred pigs infected with Actinobacillus pleuropneumoniae following intravenous (i.v.), intramuscular (i.m.), or oral (p.o.) administration of a single dose of 20 mg/kg. The disease model was confirmed by clinical signs, X rays, pathohistologic examinations, and organism isolation. Florfenicol concentrations in plasma were determined by a validated high-performance liquid chromatography method with UV detection at a wavelength of 223 nm. Pharmacokinetic parameters were calculated by using the MCPKP software (Research Institute of Traditional Chinese Veterinary Medicine, Lanzhou, China). The disposition of florfenicol after a single i.v. bolus was described by a two-compartment model with values for the half-life at α phase (t1/2α), the half-life at β phase (t1/2β), the area under the concentration-time curve (AUC0-∞), and the volume of distribution at steady state (Vss) of 0.37 h, 2.91 h, 64.86 μg · h/ml, and 1.2 liter/kg, respectively. The concentration-time data fitted the one-compartment (after i.m.) and two-compartment (after p.o.) models with first-order absorption. The values for the maximum concentration of drug in serum (Cmax), t1/2α, t1/2β, and bioavailability after i.m. and p.o. dosing were 4.00 and 8.11 μg/ml, 0.12 and 3.91 h, 13.88 and 16.53 h, and 122.7 and 112.9%, respectively, for the two models. The study showed that florfenicol was absorbed quickly and completely, distributed widely, and eliminated slowly in the infected pigs, and there was no statistically significant difference between the pharmacokinetic profiles for the infected and healthy pigs.
Florfenicol is a novel antibiotic for use only with animals that belongs to the chloramphenicol family. Florfenicol has a mechanism for inhibiting the protein synthesis of susceptible bacteria by combining simultaneously with the 50S and 70S subunits in the ribosome to abolish the activity of peptidyl transferase (5, 7). Florfenicol has been shown to not only circumvent the bacterial acetyltransferase-mediated drug resistance but also to ameliorate the risk of inducing drug-related aplastic anemia in humans (9, 11). The potent activities of florfenicol against most of the susceptible gram-negative and -positive bacteria frequently occurring in animal herds have been reported by several groups (3, 5, 8, 10, 12). For Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus, the MICs were 3.1 μg/ml (8). For 42 isolates of Actinobacillus pleuropneumoniae, the MICs were ∼0.2 to ∼0.39 μg/ml with seven thiamphenicol-resistant strains (12). An average MIC of 0.25 μg/ml was reported for 108 A. pleuropneumoniae strains isolated from pig lungs in Italy (3). The present study was designed to provide a better understanding of florfenicol deposition in vivo in a well-established pig model with experimental infection with A. pleuropneumoniae following intravenous (i.v.), intramuscular (i.m.), or oral (p.o.) administration of a single dose of 20 mg/kg.
Eighteen healthy crossbred pigs (Duroc × Landrace × Yorkshire), approximately 7 weeks of age, were enrolled in this study, housed in a self-contained animal unit, and fed a commercial nonmedicated feed. Water was always available ad libitum. Negative responses for A. pleuropneumoniae were determined for all animals with a horseradish peroxidase-staphylococcal protein A enzyme-linked immunosorbent assay (16) during a 2-week quarantine period. The pigs, randomly divided into three pens with limited air space (6 in each), were designated to undergo the i.v., i.m., or p.o. administration. With a subsequent 2-week washout interval, the animals were each given an A. pleuropneumoniae inoculum and subjected to the same pharmacokinetic study. After the final sampling, all pigs were immediately euthanized for animal welfare; this was followed by necropsy and collection of tissue samples. All procedures involving animals complied with the local animal ethics regulations and were conducted under the close supervision and guidance of an experienced veterinarian.
Aseptic technique was required for handling the bacteria. An aliquot of A. pleuropneumoniae serotype 1 (State Administration of Inspection and Quarantine, Qingdao, China), after purification, sterility testing, and observation for the staining characteristic, was inoculated into sterilized medium prepared with PPLO broth (Difco), tryptone (Oxoid), and polypeptone (Nihon Pharmaceutical Co., Tokyo, Japan). The starter culture, with a bacterial growth turbidity of 5 × 108 CFU/ml (by PPLO agar plate count) after incubation at 37°C for ∼6 to ∼8 h, was further diluted with sterile phosphate-buffered saline (0.1 M, pH 7.4, prewarmed to 37°C) to a titer of ∼3.5 × 107 to ∼4 × 107 CFU/ml for use as the final bacterial inoculum just prior to the inoculation of pigs. In each case, local infiltration anesthesia was performed on the inoculation site with 1% procaine, with the animal fixed to a special surgery rack in the face-up position. The inoculum was injected into the trachea with a disposable 35-mm, 18-gauge needle via the joint of two adjacent tracheal rings at an injection volume of 0.18 ml/kg. Clinical signs and rectal temperature were recorded immediately prior to inoculation, hourly thereafter, immediately prior to drug treatment, and at 0.5, 1, 2, 4, 8, 12, 16, 24, 36, and 48 h thereafter. The lung X-ray (model 85/30-4; Guangzhou Medical Apparatus Co., Guangzhou, China) examination was specifically conducted at 8, 12, 24, and 48 h after inoculation. Isolation of the subject organism and histological examinations by light microscopy following staining with hematoxylin and eosin were carried out on the excised samples of the pneumonic lung, liver, and kidneys. For monitoring liver and renal function, blood samples (5 ml), collected from 12 randomly selected animals immediately prior to the inoculation and the florfenicol treatment, were analyzed with a semiautomatic biochemical analyzer (UV-VIS2; Biomerieux Co., Marcy l'Etoile, France) for the levels of urea nitrogen in blood, serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, creatinine, albumin, total protein, glucose, calcium, and phosphorus.
One hour after inoculation, the animals manifested the prospective signs, including diminished appetite, reduction of movement, crouching posture, dyspnea, and cough. A significantly elevated rectal temperature (average, 41.5°C; P value of <0.05 when compared with normal value) was found ∼3 to ∼4 h after inoculation and returned to normal by ∼12 to ∼16 h thereafter. The pulmonary X-ray images showed a time-dependent progressive inflammation with infiltrative and confluent shadows of different densities sporadically or extensively outlined in the central lung area and heart-diaphragm cornu. The subject bacterium, characterized as a tiny gram-negative coccobacillus with bipolar coloration, caused a unilateral or bilateral fibrinous pleuropneumonia in all infected animals. Differences between the biochemical indices in serum or blood from the healthy and infected animals did not reach statistical significance (serum aspartate aminotransferase, 48.2 versus 54.4 U/liter; alanine aminotransferase, 49.6 versus 55.6 U/liter; alkaline phosphatase, 63.0 versus 58.2 U/liter, urea nitrogen in blood, 7.63 versus 6.64 mmol/liter; creatinine, 177.64 versus 159.6 μmol/liter; albumin, 39.82 versus 35.21 g/liter; total protein, 58.09 versus 63.21 g/liter; glucose, 5.42 versus 4.96 mmol/liter; calcium, 2.92 versus 2.76 mmol/liter; phosphorus, 2.32 versus 2.09 mmol/liter). To mimic the clinical settings, florfenicol was administered approximately 3.5 h after inoculation, when fever with an average elevation of 1.2°C appeared in the subject.
Florfenicol injection (300 mg/ml) (lot 990818; Huihua Animal Health, Guangzhou, China) was administered i.v. via the auricular vein or i.m. deeply into the side of the neck with a disposable 30-mm, 20-gauge needle. For p.o. administration, an amylum-based drug mixture (containing 15% florfenicol) (lot 981001; Huihua Animal Health) diluted in 300 ml of saline was delivered into an empty stomach after a 16-h fast by gavage by using a mouth gag. Blood samples were collected immediately prior to treatment and at approximately 5, 10, 15, 30, and 45 min and at 1, 2, 4, 8, 12, 16, 24, 36, and 48 h after the i.v. bolus; at 5, 15, and 30 min and at 1, 2, 4, 8, 12, 16, 24, 36, and 48 h after the i.m. administration; and at 10, 30, and 45 min and at 1, 2, 4, 8, 12, 16, 24, 36, and 48 h after the p.o. dose. In each case, the animal was fixed in the same position as that for the inoculation procedure. Each 5-ml blood sample was directly aspirated via the anterior vena cava by a vein puncture with a sterile 40-mm, 18-gauge needle and was deposited in a heparinized glass tube. Plasma was separated immediately and pipetted into the clean glass tube and stored at −20°C until analysis. A high-performance liquid chromatography method was established in this study for the measurement of florfenicol levels in the pig plasma. On the day of analysis, the thawed plasma sample was spiked with the internal standard and extracted with ethyl acetate by agitation and centrifugation (Universal-16; Mettich, Tuttlingen, Germany). After evaporation of the organic material under a 60°C nitrogen flow, the residue was reconstituted in the mobile phase and injected onto an HP1100 series system liquid chromatograph (Hewlett-Packard) consisting of a variable-wavelength detector set at 223 nm for monitoring the florfenicol signal. A reversed-phase Nova-pak C18 column (4 μm, 4.6 × 250 mm; Yiweike, Dalian, China) was fitted and eluted with aqueous acetonitrile (77:23, vol/vol) at a flow rate of 1 ml/min at 25°C. A linear relationship existed in the calibration curve over the range of 0.05 to 20 μg/ml, which always yielded a correlation coefficient exceeding 0.9996. The within-run and interrun precision and recovery rates for florfenicol were ∼2.86 to ∼5.26% and 95.42%, respectively. This method was comparable with those reported by De Craene et al. (6) and Lobell et al. (7), in which the average plasma recovery rates for florfenicol in calves and cattle were 93.46 and 99.0% with the corresponding within-run precision rates of 1.22 to 3.70% and 1.5 to 5.0%, respectively.
Pharmacokinetic analysis was performed on the plasma data for individual animals using the MCPKP software described elsewhere (15). The elimination half-life at β phase (t1/2β) was calculated with the equation t1/2β = 0.693/kel, where kel was the elimination rate constant calculated by linear regression from the terminal linear portion of the plasma concentration-time curve. The area under the plasma concentration-time curve extrapolated to infinity (AUC0-∞) was calculated with the corresponding equation for individual administration routes. The body clearance following i.v. administration was calculated as the total dose administered divided by the AUC0-∞. The apparent volume of distribution (V), the maximal plasma concentration (Cmax), and the time to maximal concentration (Tmax) after the extravascular routes were calculated accordingly. Calculation for the duration of therapeutic plasma level [tcp(ther)] was based on an MIC of 0.25 μg/ml as described above (3). We used Prism 3.0 software (GraphPad Software, San Diego, Calif.) to perform statistical analysis, data processing, and graph representation. In each case, a two-tailed t test was used to assess the significance of difference in the parameter values between the healthy and infected pigs. A P value of <0.05 was regarded as significant.
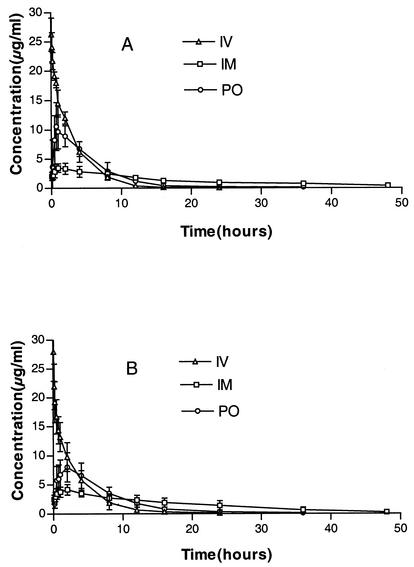
The blood samples at all time points were eligible for analysis. The plasma drug concentration-time profiles of florfenicol for the three treatment routes are illustrated in Fig. 1. The drug level in the plasma was quantifiable at the first time point but was no longer so at 36 h after i.v. administration in 8 of 12 animals and at 48 h after the p.o. dose in 6 of 12 animals, based on a quantification limit of 0.05 μg/ml. The main pharmacokinetic parameters of florfenicol in pigs following i.m., i.v., and p.o. administrations are shown in Table 1. No statistically significant differences were found between the pair of parameter values for the healthy and infected pigs.
FIG. 1.
Plasma concentration-time profiles of florfenicol following i.v., i.m., and p.o. of a single dose of 20 mg/kg in the healthy pigs (A) and in pigs infected with A. pleuropneumoniae (B). Drug concentrations are expressed as means ± standard deviations.
TABLE 1.
Pharmacokinetic parameters of florfenicol following i.v., i.m., or p.o. administration of a single dose of 20 mg/kg in healthy and A. pleuropneumoniae-infected pigs
| Parameterb | Value for indicated group and method of administrationa
|
|||||
|---|---|---|---|---|---|---|
| i.v.
|
i.m.
|
p.o.
|
||||
| Healthy | Infectedc | Healthy | Infectedc | Healthy | Infectedc | |
| Cmax (μg/ml) | 3.20 ± 0.61 | 4.00 ± 0.76 | 10.84 ± 2.71 | 8.11 ± 2.72 | ||
| Tmax (μg/ml) | 0.91 ± 0.32 | 0.78 ± 0.27 | 1.35 ± 0.71 | 1.92 ± 0.54 | ||
| t1/2α (h) | 0.64 ± 0.39 | 0.37 ± 0.29 | 0.15 ± 0.02 | 0.12 ± 0.05 | 2.87 ± 1.64 | 3.91 ± 1.4 |
| t1/2β (h) | 2.63 ± 0.51 | 2.91 ± 0.81 | 14.27 ± 2.69 | 13.88 ± 4.09 | 12.39 ± 7.23 | 16.53 ± 4.21 |
| V (liter/kg) | 1.17 ± 0.32 | 1.41 ± 0.93 | ||||
| Vss (liter/kg) | 0.95 ± 0.07 | 1.20 ± 0.39 | ||||
| CL (liter/kg/h) | 0.31 ± 0.02 | 0.32 ± 0.07 | ||||
| CL/F (liter/kg/h) | 0.30 ± 0.02 | 0.26 ± 0.06 | 0.31 ± 0.02 | 0.28 ± 0.06 | ||
| AUC0-∞ (μg · h/ml) | 66.17 ± 7.13 | 64.86 ± 14.43 | 68.61 ± 10.36 | 79.63 ± 26.8 | 65.89 ± 12.79 | 73.28 ± 14.67 |
| F (%) | 103.74 ± 26.75 | 122.77 ± 44.43 | 99.57 ± 19.33 | 112.9 ± 8.82 | ||
| tcp(ther)d (h) | 14.50 ± 1.39 | 15.95 ± 3.15 | 53.0 ± 10.53 | 53.88 ± 19.2 | 21.83 ± 10.28 | 22.78 ± 8.81 |
Values are means ± standard deviations (n = 6).
CL, clearance; F, bioavailability; the other abbreviations are defined in the text.
No significant difference compared with results for the healthy group (two-tailed t test, P < 0.05).
Based on the average MIC of 0.25 μg/ml for A. pleuropneumoniae in pigs (6).
The drug level showed a rapid distribution and a slow elimination phase with greater AUC0-∞, volume of distribution at steady state (Vss), and elimination half-life values than those for chloramphenicol in pigs after a single i.v. bolus (31.23 mg · h/liter, 0.108 liter/kg, and 1.59 h, respectively) (17). This likely resulted from the replacement of the hydroxyl group by a fluorine atom that postponed the in vivo metabolic glucuronidation (4). Consistently, the AUC, Vss, and half-life values for florfenicol given to lactating cows, cattle, and veal calves i.v. were 128.4, 89.5, and 63.85 mg · h/liter; 0.35, 0.767, and 0.872 liter/kg; and 3, 2.65, and 3.71 h, respectively, in spite of the use of the triexponential or noncompartmental model in some of them (1, 7, 9).
However, the drug deposition after the i.m. administration was well described by the one-compartment model with first-order absorption, exhibiting a markedly elongated t1/2β and a high bioavailability, indicating that florfenicol underwent a slow elimination phase but a completely systematic utilization in both healthy and infected pigs. Voorspoels et al. (14) reported a fairly comparable half-life of 14.6 h and a high AUC0-∞ of 110.6 μg · h/ml in pigs given a single i.m. dose of florfenicol. However, no dose-dependent increase in Cmax was found in the present study compared with that (7.3 ± 6.0 μg/ml) achieved by Voorspoels et al. using a dose of 15 mg/kg instead (14). This may be due to the biases introduced by individual animals or pharmaceutical factors in the special formula. t1/2β values of 12.5 and 18.3 h were obtained for lactating cows and cattle given a dose of 20 mg/kg i.m., with 38 and 78.5% of florfenicol systemically available, respectively (7, 9). These results suggested that the organic long-acting formulation caused a delay in the drug absorption from the injection site in the muscle. To address this point, we measured the florfenicol concentrations in the muscle tissue samples excised from the needle insertion sites at 48, 72, and 96 h after an i.m. dose of 20 mg/kg, which were 8.86, 4.95, and 2.33 μg/g, respectively. The drug depot apparently introduced a reasonable Cmax, followed by a sustained drug level well above the MIC for A. pleuropneumoniae, thereby inducing a long tcp(ther) of 53.88 h.
Florfenicol was absorbed rapidly through the gastrointestinal tract with short lag times of 7.2 min in healthy pigs and of 1.2 min in infected pigs under fasting conditions. Strikingly, a two- to threefold-higher maximal concentration was observed in the present study after the p.o. dose than after the i.m. dose, while about twofold-higher concentrations (14.8 μg/ml to 7.3 μg/ml) were also reported by Voorspoels et al. (14) in pigs. The two-compartment model fitted by the plasma concentration-time profiles after an oral dose in our study indicated that florfenicol underwent a fast deposition phase lasting for a long period of time after the peak level with a t1/2α of 3.91 h, which was more comparable to an elimination half-life of 5.5 h (standard deviation, ±4.2 h) reported in pigs given florfenicol p.o. in a pellet formulation (gelatin capsules, type 12-35 ov; Willi Krueger) (14). However, the terminal elimination phase with an extended half-life did not make much clinical sense, as shown by a tcp(ther) value of 22.78 h in our study. This feature should be taken into account when investigating an optimal oral regimen for pigs. The high corresponding bile/serum florfenicol level ratio (up to 2) in calves after seven continuous oral treatments with a dose of 11 mg/kg suggested the possibility that florfenicol underwent enterohepatic circulation to a certain extent (1). This could contribute to the oral absorption of florfenicol in pigs in some way. Nevertheless, it needs to be demonstrated with more kinetic evidence in a biliary excretion study because of the complexities and the potentially large interanimal variations. Additionally, it should also be mentioned that in our previous experiment feed intake did not significantly change the pharmacokinetics of florfenicol after p.o. administration in pigs (data not shown). In contrast, for calves, the intake of milk replacer at 5 min prior to oral treatment significantly reduced the AUC, F, and Cmax values from 105.65 mg · h/liter, 88%, and 11.32 μg/ml to 72.42 mg · h/liter, 65%, and 9.41 μg/ml, respectively (13).
In our study, the A. pleuropneumoniae infection in pigs with administration by the three different routes did not result in statistically significant differences in the pharmacokinetic profiles of florfenicol in plasma. The reports on the metabolism of florfenicol in animals in vivo are sparse. In calves, most of the dosed drug is excreted in urine in the parent form, indicating a major kidney clearance (13). A residue study was conducted by the European Agency for the Evaluation of Medicinal Products on pigs given multiple doses of radioisotope-labeled florfenicol at 20 mg/kg p.o. or i.m. The results showed that ∼45 to ∼60% of florfenicol excreted in urine was in unchanged form, ∼11.2 to ∼17% was excreted as a florfenicol amine, <10% was excreted as florfenicol oxamic acid, and 1.1% was excreted as florfenicol alcohol (2). This was unlike the results showing that chloramphenicol was predominantly cleared by the liver (4). On the other hand, pathohistologically and biochemically, A. pleuropneumoniae intrinsically and acutely caused an extensive lesion in the respiratory system rather than in the kidneys and liver, whose normal functions were crucial to the disposition of florfenicol.
In conclusion, this study elucidated the pharmacokinetic characteristics of florfenicol in vivo in pigs infected with A. pleuropneumoniae. Since florfenicol is an emerging antibiotic that is widely used clinically, investigations on the pharmacodynamics and tissue pharmacokinetics of the drug when given by multiple i.m. administrations or in medicated feed with specific formulations in the disease model are warranted.
Acknowledgments
This work was supported by a grant (number 980129) from the Guangdong Scientific Research Committee of China.
Scientific and technical help from the staff in our laboratories is gratefully acknowledged.
REFERENCES
- 1.Adams, P. E., K. J. Varma, T. E. Powers, and J. F. Lamendola. 1987. Tissue concentrations and pharmacokinetics of florfenicol in male veal calves given repeated doses. Am. J. Vet. Res. 48:1725-1732. [PubMed] [Google Scholar]
- 2.Anonymous. 1999. Florfenicol (extension to pigs)—summary report, p. 1-4. In EMEA/MRL/591/99-FINAL. Committee for Veterinary Medicinal Products/The European Agency for the Evaluation of Medicinal Products. Cannary Wharf, London, United Kingdom.
- 3.Barigazzi, G., P. Candotti, and E. Foni. 1996. In vitro susceptibility of 108 isolated Actinobacillus pleuropneumoniae strains to 17 antimicrobial agents from pig lungs in Italy in 1994-1995, p. 207. In Proceedings of the 14th International Pig Veterinary Society Congress, Bologna, Italy.
- 4.Bretzlaff, K. N., C. A. Neff-Davis, R. S. Ott, G. D. Koritz, B. K. Gustafsson, and L. E. Davis. 1987. Florfenicol in non-lactating dairy cows: pharmacokinetics, binding to plasma proteins, and effects on phagocytosis by blood neutrophils. J. Vet. Pharmacol. Ther. 10:233-240. [DOI] [PubMed] [Google Scholar]
- 5.Cannon, M., S. Harford, and J. Davies. 1990. A comparative study on the inhibitory actions of chloramphenicol, thiamphenicol and some fluorinated derivatives. J. Antimicrob. Chemother. 26:307-317. [DOI] [PubMed] [Google Scholar]
- 6.De Craene, B. A., P. Deprez, E. D'Haese, H. J. Nelis, W. Van den Bossche, and A. P. De Leenheer. 1997. Pharmacokinetics of florfenicol in cerebrospinal fluid and plasma of calves. Antimicrob. Agents Chemother. 41:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobell, R. D., K. J. Varma, J. C. Johnson, R. A. Sams, D. F. Gerken, and S. M. Ashcraft. 1994. Pharmacokinetics of florfenicol following intravenous and intramuscular doses to cattle. J. Vet. Pharmacol. Ther. 17:253-258. [DOI] [PubMed] [Google Scholar]
- 8.Neu, H. C., and K. P. Fu. 1980. In vitro activities of chloramphenicol and thiamphenicol analogs. Antimicrob. Agents Chemother. 18:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soback, S., M. J. Paape, R. Filep, and K. J. Varma. 1995. Florfenicol pharmacokinetics in lactating cows after intravenous, intramuscular and intramammary administration. J. Vet. Pharmacol. Ther. 18:413-417. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki, S., K. Ohmae, and K. Ohishi. 1989. Antimicrobial susceptibility of Actinobacillus (Haemophilus) pleuropneumoniae isolated from pigs with pleuropneumonia. Jpn. J. Vet. Sci. 51:450-452. [DOI] [PubMed] [Google Scholar]
- 11.Syriopoulou, V. P., A. L. Harding, D. A. Goldmann, and A. L. Smith. 1981. In vitro antibacterial activity of fluorinated analogs of chloramphenicol and thiamphenicol. Antimicrob. Agents Chemother. 19:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda, Y., and I. Suenaga. 1995. In vitro antibacterial activity of florfenicol against Actinobacillus pleuropneumoniae. J. Vet. Med. Sci. 57:363-364. [DOI] [PubMed] [Google Scholar]
- 13.Varma, K. J., P. E. Adams, T. E. Powers, J. D. Powers, and J. F. Lamendola. 1986. Pharmacokinetics of florfenicol in veal calves. J. Vet. Pharmacol. Ther. 9:412-425. [DOI] [PubMed] [Google Scholar]
- 14.Voorspoels, J., E. D'Haese, B. A. De Craene, C. Vervaet, D. De Riemaecker, P. Deprez, H. Nelis, and J. P. Remon. 1999. Pharmacokinetics of florfenicol after treatment of pigs with single oral or intramuscular doses or with medicated feed for three days. Vet. Rec. 145:397-399. [DOI] [PubMed] [Google Scholar]
- 15.Xia, W. J., and Z. R. Chen. 1988. MCPKP—a microcomputer program specialized for pharmacokinetic compartment analysis. Acta Pharmacol. Sin. 9:188-192. [PubMed] [Google Scholar]
- 16.Xu, R., S. S. Zhu, and H. H. Du. 1989. Application of horseradish peroxidase-staphylococcal protein A enzyme-linked immunosorbent assay to detect the antibody of Actinobacillus pleuropneumoniae. Chinese J. Anim. Quarant. 3:1-9. [Google Scholar]
- 17.Ye, X. S., and G. Z. Lu. 1985. The pharmacokinetic study of chloramphenicol in pigs. J. S. China Agric. Univ. 1:42-45. [Google Scholar]