Abstract
Lysostaphin is a 27-kDa endopeptidase that enzymatically disrupts the cell wall of Staphylococcus aureus and is a promising candidate for treating S. aureus blood-borne infections. It would be extremely useful to define conditions that would both increase lysostaphin's in vivo half-life to allow for more effective tissue distribution and reduce its immunogenicity. Conjugation of polyethylene glycol (PEG) to lysostaphin (PEGylation) was investigated as a means to accomplish these goals. Rather than using linear forms of PEG, branched PEGs were chosen as the initial candidates because their large spatial volumes prevent entry of the polymer into the enzyme's active sites, which could potentially reduce enzymatic function. Enzymatic activity for most PEGylated lysostaphins was reduced, but these compounds were still considerably active compared to unconjugated lysostaphin, with conjugates that had lower degrees of PEG modification having greater activity than those with higher degrees. PEGylated lysostaphin injected intravenously had a serum drug half-life of up to 24 h and resulted in much higher plasma drug concentrations than an equal dose of unconjugated lysostaphin, which had a half-life of less than 1 h. Finally, reduced binding affinity was shown for PEGylated lysostaphin in an antilysostaphin capture enzyme-linked immunosorbent assay, with some PEG-lysostaphin conjugates having binding affinities that were reduced more than 10-fold compared to unconjugated lysostaphin. These findings demonstrate that PEGylation of lysostaphin, while diminishing its S. aureus killing activity, results in prolonged serum drug persistence and reduced antibody binding. These features should significantly enhance lysostaphin's therapeutic value as an intravenous “antibiotic” against S. aureus.
The advent of recombinant technologies combined with the increased incidence of microbial resistance to both old and new antibiotics has led to intense research to develop novel proteins as new therapeutic agents. However, many of these antimicrobial proteins have limited usefulness because of their poor in vivo half-life, due to rapid renal filtration, proteolysis, and increased intracellular uptake by the reticuloendothelial system (3, 4, 13). Additionally, many of these proteins are derived from nonhuman sources and elicit strong immune responses. Repeat administrations could therefore possibly result in drug neutralization and rapid clearance by antibodies. These limitations can potentially be overcome by conjugation of these protein agents to polyethylene glycol (PEG). PEG has been conjugated to many proteins for a variety of therapeutic applications: uricase for hyperuricemia (12), alpha interferon 2a for hepatitis C (1), tumor necrosis factor alpha and concanavalin A as antitumor agents (14, 15), factor VIII for hemophilia (10), and insulin (6). PEG conjugation reduces renal ultrafiltration by increasing molecular size and prevents the approach of antibodies and proteolytic enzymes by creating a molecular “shield” of PEG around the protein (16). Furthermore, PEG conjugation reduces uptake by dendritic cells and interferes with antigen processing, thereby greatly reducing or eliminating a foreign protein's immunogenicity.
Lysostaphin is an antibacterial enzyme that shows great promise in treating staphylococcal infections, particularly those involving multidrug-resistant strains. It is a 27-kDa endopeptidase produced by Staphylococcus simulans that is capable of specifically cleaving the cross-linking pentaglycine bridges in the cell walls of staphylococci (9, 11). Lysostaphin is highly effective in lysing Staphylococcus aureus because the cell wall bridges of this species contain a high proportion of pentaglycine cross bridges. Activity against other species of staphylococci has also been demonstrated (17). One of the many potential applications for lysostaphin is systemic infusion for the treatment of invasive staphylococcal diseases, including organ abscesses, osteomyelitis, and endocarditis (2, 8). We and others have shown that systemic infusion of lysostaphin in a number of different animal models leads to eradication of disease and is an alternative to currently available therapy (7, 8). However, greater than 95% of the enzyme is cleared from the serum circulation within 1 h after injection, and this rapid clearance may necessitate more frequent dosing to maintain the serum drug concentrations necessary to effectively treat infections. Lysostaphin is also immunogenic, and patients who have been previously exposed to lysostaphin may produce antibodies that could potentially neutralize the activity of this enzyme or increase the rate of drug clearance (5, 17).
Lysostaphin has a high net charge of +10.5 at pH 7 that is attributable to the 16 lysine and 6 arginine residues in the polypeptide chain. The amine groups on the side chains of these lysines are ideal targets to covalently link PEG that has been activated with N-hydroxysuccinimide (NHS). Branched PEGs were chosen because their larger spatial volume makes them less likely to penetrate protein crevices, which are often the binding motifs and active sites of enzymes. This study describes the conjugation of PEG to lysostaphin (PEGylation) with primary goals of increasing the serum drug half-life and reducing its binding to antilysostaphin antibodies while also maintaining the enzyme's staphylolytic activity. Addition of these two properties to lysostaphin may enhance its value as an antibacterial agent by reducing both the size and frequency of dosing and may also protect the enzyme from immune neutralization and clearance.
MATERIALS AND METHODS
Materials.
Lysostaphin (Ambicin L) was obtained from AMBI, Inc. The mPEG2-NHS esters, 10 and 40 kDa, were purchased from Shearwater Corporation (Huntsville, Ala.). Sodium borate, dimethyl sulfoxide (DMSO), bovine serum albumin (BSA), and extravidin-horseradish peroxidase (HRP) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Glycine was purchased from EM Science (Gibbstown, N.J.). The NuPage electrophoresis system and Colloidal Blue stain were purchased from Invitrogen (Carlsbad, Calif.). Sephacryl S-100HR and HiTrap SP FF were purchased from Amersham-Pharmacia (Piscataway, N.J.). Tryptic soy broth and cation-adjusted Mueller-Hinton broth were purchased from Becton Dickinson (Sparks, Md.). TMB Microwell and 450 STOP reagent were purchased from BioFX (Owings Mills, Md.).
Lysostaphin PEGylation.
Lysostaphin at 0.27, 1, or 5 mg/ml was dissolved in either 0.2 M borate buffer, pH 8.5, or DMSO. The mPEG2-NHS esters were prepared in DMSO and added to the lysostaphin solution in molar excess at ratios of 40, 20, 10, 5, or 2.5:1. PEGylation was performed with three different buffer conditions at room temperature for 1, 2, or 3 h: borate buffer (with <10% DMSO contributed by adding PEG), 50% borate-50% DMSO, and 100% DMSO. All reactions were quenched by addition of glycine to 25 mM and vortexing.
PEG conjugation to lysostaphin was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with the NuPage electrophoresis system. Nonreduced samples (300 ng) were run on a Novex 4-to-12% bis-Tris gel at 115 V and stained with Colloidal Blue. PEGylated lysostaphin was separated from unreacted lysostaphin by running the reaction mixture over a Sephacryl S-100HR spin column. PEG-lysostaphin conjugates elute in the void volume, whereas the resin retains unconjugated lysostaphin. Purified PEG-lysostaphin was concentrated and stored for activity assays.
Alternatively, unconjugated lysostaphin was removed from the sample by ion-exchange chromatography. Lysostaphin, but not PEG-lysostaphin, was bound onto a HiTrap SP FF column in 50 mM sodium phosphate buffer, pH 7.0. The column was washed with the same buffer until the optical density at 280 nm (OD280) of the eluate was reduced to background levels. Bound lysostaphin was then removed, and the column was regenerated by washing with 50 mM sodium phosphate plus 1 M NaCl, pH 7.0. This process was repeated several times until the PEG-lysostaphin fraction (unbound) was at least 98% pure of unconjugated lysostaphin, as determined by SDS-PAGE, which is sensitive to 10 ng of lysostaphin. Purity and the relative concentration of gel bands were quantitatively determined by measuring band densities of digitally scanned gels, using Scion Image software (Scion Corporation).
PEG-lysostaphin conjugates with the 40-kDa branched PEG were also synthesized under contract by Shearwater Corporation. These samples were purified and fractionated by Shearwater Corporation using ion-exchange chromatography, yielding two different conjugates: one with a single PEG per lysostaphin molecule (1-mer), and the other with two PEGs per lysostaphin molecule (2-mer). Both conjugates were greater than 99% pure of unconjugated lysostaphin as determined by high-performance liquid chromatography (HPLC) and SDS-PAGE. The relative purities of the mono- and di-PEGylated species of lysostaphin were determined by size exclusion chromatography HPLC. The 40-kDa PEG-lysostaphin 1-mer was determined to be greater than 99% of the mono-PEGylated species, and the 2-mer was about 93% of the di-PEGylated species, with the remainder being mono-PEGylated.
In vitro activity of PEG-lysostaphin.
Lysostaphin's ability to lyse S. aureus serotype 5 (SA5) was tested by measuring the drop in absorbance at 650 nm of a solution containing heat-killed SA5 (HKSA5). HKSA5 was prepared by incubating live bacteria (grown in an overnight culture of tryptic soy broth and washed once with phosphate-buffered saline [PBS]) in PBS at 62°C for 2 h followed by dilution in PBS such that the initial absorbance at 650 nm was about 1. Lysostaphin was then added at a concentration of 32 μg/ml, and absorbance readings were taken every 60 s for 10 min. Lysis of live SA5 bacteria was measured by adding lysostaphin from 0 to 20 μg/ml to an SA5 suspension in PBS (percent transmittance = 40 at 650 nm; Spectonic 200+; Spectonic Instruments). The samples were incubated at 37°C for 1 h and then streaked onto blood agar plates to determine viability after treatment. After overnight culture at 37°C, colonies were counted and compared to untreated samples.
Antilysostaphin binding activity.
A lysostaphin capture enzyme-linked immunosorbent assay (ELISA) was performed to determine if PEGylated lysostaphin shields the protein from antibody binding. Ninety-six-well microtiter plates were coated overnight with a polyclonal rabbit antilysostaphin antibody. The wells were blocked with 1% BSA in PBS followed by incubation with the lysostaphin samples in PBS-0.5% Tween 20 plus 0.1% BSA. Lysostaphin binding was detected with biotin-labeled, polyclonal rabbit antilysostaphin followed by extravidin-HRP incubation and tetramethyl benzidine (TMB) colorimetric detection. The plates were measured at an absorbance of 450 nm in a SpectraMAX Plus plate reader (Molecular Devices, Sunnyvale, Calif.). The detection limit for this assay was 0.63 ng of lysostaphin/ml, which corresponds to twice the background OD value. The percent coefficient of variation was calculated for a series of standard curves and determined to be 9.12%.
Serum drug pharmacokinetics of PEG-lysostaphin.
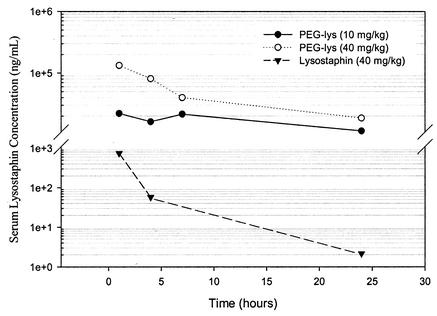
Three CF1 mice for each sample were injected intravenously via the tail vein with S-100HR-purified PEG-lysostaphin, which was synthesized with a 2:1 molar ratio of the 40-kDa branched PEG in 50% DMSO, at a dose of 40 or 10 mg/kg of body weight. This lysostaphin sample had a mixture of conjugates with one to four PEG chains per lysostaphin molecule. The relative ratios were determined by measuring the OD of SDS-PAGE gel bands using Scion Image software and were as follows: 1-mer, 38%; 2-mer, 45%; 3-mer, 13%; and 4-mer, 4%. Control mice were injected with 40 mg of unconjugated lysostaphin/kg. Blood was collected by orbital bleeding at 1, 4, 7, and 24 h postadministration. The blood was incubated at 37°C for 30 min followed by 4°C for 30 min. Serum was then separated by centrifugation at 1,000 × g for 10 min. Serum samples from each time point were pooled before the ELISA, so each data point in Fig. 3, below, represents the average serum lysostaphin concentration of three mice. The concentration of lysostaphin in serum was determined by ELISA as described above. Since PEGylated lysostaphin has less binding activity towards antilysostaphin antibodies, unconjugated lysostaphin cannot be used as an ELISA standard to determine the concentration of PEG-lysostaphin in serum. Therefore, two lysostaphin standards were used in the ELISA: a purified PEGylated lysostaphin standard of known concentration that is identical to the PEG-lysostaphin injected into the mice, and an unconjugated lysostaphin standard for those mice injected with native lysostaphin. The concentration of the PEG-lysostaphin standard was determined by measuring its OD280. PEG does not absorb at this wavelength and therefore does not interfere with the determination of the protein concentration.
FIG. 3.
Serum pharmacokinetics of PEGylated lysostaphin in mice. PEG-lysostaphin conjugates (200 μl) injected at a dose of 0.8 or 0.2 mg show much higher serum drug concentrations and increased serum drug half-life than a 0.8-mg dose of unconjugated lysostaphin. Each data point is the average lysostaphin concentration from pooled sera of three mice.
RESULTS AND DISCUSSION
PEG conjugation to lysostaphin.
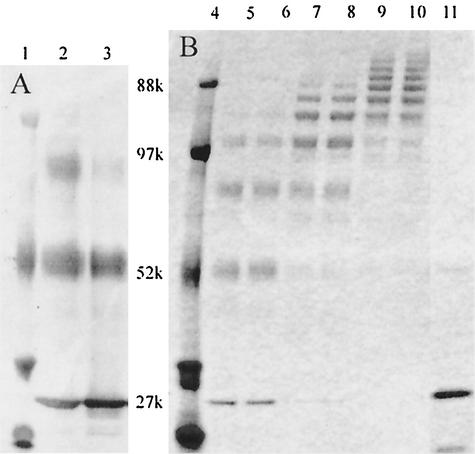
PEG was initially conjugated to lysostaphin in an aqueous borate buffer, resulting in only partial protein PEGylation, most likely due to rapid hydrolysis of the NHS ester (Fig. 1A). The activated PEG is much more stable in solutions containing a higher percentage of organic solvent, such as DMSO, and subsequent reactions were therefore performed in either 50 or 100% DMSO solutions. Conjugation with a 10-kDa branched PEG in 50% DMSO is shown in Fig. 1B, with unconjugated lysostaphin having a molecular mass of about 27 kDa. Greater than 90% PEG labeling efficiency was achieved under most reaction conditions when using DMSO as a solvent. Each successive addition of PEG onto a lysostaphin molecule increased the molecular weight, as evidenced by a ladder of lysostaphin bands in the SDS-PAGE gel. The number of PEG chains conjugated to each lysostaphin molecule was found to be as few as 1 and as many as 10. The number and pattern of conjugation was controlled by varying two of the reaction conditions: (i) the concentration of the reactants and (ii) the molar ratio of PEG to lysostaphin. The degree of PEG conjugation and the polydispersity of the products increased as the ratio of PEG and the concentration of reactants were increased.
FIG. 1.
Conjugation of a 10-kDa mPEG2-NHS ester to lysostaphin. Unconjugated lysostaphin runs as a single band of 27 kDa. All wells were loaded with 300 ng of total protein. Lanes 1 and 4, molecular mass marker; lanes 2 and 3, lysostaphin conjugated at a 10:1 or 2.5:1 molar ratio of PEG in aqueous, borate buffer; lanes 5 to 10, lysostaphin conjugated at a 10:1 molar ratio of PEG in 50% DMSO-50% borate buffer with low (lanes 5 and 6), medium (lanes 7 and 8), and high (lanes 9 and 10) reactant concentrations; lane 11, native lysostaphin.
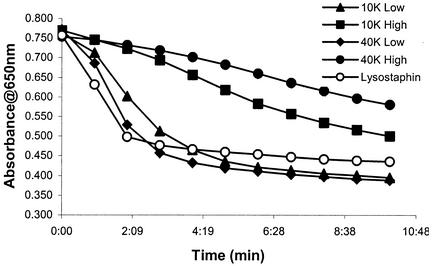
The in vitro enzymatic activity of 10- and 40-kDa PEGylated lysostaphin was tested by measuring lysis of HKSA5. The kinetics of bacterial lysis with various PEG-lysostaphin conjugates is shown in Fig. 2. All samples were positive for enzyme activity, although there was considerable variability in activity levels. The two samples with high degrees of PEG conjugation retained only partial enzyme function and did not achieve complete lysis by 10 min. These two conjugates were reacted with a higher PEG/lysostaphin ratio and thus have a greater number of PEG chains (three to seven) per molecule of lysostaphin. The reduction in activity with increasing degrees of PEGylation suggests either that the PEG is small enough to gain access to lysostaphin's active site or its peptidoglycan binding domain, thus reducing its enzymatic activity, or that the molecular size of the conjugate simply becomes too large for the enzyme to access the bacterial cell wall. In contrast, samples with lower degrees of PEG conjugation (one to four per molecule) were able to lyse HKSA5 as efficiently as unconjugated lysostaphin, although with slightly slower kinetics, demonstrating little loss in enzymatic activity under these PEGylation conditions. There appeared to be no difference in activity when comparing the 40-kDa conjugates to the 10-kDa conjugates; rather, the degree of PEGylation was the determining factor for whether a conjugate retained or lost activity, with low degrees of modification retaining activity for both 10- and 40-kDa forms. These results demonstrate that PEG can be conjugated onto lysostaphin without compromising bactericidal activity but that the degree of PEG modification is a critical parameter in preserving this function.
FIG. 2.
Lysis of HKSA5. Lysostaphin was added at t = 0 h, and bacterial lysis was monitored by measuring the decrease in absorbance with time. High degrees of lysostaphin conjugation with either the 10- or 40-kDa PEG (10K High and 40K High) led to reduced SA5 lysis, whereas enzymatic activity was preserved for low degrees of PEG conjugation (10K Low and 40K Low).
Prolonged serum drug half-life of PEGylated lysostaphin.
Conjugation of PEG onto protein drugs enables them to avoid the normal clearance mechanisms of the body and thereby leads to an increased plasma drug half-life. We determined the pharmacokinetic profile of lysostaphin conjugated to the 40-kDa branched PEG (one to four PEG chains per molecule) in mice and compared it to clearance of unconjugated lysostaphin (Fig. 3). Two enhancements due to PEGylation are apparent from the graph: (i) the half-life of lysostaphin has been dramatically increased, and (ii) the total serum drug concentration achieved is much greater than that for unconjugated lysostaphin. The serum drug concentration of the PEG-lysostaphin conjugates drops by only 2- to 10-fold over 24 h, whereas that of unconjugated lysostaphin falls by nearly 500-fold over the same time period. This improved retention of lysostaphin should reduce the dosing quantities and frequency needed to maintain plasma drug concentrations above therapeutically effective drug concentrations. Maintaining these high levels of lysostaphin for longer periods of time may also result in more rapid clearance of bacterial infections and decrease the probability that lysostaphin resistance will emerge. Total serum drug concentrations were also much greater with the PEG-lysostaphin conjugates. At 1 h postadministration, serum drug concentrations of PEG-lysostaphin were more than 30 times the concentration of native lysostaphin, even when the initial PEG-lysostaphin dose was one-fourth that of unconjugated lysostaphin. This result suggests that a much lower dose of PEG-lysostaphin may be used to achieve the same clinical benefit as with unconjugated lysostaphin, which could result in lower cost of therapy and minimize potential toxic or allergic reactions to the drug.
Antilysostaphin antibody activity for PEGylated lysostaphin.
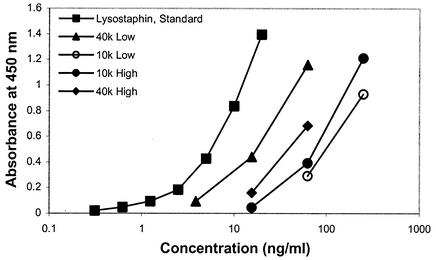
The ability of PEG to shield lysostaphin from antibodies was tested in vitro with a lysostaphin capture immunoassay (Fig. 4). Unconjugated lysostaphin shows a standard antibody binding response from 0.3 to 20 ng/ml. A heterogeneous response is seen with the binding of PEG-lysostaphin to antilysostaphin antibody, but all bind less efficiently than unconjugated lysostaphin. The best shielding observed resulted in a greater-than-10-fold reduction in antibody affinity to the PEGylated lysostaphin. It must be kept in mind that this assay environment is different from binding to antibodies on mucosal surfaces or flowing serum, but it does demonstrate that PEG conjugation onto the surface of lysostaphin can at least partially shield the enzyme from antibody binding. There does not appear to be any correlation between enzyme activity and reduced antibody binding, but differences in antibody binding to the 40- and 10-kDa conjugates may be explained by differing degrees of PEGylation. In general, fewer PEG chains, one to four, are attached to lysostaphin for the 40-kDa PEG, so it may have a more open structure that does not exclude antibodies as well as the 10-kDa forms do, which have many more PEG chains, 3 to 10, per lysostaphin molecule. Nevertheless, antibody binding to the 40-kDa conjugate is sufficiently reduced compared to unconjugated lysostaphin that, when combined with activity and pharmacokinetic data, makes it the most attractive candidate for a new lysostaphin formulation.
FIG. 4.
Lysostaphin capture immunoassay with PEGylated lysostaphin. All of the PEG-lysostaphin samples have reduced antilysostaphin binding activity (from 3 to >10 times less binding). Low degrees of lysostaphin conjugation with both 10- and 40-kDa PEGs did not shield antibody as well as the higher-degree modifications.
Fractionation of PEG-lysostaphin conjugates.
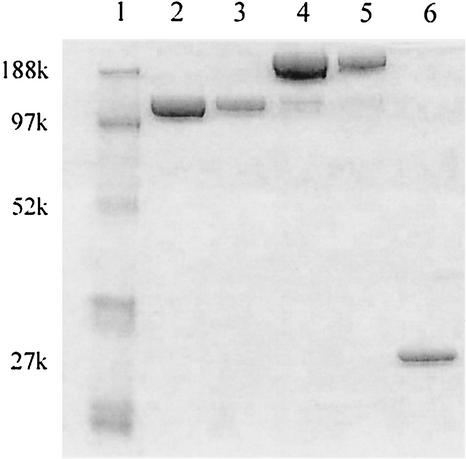
Varying reaction conditions is a reliable approach to control the degree of lysostaphin PEGylation between high, medium, and low degrees of PEGylation. However, samples in each of these ranges are still polydisperse with respect to PEG number (12, 15). For example, enzymes with a low average degree of modification (2:1 PEG/lysostaphin molar ratio) have an equal number of lysostaphin molecules with one to three PEG chains and a small fraction with four chains. Since we are examining the staphylolytic activity of the entire population of substituted lysostaphins, we cannot attribute the aggregate level of activity to specific levels of PEG conjugation of the lysostaphin molecule. It is possible that lysostaphin with one PEG chain retains the majority of its enzymatic activity, but those with two or three chains may have significantly reduced activities. For this reason we wished to isolate enzyme conjugates that are monodispersed with respect to PEG number. Fractionation of the various PEG-lysostaphin species was performed by ion-exchange chromatography as a means to test enzyme activity as a function of PEG conjugation number. The results of the fractionation are shown in Fig. 5 and, although perfect resolution was not achieved, fractions tended to be enriched in just one specific band. The mono-PEGylated form was purified to greater than 99% 1-mer, while the di-PEGylated form was purified to 93% 2-mer with the remainder contributed mostly by the 1-mer.
FIG. 5.
SDS-PAGE of a 40-kDa PEG conjugate purified into predominantly mono- or di-PEGylated lysostaphin (1-mer and 2-mer). Lane 1, molecular mass marker; lanes 2 and 3, PEG-lysostaphin 1-mer loaded at 1 and 0.3 μg of total protein; lanes 4 and 5, PEG-lysostaphin 2-mer loaded at 1 and 0.3 μg of total protein; lane 6, native lysostaphin (0.3 μg).
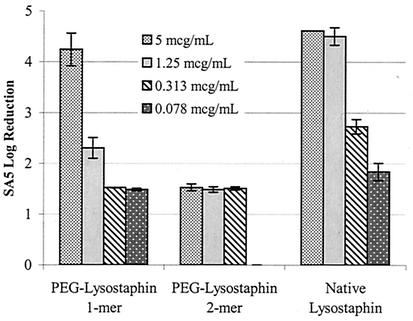
These two PEG-lysostaphin fractions were tested in a killing assay against live SA5 to determine their relative activities (Fig. 6). The lysostaphin 1-mer, which is 99% mono-PEGylated lysostaphin, was clearly active but with slightly reduced levels of lysostaphin killing activity compared to unconjugated lysostaphin. However, conjugation of two PEG chains onto lysostaphin rendered the enzyme virtually inactive, with less than 1% of the killing activity of unconjugated lysostaphin. There are two possible explanations for the loss of activity with the 2-mer. Although there may be as many as 10 lysine residues available for PEG conjugation onto lysostaphin, each will have a different reactivity, and it is likely that only one or two lysine residues are preferentially PEGylated for a given reaction condition. The preferred site for conjugation of the first PEG chain might lie in a region that is not critical for enzyme function and may explain why there is little loss in activity for the 1-mer. However, the next-most-preferred lysine for PEGylation may reside in or near the active or cell wall binding sites of lysostaphin, and attachment of PEG to these regions is likely to seriously disrupt enzyme function. Another possible explanation for the loss of activity with the 2-mer relates to the increased spatial volume of lysostaphin due to PEGylation. Lysostaphin does not act on a soluble, diffusible substrate but rather must be able to penetrate the thick, solid peptidoglycan scaffold of the bacterial cell wall. Each successive addition of PEG raises the molecular weight of lysostaphin, and the increase in spatial volume from the 1-mer to the 2-mer may hinder enzyme access to the pentaglycine cross bridges in the cell wall, thus eliminating its killing activity.
FIG. 6.
Enzymatic killing activity in a high inoculum of live SA5 with various concentrations of PEG-lysostaphin (1-mer and 2-mer) or native lysostaphin. A log10 reduction of 4.6 represents complete clearance of bacteria from the culture. Enzyme killing activity is preserved with mono-PEGylated lysostaphin, although it is slightly reduced compared to native lysostaphin. However, lysostaphin activity was virtually eliminated for the 2-mer.
The results from fractionating lysostaphin conjugates into mono- or di-PEGylated forms suggest that future studies should concentrate on conjugating a single PEG chain onto lysostaphin. We chose to focus our studies on the mPEG2 40-kDa branched PEG for two reasons. The bulky structure of branched PEGs makes them less likely than linear PEGs to enter the protein crevices of active and binding domains and therefore reduces the risk of eliminating enzymatic activity. Furthermore, the enhancements in pharmacokinetics and antibody reactivity are greater when higher-molecular-weight PEGs are used than when lower-molecular-weight PEGs are used. Although the S. aureus killing activity of our 40-kDa PEG-lysostaphin 1-mer is slightly reduced compared to unconjugated lysostaphin, it is more than offset by the enhancements to circulating serum drug concentrations, increased serum drug half-life, and decreased antibody binding, which we hope will improve the efficacy of intravenously administered lysostaphin for the treatment of systemic staphylococcal infections.
Acknowledgments
We thank Xuan “Simon” Zhao and Ping Zhang for synthesis of PEG-lysostaphin 1- and 2-mers and technical expertise. Jon de la Harpe is also acknowledged for reading the manuscript.
REFERENCES
- 1.Algranati, N. E., S. Sy, and M. Modi. 1999. A branched methoxy 40-kDa polyethylene glycol (PEG) moiety optimizes the pharmacokinetics of PEG-interferon alpha-2a (PEG-IFN) and may explain its enhanced efficacy in chronic hepatitis C. Hepatology 40(Suppl.):190A.
- 2.Climo, M. W., R. L. Patron, B. P. Goldstein, and G. L. Archer. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 42:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donohue, J. H., and S. A. Rosenberg. 1983. The fate of interleukin-2 after in vivo administration. J. Immunol. 130:2203-2208. [PubMed] [Google Scholar]
- 4.Francis, G. E., C. Delgado, and D. Fisher. 1992. PEG-modified proteins, p. 235-263. In M. C. Manning (ed.), Stability of protein pharmaceuticals: in vivo pathways of degradation and strategies for protein stabilization. Plenum Press, New York, N.Y.
- 5.Harrison, E. F., M. E. Fuquay, and W. A. Zygmunt. 1975. Antigenic response to topically applied proteins. Infect. Immun. 11:309-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinds, K., J. J. Koh, L. Joss, F. Liu, M. Baudys, and S. W. Kim. 2000. Synthesis and characterization of poly(ethylene glycol)-insulin conjugates. Bioconjug. Chem. 11:195-201. [DOI] [PubMed] [Google Scholar]
- 7.Kiri, N., G. Archer, and M. W. Climo. 2002. Combinations of lysostaphin with beta-lactams are synergistic against oxacillin-resistant Staphylococcus epidermidis. Antimicrob. Agents Chemother. 46:2017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patron, R. L., M. W. Climo, B. P. Goldstein, and G. L. Archer. 1999. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 43:1754-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quickel, K. E., Jr., R. Selden, J. R. Caldwell, N. F. Nora, and W. Schaffner. 1971. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl. Microbiol. 22:446-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rostin, J., A. L. Smeds, and E. Akerblom. 2000. β-Domain deleted recombinant coagulation factor VIII modified with monomethoxy polyethylene glycol. Bioconjug. Chem. 11:387-396. [DOI] [PubMed] [Google Scholar]
- 11.Schaffner, W., M. A. Melly, J. H. Hash, and M. G. Koenig. 1967. Lysostaphin: an enzymatic approach to staphylococcal disease. I. In vitro studies. Yale J. Biol. Med. 39:215-229. [PMC free article] [PubMed] [Google Scholar]
- 12.Schiavon, O., P. Caliceti, P. Ferruti, and F. M. Veronese. 2000. Therapeutic proteins: a comparison of chemical and biological properties of uricase conjugated to linear or branched poly(ethylene glycol) and poly(N-acryloylmorpholine). Farmaco 55:264-269. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka, H., and T. Tokiwa. 1990. Influence of renal and hepatic failure on the pharmacokinetics of recombinant human granulocyte colony-stimulating factor (KRN8601) in the rat. Cancer Res. 50:6615-6619. [PubMed] [Google Scholar]
- 14.Tsunoda, S., T. Ishikawa, Y. Yamamoto, H. Kamada, K. Koizumi, J. Matsui, Y. Tsutsumi, T. Hirano, and T. Mayumi. 1999. Enhanced antitumor potency of polyethylene glycolylated tumor necrosis factor-alpha: a novel polymer-conjugation technique with a reversible amino-protective reagent. J. Pharmacol. Exp. Ther. 290:368-372. [PubMed] [Google Scholar]
- 15.Ueno, T., K. Ohtawa, Y. Kimoto, K. Sakurai, Y. Kodera, M. Hiroto, A. Matsushima, H. Nishimura, and Y. Inada. 2000. Polyethylene glycol-modified concanavalin A as an effective agent to stimulate anti-tumor cytotoxicity. Cancer Detect. Prev. 24:100-106. [PubMed] [Google Scholar]
- 16.Veronese, F. M. 2001. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials 22:405-417. [DOI] [PubMed] [Google Scholar]
- 17.Zygmunt, W. A., and P. A. Tavormina. 1972. Lysostaphin: model for a specific enzymatic approach to infectious disease. Prog. Drug Res. 16:309-333. [DOI] [PubMed] [Google Scholar]