Abstract
Three antimalarial drugs, artesunate, pyrimethamine, and pamaquine, were evaluated for their growth-inhibitory effects against Babesia equi and Babesia caballi in in vitro culture. B. equi was more resistant to pyrimethamine than B. caballi. B. equi was also found to be more sensitive to artesunate and pamaquine than B. caballi. Of the three compounds, pyrimethamine gave the most promise for in vivo effectiveness.
Equine babesiosis caused by Babesia equi and Babesia caballi is an economically important protozoan disease in horses in the tropical and subtropical regions, including Central and South America, Africa, Asia, and Southern Europe (18). Babesia parasites invade and destroy red blood cells (RBC) and induce severe clinical symptoms, such as fever, anemia, jaundice, and edema, in infected horses (4). Babesiacidal drugs used in the treatment of equine babesiosis are limited and are either ineffective in completely eliminating the parasites and/or cause pronounced, severe, and drastic side effects (11, 21). Considering an expanding horse industry where horses are traded worldwide, the risk of parasite transmission and spread of infection is inevitable. In Japan in particular, while equine babesiosis is nonexistent to this day, there is a growing apprehension that the situation may be reversed, given the importation of an increasing number of horses from countries where the infection is endemic. Thus, a continuous search for alternative and effective chemotherapeutic drugs is necessary.
Artesunate is a semisynthetic derivative of artemisinin, the active component of the Chinese herb Artemisia annua, and consists of the sodium succinyl salt of dihydroartemisinin (9). Artemisinin-type compounds reduce malaria parasitemia more rapidly than any other known antimalarial drugs and are effective against multidrug-resistant malaria parasites (13, 16). Pyrimethamine is a potent inhibitor of dihydrofolate reductase and disrupts folate metabolism (17). Pamaquine, an 8-aminoquinoline, is effective against preerythrocytic malaria parasites (20) and theileriosis (7). Moreover, pamaquine and primaquine, also an 8-aminoquinoline, are more effective against chloroquine-resistant strains of Plasmodium falciparum and are very likely to principally target the asexual erythrocytic or blood stages (6). In the present study, taking into account the biological similarities and presumably close phylogenetic relationship of the intraerythrocytic Plasmodium and Babesia parasites, we evaluated the efficacy of those three compounds.
USDA strains of B. equi and B. caballi parasites were grown in equine RBC using a previously established continuous microaerophilous stationary phase culture system (1). Stock solutions of 60 mg of artesunate (Gurin No. 2 Pharmaceutical Factory, Guanaxi, China)/ml in 5% sodium bicarbonate, 2.49 mg of pyrimethamine (Sigma Chemical Co.)/ml in 2.5% dimethyl sulfoxide (WAKO Pure Chemical Industrial, Ltd., Osaka, Japan), and 200 mg/ml of pamaquine in 0.5% vegetal oil (Nihon Zenyaku Kougyou Kabusikigaisha, Fukushima, Japan) were kept at −20°C until use. The range of drug concentrations used was based on the results of preliminary assays we had earlier conducted (data not shown). The in vitro growth-inhibitory assay described earlier was adopted (2, 8). Babesia parasite cultures that had reached 3 to 5% parasitemia mixed with normal horse RBC were used in the preparation of initial cultures containing 1% parasitized RBC. To each well, 100 μl of the parasite-RBC mixture was dispensed, and 1 ml of the growth medium with the appropriate concentration of the indicated drug was added. The evaluation of the growth-inhibitory effect per drug concentration per parasite species was monitored in triplicate and in three separate trials. Culture plates were kept in a humidified 5% CO2 incubator at 37°C. Per well, 1 ml of the culture medium was replaced daily with fresh medium plus the indicated drug. Daily percent parasitemia in Giemsa-stained culture smears was calculated based on four to six microscopic fields covering approximately 1,000 cells. Following the last day of drug administration, 1 ml of drug-free medium and 70 μl of normal equine RBC in fresh growth medium were added to 30 μl of the previously drug-treated cultures, and parasite viability or regrowth (=recrudescence) was checked for four days after withdraw of drugs.
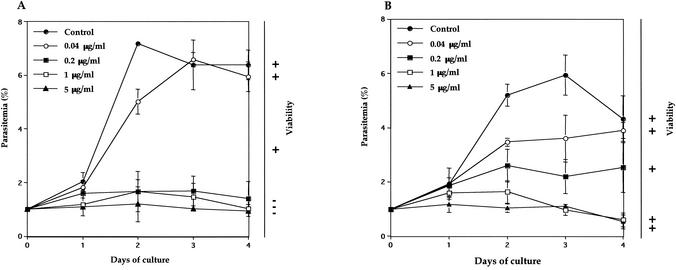
B. equi and B. caballi were grown in in vitro culture from 1% parasitemia under indicated concentrations of artesunate, and the parasitemia was compared with the control. Complete growth inhibition of B. equi and B. caballi was observed at more than 0.2 and 1.0 μg/ml, respectively (Fig. 1). We also confirmed that the growth medium containing 0.2% sodium bicarbonate, which was used to dilute the artesunate, did not influence the growth of either parasite (data not shown). Values of 50% inhibitory concentration (IC50) of artesunate against B. equi and B. caballi were 0.1 and 0.18 μg/ml, respectively, and these values are higher than that for P. falciparum (3). The viability tests showed that B. equi did not grow but B. caballi survived and regrew after withdrawal of the drug (Fig. 1). Therefore, artesunate is able to destroy B. equi but unable to destroy B. caballi.
FIG. 1.
Growth curves of B. equi (A) and B. caballi (B) in in vitro culture treated with different concentrations of artesunate and the determination of parasite viability or recrudescence after withdrawal of the drug. Cultures were initiated at 1% parasitemia, and smears were made every day. Parasitemias were determined in Giemsa-stained thin blood smears. Viability was indicated as + when parasites were still grown and as − when parasites did not grow again during 4 days in the absence of drug after the growth-inhibitory assay. Each value represents the mean and standard deviation from the three blood film counts. Data are representative of three separate experiments.
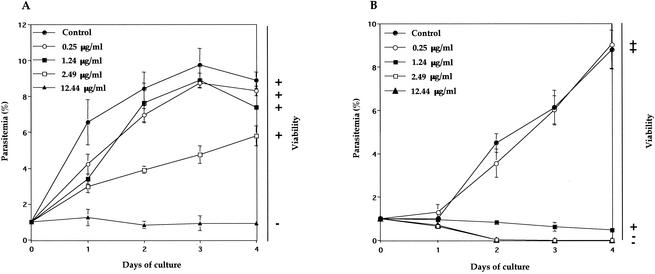
Both parasites were grown in in vitro culture from 1% parasitemia under various concentrations of pyrimethamine. Complete growth inhibition was observed for B. equi at 12.44 μg/ml (Fig. 2A). In contrast, the inhibition was observed for B. caballi at 1.24 μg/ml and higher concentrations (Fig. 2B). A growth medium containing 2.5% dimethyl sulfoxide did not show any inhibitory effects against either parasite (data not shown). B. equi, whose growth was inhibited in the presence of the drug, did not grow again after the withdrawal of the drug (Fig. 2A). However, a few B. caballi parasites cultured at a pyrimethamine concentration of 1.24 μg/ml were still alive (Fig. 2B). IC50s of pyrimethamine against B. equi and B. caballi were 3.2 and 0.8 μg/ml, respectively, and these values are higher than that against B. bovis (14) and P. falciparum (IC50 = 248 ng/ml) (5).
FIG. 2.
Growth curves of B. equi (A) and B. caballi (B) in in vitro culture treated with different concentrations of pyrimethamine and the determination of parasite viability or recrudescence after withdrawal of the drug. Cultures were initiated at 1% parasitemia, and smears were made every day. Parasitemias were determined in Giemsa-stained thin blood smears. Viability was indicated as + when parasites were still grown and as − when parasites did not grow again during 4 days in the absence of drug after the growth-inhibitory assay. Each value represents the mean and standard deviation from the three blood film counts. Data are representative of three separate experiments.
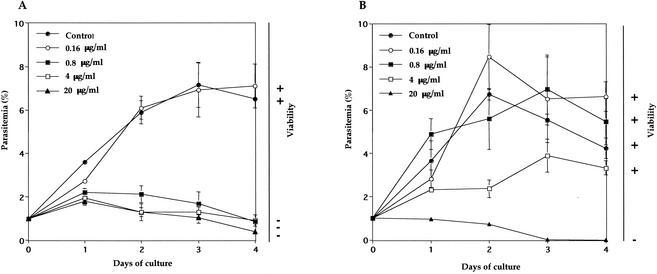
Both parasites were grown in in vitro culture from 1% parasitemia under indicated concentrations of pamaquine. As shown in Fig. 3, complete growth inhibition of B. equi and B. caballi was observed at concentrations exceeding 0.8 and 20 μg/ml, respectively (Fig. 3). IC50s of pamaquine for B. equi and B. caballi were 0.4 and 2.4 μg/ml, respectively, and they are higher than that for P. falciparum (20). A growth medium containing 0.5% vegetal oil did not show any inhibitory effect against either of the parasites (data not shown). In the viability tests, the parasites whose growth was inhibited did not grow again (Fig. 3).
FIG. 3.
Growth curves of B. equi (A) and B. caballi (B) in in vitro culture treated with different concentrations of pamaquine and the determination of parasite viability or recrudescence after withdrawal of the drug. Cultures were initiated at 1% parasitemia, and smears were made every day. Parasitemias were determined in Giemsa-stained thin blood smears. Viability was indicated as + when parasites were still grown and as − when parasites did not grow again during 4 days in the absence of drug after the growth-inhibitory assay. Each value represents the mean and standard deviation from the three blood film counts. Data are representative of three separate experiments.
The mechanism of action of these compounds is not yet fully understood. Artemisinin-type compounds are found to block heme polymerization, causing a buildup of monomeric (=ferrous) free heme toxic to the parasites (16). In the present study, artesunate showed the growth-inhibitory effect on equine Babesia parasites, although Babesia parasites do not make the hemozoin pigment (10), suggesting an alternative mechanism of action including the role of reactive oxygen species. Pyrimethamine inhibited incorporation of [3H]hypoxanthine by B. bovis due to the perturbation of the parasite's dihydrofolate reductase, an enzyme essential in the de novo synthesis of thymidylate (14). Therefore, pyrimethamine may act similarly against equine Babesia parasites. Although several modes of action of 8-aminoquinolines against P. falciparum have been examined (12, 15, 20), recent studies suggest that primaquine's effect is on receptor recycling due to interference with calmodulin function at the endosome (19). Further studies are necessary to examine the mode of action of these agents against equine Babesia parasites.
In conclusion, we demonstrated that artesunate, pyrimethamine, and pamaquine have inhibitory effects on the in vitro growth of B. equi and of B. caballi. Pyrimethamine gave the most promise for in vivo application. Further studies will be required to examine various drug combinations and their side effects before these drugs are used on infected animals in the field.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Avarzed, A., I. Igarashi, T. Kanemaru, K. Hirumi, Y. Omata, A. Saito, T. Oyamada, H. Nagasawa, Y. Toyoda, and N. Suzuki. 1997. Improved in vitro cultivation of Babesia caballi. J. Vet. Med. Sci. 59:479-481. [DOI] [PubMed] [Google Scholar]
- 2.Brockelman, C., and P. Tan-ariya. 1991. Development of an in vitro microtest to assess drug susceptibility of Babesia bovis and Babesia bigemina. J. Parasitol. 77:994-997. [PubMed] [Google Scholar]
- 3.Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brüning, A. 1996. Equine piroplasmosis an update on diagnosis, treatment and prevention. Br. Vet. J. 152:139-151. [DOI] [PubMed] [Google Scholar]
- 5.Fidock, D. A., and T. E. Wellems. 1997. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR 992210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA 94:10931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geary, T. G., A. A. Divo, and J. B. Jensen. 1987. Activity of quinoline-containing antimalarials against chloroquine-sensitive and -resistant strains of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 81:499-503. [DOI] [PubMed] [Google Scholar]
- 7.Hagiwara, K., M. Tsuji, C. Ishihara, M. Tajima, T. Kurosawa, H. Iwai, and K. Takahashi. 1993. The Bo-RBC-SCID mouse model for evaluating the efficacy of anti-theilerial drugs. Int. J. Parasitol. 23:13-16. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi, I., F. K. Njonge, Y. Kaneko, and Y. Nakamura. 1998. Babesia bigemina: in vitro and in vivo effects of curdlan sulfate on growth of parasites. Exp. Parasitol. 90:290-293. [DOI] [PubMed] [Google Scholar]
- 9.Ittarat, W., R. Udomsangpeth, K. T. Chotivanich, and S. Looareesuwan. 1999. The effects of quinine and artesunate treatment on plasma tumor necrosis factor levels in malaria-infected patients. Southeast Asian J. Trop. Med. Public Health 30:7-10. [PubMed] [Google Scholar]
- 10.Kawai, S., I. Igarashi, A. Avarzed, K. Miyazawa, H. Ikadai, H. Nagasawa, K. Fujisaki, T. Mikami, N. Suzuki, and H. Matsuda. 1999. Ultrastructural characteristics of Babesia caballi in equine erythrocytes in vitro. Parasitol. Res. 85:794-799. [DOI] [PubMed] [Google Scholar]
- 11.Kuttler, K. L., J. L. Zaugg, and C. A. Gipson. 1987. Imidocarb and parvaquone in the treatment of piroplasmosis (Babesia equi) in equids. Am. J. Vet. Res. 48:1613-1616. [PubMed] [Google Scholar]
- 12.Lanners, H. N. 1991. Effect of the 8-aminoquinoline primaquine on culture-derived gametocytes of the malaria parasite Plasmodium falcipalm. Parasitol. Res. 77:478-481. [DOI] [PubMed] [Google Scholar]
- 13.Meshnick, S. R., T. E. Taylor, and P. Kamchonwongpaisan. 1996. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol. Rev. 60:301-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nott, S. E., and A. S. Bagnara. 1993. The toxicity of antifolates in Babesia bovis. Int. J. Parasitol. 23:399-402. [DOI] [PubMed] [Google Scholar]
- 15.Olenick, J. G. 1975. Primaquine, p. 516-520. In J. W. Corcoran and F. E. Hahn (ed.), Antibiotics III: mechanism of action of anti-microbial and anti-tumor agents. Springer-Verlag, New York, N.Y.
- 16.Olliaro, P. L., R. K. Haynes, B. Meunier, and Y. Yuthavong. 2001. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 17:122-126. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds, M. G., J. Oh, and D. S. Roos. 2001. In vitro generation of novel pyrimethamine resistance mutations in the Toxoplasma gondii dihydrofolate reductase. Antimicrob. Agents Chemother. 45:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schein, E. 1988. Equine babesiosis, p. 197-208. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Boca Raton, Fla.
- 19.van Weert, A. W., H. J. Geuze, B. Groothuis, and W. Stoorvogel. 2000. Primaquine interferes with membrane recycling from endosomes to the plasma membrane through a direct interaction with endosomes which does not involve neutralisation of endosomal pH nor osmotic swelling of endosomes. Eur. J. Cell Biol. 79:394-399. [DOI] [PubMed] [Google Scholar]
- 20.Vennerstrom, J. L., E. O. Nuzum, R. E. Miller, A. Dorn, L. Gerena, P. A. Dande, W. Y. Ellis, R. G. Ridley, and W. K. Milhous. 1999. 8-Aminoquinolines active against blood stage Plasmodium falciparum in vitro inhibit hematin polymerization. Antimicrob. Agents Chemother. 43:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaugg, J. L., and V. M. Lane. 1992. Efficacy of buparvaquone as a therapeutic and clearing agent of Babesia equi of European origin in horses. Am. J. Vet. Res. 53:1396-1399. [PubMed] [Google Scholar]