Abstract
Embryos carrying null mutations of both retinoid X receptors α and β (RXRα−/−/RXRβ−/− mutants) were generated. These mutant embryos die between 9.5 and 10.5 days of gestation and display a wide range of abnormalities. The cause of the lethality appears to be the lack of formation of the labyrinthine zone of the chorioallantoic placenta. In a thorough analysis of mutant conceptuses, we establish that RXRs, through heterodimerization with retinoic acid receptors, are essential for postimplantation embryonic development before placentogenesis. RXRs are also essential for the formation of the chorioallantoic placenta, most probably through RXR/peroxisomal proliferator-activated receptor-γ heterodimers. Interestingly, as a RXR ligand appears dispensable, placentogenesis must be controlled by a yet unknown hormonal ligand(s) activating the heterodimerization partner(s) of RXRs.
Keywords: nuclear receptor, heterodimerization, vitamin A, mouse embryogenesis, placenta
Retinoid X receptors RXRα, RXRβ, and RXRγ are thought to be key actors in several nuclear receptor signaling pathways. They specifically bind 9-cis-retinoic acid and thus may be directly involved in the transduction of retinoid signals. In transfected cultured cells and in established cell lines, RXRs can act either as homodimers or heterodimeric partners of a number of other nuclear receptors, including retinoic acid receptors (RARα, -β, and -γ), thyroid hormone receptors (TRα and -β), the vitamin D3 receptor, peroxisomal proliferator-activated receptors (PPARα, -β, and -γ), and a number of orphan receptors (reviewed in refs. 1 and 2).
RXR knockouts in the mouse therefore should generate defects that are also associated with null mutations of their heterodimerization partners. RXRα−/− fetuses die at embryonic days (E) 12.5–16.5 and display a myocardial hypoplasia, as well as conotruncal and ocular defects that have been observed in RAR/RAR compound mutants and belong to the fetal vitamin A deficiency syndrome (3, 4). Moreover, compound mutants in which either a RXRα null mutation or a mutation (RXRαAF2°) deleting the last helical α structure of the RXRα ligand-binding domain (AF-2 AD core) is associated with a null mutation of one of the three RAR isotypes (α, β, or γ) altogether recapitulate most of the abnormalities exhibited by RAR/RAR compound mutants (refs. 3, 5, and 6 and references therein). In contrast, there is no apparent synergism during development between RARs (α, β, or γ) and RXRβ or RXRγ inactivations (5). Thus, RXRα/RAR (α, β, and γ) heterodimers are the main functional units that transduce retinoid signals during ontogenesis.
The inactivation of RXRγ in a RXRα−/− genetic background does not result in additional abnormalities (7), whereas RXRβ−/−/RXRγ−/− mice are morphologically normal while exhibiting locomotor deficiencies also observed in RARβ−/− mice (8). We have reported previously that RXRα−/−/RXRβ−/− mutant embryos die earlier than RXRα−/− mutants and display a more severe myocardial hypoplasia, thus indicating that RXRβ can perform certain developmental functions (9). We have analyzed here in greater detail the phenotype of RXRα/RXRβ compound mutants to further define the role of RXRs during mouse ontogenesis.
MATERIALS AND METHODS
Mice.
RXRα and RXRβ single mutant mouse lines have been described (3, 10). RXRα/RXRβ double null embryos were obtained by crosses of RXRα+/−/RXRβ+/− males with RXRα+/−/RXRβ+/− or RXRα+/−/RXRβ−/− females. Genotypes were determined by PCR on tail (postnatally) or yolk sac (embryos) DNA (primers and PCR conditions are available upon request).
In Situ Hybridization.
Placentas were embedded in OCT medium and frozen immediately on dry ice. In situ hybridization was performed on 10-μm-thick sections mounted on gelatin-coated slides. Sections were postfixed in 4% paraformaldehyde in PBS for 30 min at 4°C, washed twice in PBS, and hybridized with digoxigenin-labeled probes for 4311, placental lactogen-1 (pl-1), and Mash-2 cDNAs (ref. 20 and references therein) as described (11).
RESULTS AND DISCUSSION
RXRα−/−/RXRβ−/− Compound Mutants Die Between E9.5 and E10.5.
RXRα−/−/RXRβ−/− mutants were generated by intercrossing RXRα+/−/RXRβ+/− males with either RXRα+/−/RXRβ+/− or RXRα+/−/RXRβ−/− females. These matings did not yield any double homozygote among 58 fetuses at E13.5 (i.e., about a day before the death of most RXRα−/− mutants) (3). E9.5 (Fig. 1 b–g) and E10.5 double homozygotes were found, but at a ratio decreased by ≈30% when compared with the Mendelian distribution (data not shown). Three of 20 E9.5 double homozygotes were dead (as judged from the absence of heart beats), two of which were obviously necrotic. Out of the four E10.5 double homozygotes, one was alive, one was dead but still well preserved (see EM, Fig. 2f), and the two others were necrotic. No double homozygotes were recovered at E11.5 among 32 embryos from three heterozygote matings. Thus, the vast majority of RXRα−/−/RXRβ−/− mutants die between E9.5 and E10.5.
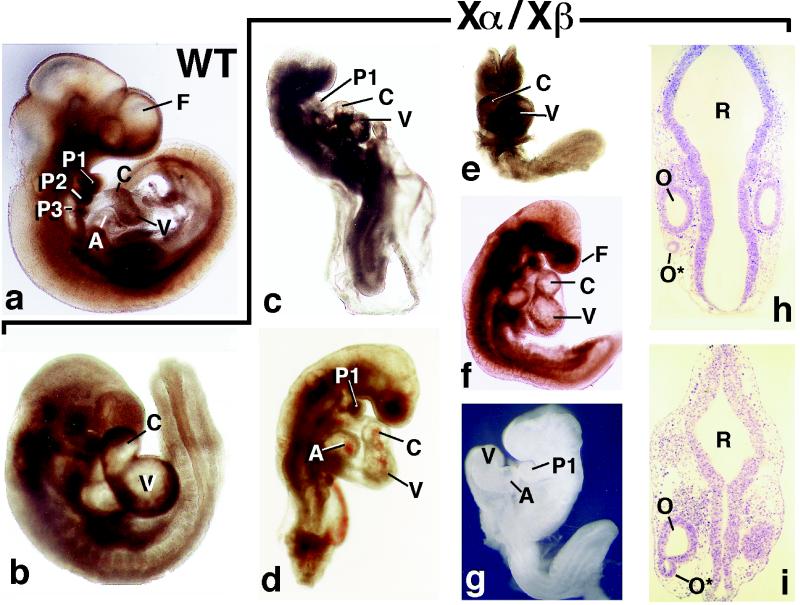
Figure 1.
Morphology of E9.5 wild-type (WT) and RXRα−/−/RXRβ−/− (Xα/Xβ) mutants. (a–g) External views. In b, note that the extremity of the tail is oriented perpendicularly to the plane of the embryo, which reflects an abnormal turning. (h and i) Frontal histological sections of the hindbrain region. A, atrium; C, conotruncus; F, frontonasal region; O and O*, orthotopic and ectopic otic vesicles; P1–3, first, second, and third pharyngeal arches; R, rhombencephalon; V, ventricle. All embryos are displayed at the same magnification (×17).
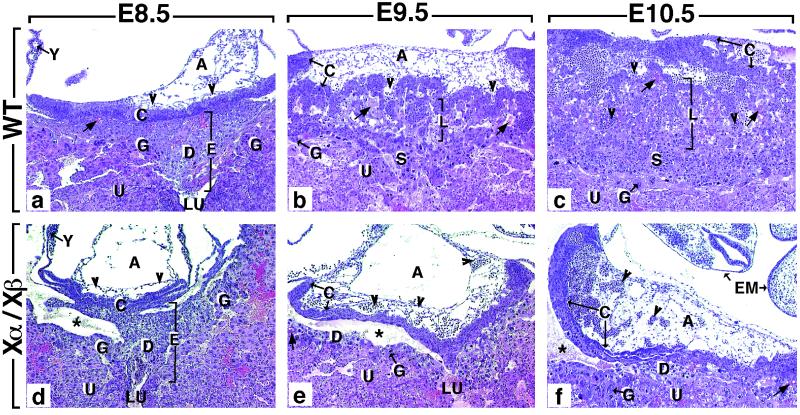
Figure 2.
Comparison of histological sections through the implantation site of wild-type (WT) (a–c) and RXRα−/−/RXRβ−/− (Xα/Xβ) mutants (d–f). (a) In E8.5 WT embryos, the polar extraembryonic ectoderm has generated the ectoplacental cone (E) and the chorionic (ectoplacental) plate (C). The ectoplacental cone penetrates into the uterine epithelium (U). It contains embryo-derived diploid and polyploid (giant) trophoblast cells (D and G, respectively), as well as maternal blood sinuses (arrows). The chorionic plate forms a solid wall of epithelial cells continuous with the ectoplacental cone and is fused to the mesoderm of the allantois (A), which contains extraembryonic blood vessels (arrowheads). (b) At E9.5, the placenta consists of four regions: (i) the chorionic plate (C), which is now traversed by allantoic capillaries (arrowheads); (ii) the labyrinthine zone (L), which is composed of strands of diploid trophoblast cells and of a network of extraembryonic capillaries interspersed with maternal blood sinuses; (iii) the spongiotrophoblast zone (S), in which only maternal blood circulates; and (iv) the giant cell zone (G). (c) At E10.5, the labyrinthine zone has enlarged markedly. (d–f) The size of the structures contributing to the mutant placenta increases between E8.5 and E10.5, but their differentiation is arrested at E8.5 (see the main text for further details). A, allantois; C, chorionic plate; D, diploid trophoblast; E, ectoplacental cone; EM, embryo; G, giant cell zone; L, labyrinthine zone; S, spongiotrophoblast zone; U, uterine epithelium; LU, uterine lumen; Y, yolk sac; arrowheads and arrow point to allantoic capillaries (containing nucleated erythrocytes) and to maternal blood sinuses, respectively. The asterisk indicates an artifactual tissue detachment generated during embedding. (×50.)
RXRα−/−/RXRβ−/− Embryos Are Severely Malformed.
E8.5 RXRα−/−/RXRβ−/− mutants (n = 4) were externally indistinguishable from their littermates (data not shown). In contrast, all E9.5 RXRα−/−/RXRβ−/− mutants displayed variable degrees of truncation of the caudal region, incomplete (Fig. 1 c–e and g) or abnormal (e.g., Fig. 1b) body turning, dilated heart cavities (C and V in Fig. 1 b, e, and f), and wavy aspect of the neural tube (not shown). Twelve of these double mutants were severely shortened (compare Fig. 1 a with c–g) and lacked the second and third pharyngeal arches (Fig. 1 a and c–g); 40% exhibited a major hypoplasia of the frontonasal region (Fig. 1 c, d, and f), and 30% had a complete failure of closure of the neural tube (Fig. 1 e and g). In four of six externally nearly normal RXRα−/−/RXRβ−/− embryos (e.g., Fig. 1b), serial histological sections revealed a small uni- or bilateral vesicle lined by epithelial cells that was located lateral to the postotic hindbrain (data not shown). In one embryo, this vesicle was continuous with the otocyst, indicating that it most probably corresponds to an ectopic otocyst (0* in Fig. 1i). With the exception of ectopic otocysts, dilated heart cavities, and precocious differentiation of cardiomyocytes (9), all of the above defects correspond to developmental arrests taking place between E8.5 and E9.5. Interestingly, a strikingly similar spectrum of developmental arrests has been reported under various conditions of energy deficiency because of malnutrition and hypoxia related to failure of erythropoiesis (12, 13), yolk sac abnormalities (14), or in vitro culture of mouse embryos (15). These defect similarities suggest that RXRs could be involved in basic metabolic processes in postimplantation embryos.
Absence of Formation of the Chorioallantoic Placenta Is Responsible for the Death of RXRα−/−/RXRβ−/− Embryos.
Lethality during the postimplantation period (i.e., E5.0–E10.5) can be the result of either defects in yolk sac or chorioallantoic placentas (16) or abnormal cardiac looping (17–19). Cardiac looping and chamber formation appeared normal in E9.5 RXRα−/−/RXRβ−/− embryos (Fig. 1 b–g). At E8.5 and E9.5, the yolk sac of RXRα−/−/RXRβ−/− conceptuses was histologically normal (Y in Fig. 2 a and d; data not shown). At E8.5, extraembryonic structures contributing to the RXRα−/−/RXRβ−/− placenta (allantois, chorionic plate, and ectoplacental cone) were indistinguishable from their wild-type homologs (compare A, C, and E in Fig. 2 a and d). In all RXRα−/−/RXRβ−/− placentas examined at E9.5 (n = 4) and E10.5 (n = 1), the number of diploid trophoblast cells appeared markedly reduced (compare S in Fig. 2b with D in Fig. 2e). Moreover, the mutant chorionic plate (C) was not pierced by allantoic capillaries (arrowheads), and the labyrinthine zone (L) was absent (Fig. 2 b, c, e, and f).
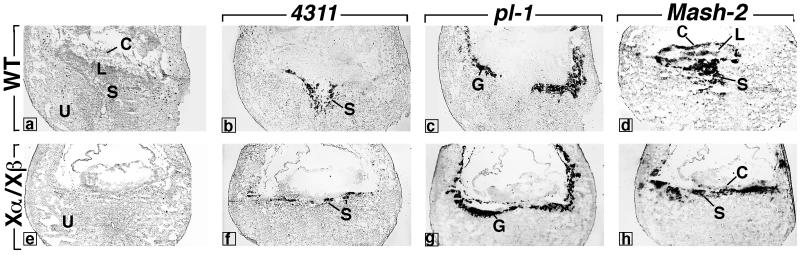
Placental defects were analyzed further at E9.5 by using markers of the trophoblast lineage: Mash-2, which normally is expressed in both spongio- and labyrinthine trophoblast cells, and the 4311 and pl-1 genes, which are expressed in the spongiotrophoblast and giant cells, respectively (ref. 20 and references therein). These markers revealed the presence of all three trophoblastic cell types in RXRα−/−/RXRβ−/− conceptuses (Fig. 3). However, the trophoblast giant cell zone was not interrupted at the implantation site, in contrast to age-matched wild-type conceptuses (Fig. 3 c and g, G), and the thickness of the spongiotrophoblast zone was severely reduced (Fig. 3 b and f, S).
Figure 3.
Expression of trophoblast marker genes in wild-type (WT) and RXRα−/−/RXRβ−/− (Xα/Xβ) placentas at E9.5. a–d and e–h are adjacent frozen sections stained with hematoxylin (a and e) or hybridized with antisense riboprobes for 4311, pl-1, and Mash-2 (b–d and f–h). C, chorionic plate; G, giant cell zone; L, labyrinthine zone; S, spongiotrophoblast; U, uterine epithelium.
Thus, in RXRα−/−/RXRβ−/− mutants, the yolk sac circulation forms normally and the allantois contacts the chorionic plate. However, the development of the chorioallantoic placenta does not progress further, resulting in a lack of labyrinthine zone. The deficiency of spongiotrophoblast cells at the implantation site of RXRα−/−/RXRβ−/− mutants cannot account for the agenesis of the labyrinth, as Mash-2 null mutants that lack spongiotrophoblast nevertheless develop a small labyrinth (20). On the other hand, changes in the spatial distribution of the trophoblast giant cells probably represents a secondary consequence of the labyrinthine agenesis, because it is widely held that trophoblastic giant cells normally migrate away from an inhibitory influence of other components of the placenta (21). Therefore, the primary cause of the labyrinthine agenesis in RXRα−/−/RXRβ−/− mutants may correspond either to a failure of allantoic capillary sprouting or to a lack of proliferation of the trophoblast cells forming the chorionic plate. This latter possibility is supported by the observation that RXRα−/−/RXRβ+/− conceptuses, which died between E10.5 and E11.5, developed a labyrinthine zone that was indistinguishable from its E9.5 wild-type counterpart, but never grew beyond this stage (data not shown). In contrast, RXRα−/− conceptuses that die at the fetal stage (E12.5–E16.5) (3) displayed milder placental alterations (22). In any event, as the labyrinth normally provides an interface for nutrient and gas exchanges between the maternal and embryonic circulations, the failure to establish a functional chorioallantoic placenta is sufficient on its own to account for the death of the RXRα−/−/RXRβ−/− conceptuses between E9.5 and E10.5, when the primary, yolk sac-derived, placenta can no longer meet the increasing metabolic demand of the embryo (20, 23–25). On the other hand, the embryonic abnormalities corresponding to developmental arrests at E8.5–E9.5 (see Fig. 1 b–g and above) cannot be a result of the placental dysfunction, as failure of fusion of the allantois with the chorionic plate does not alter the morphogenesis of E9.5 embryos (23, 26, 27).
RXR Functions in Early Embryogenesis and Placentogenesis Are Likely to Involve Heterodimerization with RARs and PPARγ, Respectively.
Because RXRs potentially can function as either homodimers or heterodimerization partners for a number of nuclear receptors, our present results raise the question of which signaling pathways could be altered upon simultaneous inactivation of RXRα and RXRβ. It is unlikely that the present defects reflect a critical function(s) of RXR homodimers at E8.5–E10.5, as we previously showed that triple mutants lacking RXRβ, RXRγ, and the ligand-inducible activation function AF-2 of RXRα (RXRαAF2°/RXRβ−/−/RXRγ−/−) survive until at least E14.5 (6) and display only minor placental defects (M.M., unpublished results). The occurrence, in RXRα−/−/RXRβ−/− mutants, of ectopic otocysts, myocardial hypoplasia, and precocious differentiation of cardiomyocytes likely reflects the involvement of these RXRs in RAR/RXR heterodimer-mediated signaling pathways, as similar defects have been found in RARα−/−/RARβ−/−, RARα−/−/RARγ−/−, and RARα−/− mutants, respectively (refs. 5 and 28; unpublished results). Along the same lines, the above cardiac muscle defects are generated in embryos or fetuses deprived from vitamin A (ref. 9 and references therein), and the developmental arrest defects seen in RXRα−/−/RXRβ−/− mutants (Fig. 1 c–g) are fully penetrant in mouse embryos lacking retinaldehyde dehydrogenase-2 (RALDH-2), an essential retinoic acid (RA)-synthesizing enzyme (K. Niederreither, V. Subbarayan, P. Dollé, and P.C., unpublished results). Interestingly, the early arrest of cardiac morphogenesis displayed by these RALDH-2−/− embryos was not observed in our present mutants, indicating that some RA signaling mediated by RXRγ/RAR (α, β, or γ) heterodimers most likely is occurring in RXRα−/−/RXRβ−/− mutants. It is also noteworthy that RXRαAF2°/RXRβ−/−/RXRγ−/− mutants lacking all RXR AF-2s (see above) never exhibit the RXRα−/−/RXRβ−/− developmental arrest defects, thus suggesting that a ligand capable of activating RXRs (9-cis-RA or RXR ligands as yet uncovered) is not required for the completion of the underlying developmental events, in marked contrast to many other events for which the integrity of RXR AF-2 is indispensable (6).
In contrast to the above embryonic defects, it appears that a critical function of RXR/RAR heterodimers in placental labyrinthine formation can be ruled out as (i) all RARα−/−/RARβ−/− and RARβ−/−/RARγ−/− mutants as well as 50% of RARα−/−/RARγ−/− mutants survive until birth (29–31) and (ii) RXRα/RAR (α, β, or γ) double null mutants do not die earlier than RXRα−/− mutants (5), as one might have expected if their labyrinthine zone were more affected. PPARγ−/− conceptuses exhibit a similar, albeit less severe, placental agenesis (Y. Barak, E. S. Ong, M. C. Nelson, Y. Z. Jones, and R. M. Evans, personal communication), which possibly may reflect a functional redundancy between PPARs. Interestingly, as the placenta of RXRαAF2°/RXRβ−/−/RXRγ−/− mutant embryos is only mildly affected, this RXR/PPARγ signaling does not appear to require an RXR ligand.
CONCLUSION
The present study establishes that RXRs through heterodimerization with RARs are essential for postimplantation embryonic development before placentogenesis. RXRs are also essential for the formation of the chorioallantoic placenta, most probably through heterodimerization with PPARγ. Interestingly, in both cases a RXR ligand appears dispensable, thus indicating that (i) RXRs can be instrumental as transcriptionally silent obligatory heterodimerization partners during early mouse embryogenesis, and (ii) mouse placentogenesis is controlled by a yet unidentified hormonal signal(s) transduced by the heterodimerization partner (most likely PPARγ).
Acknowledgments
We are grateful to Dr. F. Guillemot (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) for his gift of the Mash-2 cDNA and to Dr. J. Rossant (Mount Sinai Hospital, Toronto, Canada) for providing us with the 4311 and pl-1 probes. We thank B. Weber, C. Fisher, and I. Tilly for technical assistance and Jean-Luc Vonesch and the secretarial staff for their help in the preparation of the manuscript. This work was supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Hôpital Universitaire de Strasbourg, the Collège de France, the Association pour la Recherche sur le Cancer, the Fondation pour la Recherche Medicale, the Ligue Nationale contre le Cancer, Bristol Myers Squibb, and European Economic Community Contract FAIR-CT97-3220. O.W. was supported by a fellowship from the Ministère de l’Éducation Supérieure et de la Recherche.
ABBREVIATIONS
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- PPAR
peroxisomal proliferator-activated receptor
- AF-2
activation function-2
- E
embryonic day
References
- 1.Chambon P. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 2.Perlmann T, Evans R M. Cell. 1997;90:391–397. doi: 10.1016/s0092-8674(00)80498-5. [DOI] [PubMed] [Google Scholar]
- 3.Kastner P, Grondona J M, Mark M, Gansmuller A, Le Meur M, Decimo D, Vonesch J L, Dolle P, Chambon P. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 4.Sucov H M, Dyson E, Gumeringer C L, Price J, Chien K R, Evans R M. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 5.Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona J M, Chambon P. Development (Cambridge, UK) 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- 6.Mascrez B, Mark M, Dierich A, Ghyselinck N B, Kastner P, Chambon P. Development (Cambridge, UK) 1998;125:4691–4707. doi: 10.1242/dev.125.23.4691. [DOI] [PubMed] [Google Scholar]
- 7.Krezel W, Dupe V, Mark M, Dierich A, Kastner P, Chambon P. Proc Natl Acad Sci USA. 1996;93:9010–9014. doi: 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krezel W, Ghyselinck N, Samad T A, Dupe V, Kastner P, Borrelli E, Chambon P. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- 9.Kastner P, Messaddeq N, Mark M, Wendling O, Grondona J M, Ward S, Ghyselinck N, Chambon P. Development (Cambridge, UK) 1997;124:4749–4758. doi: 10.1242/dev.124.23.4749. [DOI] [PubMed] [Google Scholar]
- 10.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona J M, Decimo D, Krezel W, Dierich A, Chambon P. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 11.Myat A, Henrique D, Ish-Horowicz D, Lewis J. Dev Biol. 1996;174:233–247. doi: 10.1006/dbio.1996.0069. [DOI] [PubMed] [Google Scholar]
- 12.Warren A J, Colledge W H, Carlton M B, Evans M J, Smith A J, Rabbitts T H. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 13.Robb L, Lyons I, Li R, Hartley L, Kontgen F, Harvey R P, Metcalf D, Begley C G. Proc Natl Acad Sci USA. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis A C, Wims M, Spotts G D, Hann S R, Bradley A. Genes Dev. 1993;7:671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- 15.Van Maele-Fabry G, Clotman F, Gofflot F, Bosschaert J, Picard J J. Int J Dev Biol. 1997;41:365–374. [PubMed] [Google Scholar]
- 16.Rinkenberger J L, Cross J C, Werb Z. Dev Genet. 1997;21:6–20. doi: 10.1002/(SICI)1520-6408(1997)21:1<6::AID-DVG2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Fishman M C, Chien K R. Development (Cambridge, UK) 1997;124:2099–2117. doi: 10.1242/dev.124.11.2099. [DOI] [PubMed] [Google Scholar]
- 18.Charng M J, Frenkel P A, Lin Q, Yumida M, Schwartz R J, Olson E N, Overbeek P, Schneider M D. Dev Biol. 1998;199:72–79. doi: 10.1006/dbio.1998.8905. [DOI] [PubMed] [Google Scholar]
- 19.Riley P, Anson-Cartwright L, Cross J C. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 20.Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner A L. Nature (London) 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman M H. The Atlas of Mouse Development. London: Academic; 1992. [Google Scholar]
- 22.Sapin V, Dolle P, Hindelang C, Kastner P, Chambon P. Dev Biol. 1997;191:29–41. doi: 10.1006/dbio.1997.8687. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Weinstein M, Li C, Naski M, Cohen R I, Ornitz D M, Leder P, Deng C. Development (Cambridge, UK) 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- 24.Kozak K R, Abbott B, Hankinson O. Dev Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 25.Luo J, Sladek R, Bader J A, Matthyssen A, Rossant J, Giguere V. Nature (London) 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- 26.Yang J T, Rayburn H, Hynes R O. Development (Cambridge, UK) 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 27.Kwee L, Baldwin H S, Shen H M, Stewart C L, Buck C, Buck C A, Labow M A. Development (Cambridge, UK) 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 28.Mendelsohn C, Lohnes D, Decimo D, Lufkin T, Le Meur M, Chambon P, Mark M. Development (Cambridge, UK) 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- 29.Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Development (Cambridge, UK) 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 30.Ghyselinck N B, Dupe V, Dierich A, Messaddeq N, Garnier J M, Rochette-Egly C, Chambon P, Mark M. Int J Dev Biol. 1997;41:425–447. [PubMed] [Google Scholar]
- 31.Kastner P, Mark M, Chambon P. Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]