Abstract
The antibacterial properties of novel quinoline-indole (QI) agents were examined. QI agents demonstrated potent bactericidal activities against Staphylococcus aureus, killing by lytic and nonlytic mechanisms. S. aureus mutants resistant to a lytic QI agent (SEP 155342) and a nonlytic QI agent (SEP 118843) arose at frequencies of 1.4 × 10−9 and 1.2 × 10−8, respectively, by selection at four times the MICs. Mutants resistant to QI agent SEP 155342 were unstable, but mutants resistant to QI agent SEP 118843 displayed stable resistance. Mutants resistant to QI agent SEP 118843 were not cross resistant to other inhibitors, including QI agent SEP 155342. Addition of QI agents SEP 118843 and SEP 155342 at four times the MIC caused nonspecific inhibition of several macromolecular biosynthetic pathways in S. aureus. Within 10 min, QI agents SEP 118843 and SEP 155342 both interfered with bacterial membrane integrity, as measured by uptake of propidium iodide. Agents from the two classes of the QI agents probably kill staphylococci by separate mechanisms which, nevertheless, both involve interference with cytoplasmic membrane function. Precise structure-activity relationships for the division of QI agents into two classes could not be determined. However, lytic activity was often associated with substitution of a basic amine at position 4 of the quinoline nucleus, whereas compounds with nonlytic activity usually contained an aromatic ring with or without a methoxy substituent at position 4. Nonlytic QI agents such as SEP 118843 may possess selective activity against the prokaryotic membrane since this compound failed to lyse mouse erythrocytes when it was added at a concentration equivalent to four times the MIC for S. aureus.
Resistance to antibiotics is a major problem in the management of infections caused by gram-positive bacteria (9, 21, 31). The situation is particularly critical for treatment of infections caused by Staphylococcus aureus, in which methicillin-resistant (MRSA) and glycopeptide intermediate-resistant (GISA) strains have emerged; these strains are also frequently resistant to multiple classes of antibiotics (27, 45). The recent report of an MRSA isolate resistant to the new oxazolidinone antimicrobial linezolid (46) is a further disturbing trend in the evolution of antimicrobial resistance in staphylococci. New approaches are therefore required to combat staphylococcal infections (24).
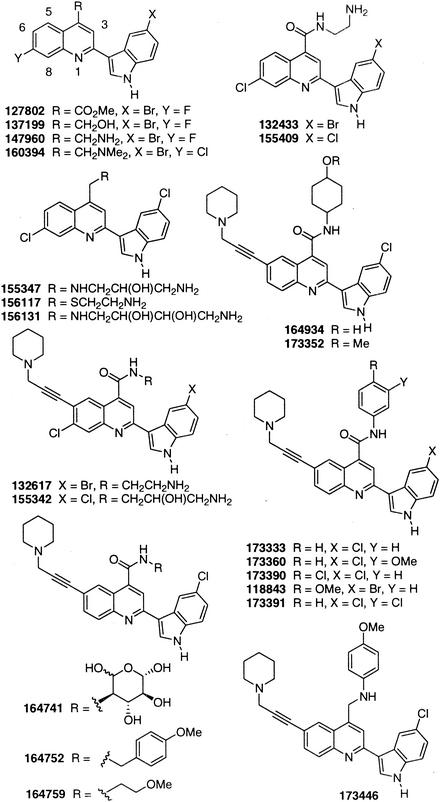
Several new strategies to control staphylococcal infections have been considered in recent years. These include the use of antibiotic combinations (8, 36, 49), the development of new members of existing antibiotic classes (5, 15, 16, 24, 27), and the introduction of novel agents (28, 44). Novel classes will be particularly advantageous since such agents, with unique modes of action, are likely to circumvent existing resistance mechanisms (3). Recently, a novel structural class of antibacterials, the quinoline-indole (QI) agents, was discovered in a library of compounds generated by combinatorial methods (19). None of the compounds in this new structural class is active against gram-negative bacteria, but potent in vitro activity against several gram-positive species was demonstrated, including MRSA and GISA. We have now examined several members of this new class (Fig. 1) in more detail to determine their antistaphylococcal properties, potential for resistance development, and modes of action.
FIG. 1.
Structures of QI agents. Me, methyl.
(Portions of this work were presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17 to 20 September 2000 [B. Oliva, K. Miller, N. Caggiano, G. D. Cuny, M. Z. Hoemann, J. R. Hauske, and I. Chopra, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2180, p. 229, 2000; B. Oliva, G. D. Cuny, M. Z. Hoemann, J. R. Hauske, and I. Chopra, 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2181, p. 230, 2000].)
MATERIALS AND METHODS
Organisms.
The S. aureus and Escherichia coli laboratory strains used in the study are listed in Table 1. The clinical bacteria used for more extensive susceptibility testing were isolates maintained in a culture collection belonging to the University of Leeds.
TABLE 1.
Laboratory strains of S. aureus and E. coli
| Strain | Description | Reference or source |
|---|---|---|
| S. aureus 8325-4 | NCTC 8325 cured of phages 11, 12, and 13 | 33 |
| E. coli | ||
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) F′ [traD36 proAB+lacIΔM15] | 38 |
| M31 | Streptolydigin-sensitive mutant of E. coli K-12 MG1655 | 17 |
| ESS | β-Lactam-hypersensitive mutant of E. coli B | 23 |
| D21f2 | ampA rpsL pro trp his lpcA | 1 |
| UB1005 | F−nalA37 metB1 | 6, 7 |
| DC2 | abs mutant of UB1005 | 6, 7 |
| DC4 | abs mutant of UB1005 | D. P. Clark |
| DC10 | abs mutant of UB1005 | D. P. Clark |
| DC12 | abs mutant of UB1005 | D. P. Clark |
| DC13 | abs mutant of UB1005 | D. P. Clark |
Growth media.
Mueller-Hinton broth (MHB) and Mueller-Hinton agar (MHA) (both conforming to specifications set by the National Committee for Clinical Laboratory Standards) were from Fisher (Loughborough, United Kingdom). Iso-Sensitest agar (used for determination of mutation frequencies) was purchased from Oxoid (Basingstoke, United Kingdom).
Murine blood.
Whole mouse blood treated with heparin was obtained from Harlan Sera Laboratories (Loughborough, United Kingdom).
Antimicrobial agents.
The QI agents were prepared in the Discovery Group, Sepracor Inc. Imipenem was purchased as Primaxin from Merck Sharp & Dohme (Hoddesdon, United Kingdom). Ciprofloxacin, mupirocin, meropenem, fosfomycin, protegrin IB-367, and linezolid were gifts from Bayer AG (Leverkusen, Germany), SmithKline Beecham Pharmaceuticals (Harlow, United Kingdom), AstraZeneca (Alderley Park, Macclesfield, United Kingdom), Biochemie GmbH (Kundl, Austria), IntraBiotics Pharmaceuticals (Mountain View, Calif.), and Pharmacia and Upjohn Inc. (Kalamazoo, Mich.), respectively. The other antibiotics tested were purchased from Sigma-Aldrich (Poole, United Kingdom).
Chemicals.
The following radiolabeled chemicals were purchased from Amersham Life Science (Little Chalfont, United Kingdom): [methyl-3H]thymidine (70 to 85 Ci/mmol), [5-3H]uridine (25 to 30 Ci/mmol), l-[3,4-3H]glutamine (20 to 50 Ci/mmol), [U-14C]glycine (>100 mCi/mol), [3H]acetic acid, sodium salt (87 mCi/mmol), and [5-3H]UTP (14 mCi/mmol). The BacLight kit (Molecular Probes, Inc., Eugene, Oreg.) for assessment of membrane damage was purchased from Cambridge Biosciences (Cambridge, United Kingdom). All other chemical and biochemical reagents were purchased from standard commercial sources.
Determination of susceptibility to antimicrobial agents.
MICs were determined by agar dilution on MHA with inocula in MHB of 106 CFU/spot for S. aureus and 104 CFU/spot for E. coli (2). The MIC was defined as the lowest concentration of compound that completely inhibited visible growth after 18 to 24 h of incubation at 37°C.
Effects of antimicrobial agents on culture turbidity and bacterial viability.
The turbidity at 600 nm of cultures growing in MHB was determined either manually by measurement of the absorbance in a Jenway 6105 UV/Vis spectrophotometer or automatically in a Molecular Devices Spectra Max Plus 384 microplate reader. The microplates were shaken and incubated at 37°C within the instrument. Studies to determine bactericidal activity were performed on exponential-phase cultures of S. aureus 8325-4. Samples were serially diluted in phosphate-buffered saline and plated onto MHA. The colonies were counted after incubation at 37°C for 18 to 24 h.
Measurement of PAE.
In vitro postantibiotic effects (PAEs) were determined by the viable count method following removal of the antimicrobials by a 10−3 dilution (10). Organisms were exposed to five times the MIC for 60 min before washout. The PAE was calculated with the standard formula of Craig and Gudmundson (10): PAE = T − C, where T is the time required for the treated cells to increase 1 log10 CFU/ml after washout of the drug with fresh medium, and C is the time required for the nontreated control to increase 1 log10 CFU/ml after washout of the drug with fresh medium.
Determination of frequencies of mutation for resistance to antimicrobial agents.
Determination of frequencies of mutation for resistance to antimicrobial agents was performed as described by O'Neill et al. (36) with Iso-Sensitest agar. Both standard and concentrated cell techniques were used, whereby mutation frequencies as low as 1 in 10−11 can be detected. Mutant colonies were normally counted after incubation of the plates for 24 h at 37°C. However, mutants resistant to ciprofloxacin were slow growing, and in this case colonies were quantified after 48 h of incubation.
Rates of bacterial lysis.
First-order rate constants for bacterial lysis were calculated from culture absorbance data (600 nm) as described by Leduc et al. (25)
Macromolecular synthesis.
DNA, RNA, protein, peptidoglycan, and lipid syntheses were monitored in mid-exponential-phase cultures of S. aureus 8325-4 (108 CFU/ml in MHB) by measurement of the incorporation of the radiolabeled precursors [methyl-3H]thymidine, [5-3H]uridine, l-[3,4-3H]glutamine, [U-14C]glycine, and [3H]acetic acid into macromolecular fractions as described previously (35, 48). Final concentrations were 1 μCi/ml for the 3H-labeled compounds and 0.1 μCi/ml for [U-14C]glycine. Precursors were added to cultures 3 min before the addition of antibiotics (at four times the MIC).
Bacterial membrane damage.
The BacLight kit from Molecular Probes, Inc., was used to assess bacterial membrane damage as described by Hilliard et al. (18), with the exception that fluorescence readings were measured with a Perkin-Elmer LS 45 luminescence spectrometer.
Preparation of protoplasts.
Protoplasts of S. aureus 8325-4 were generated with lysostaphin as described previously (4), with the exception that the buffer for protoplast preparation contained 50% (wt/vol) sucrose.
Hemolysis assays.
Erythrocytes were recovered from murine blood as described by Fernandez-Lopez et al. (12), and hemolysis was measured by the spectrophotometric procedure of Lee and Oh (26). The hemolysis that occurred following the addition of 5% (wt/vol) sodium dodecyl sulfate (SDS) was defined as 100% hemolysis.
RESULTS
In vitro antistaphylococcal activity.
In view of the growing importance of antibiotic-resistant S. aureus infections, we have focused our attention on the antistaphylococcal activities of QI agents. Several QI agents that were identified as lead compounds in primary screens were selected for further studies. The activities of these compounds against clinical isolates of S. aureus (methicillin-susceptible S. aureus [MSSA], MRSA, and GISA) were compared with those of oxacillin, linezolid, vancomycin, and quinupristin-dalfopristin (Table 2). The QI agents displayed excellent antistaphylococcal activities, including activities against strains resistant to established agents. For many of the QI agents, the MICs at which 90% of isolates are inhibited (MIC90s) were ≤1 μg/ml for MSSA and MRSA. Furthermore, the activities of the QI agents were comparable to or exceeded those displayed by vancomycin, linezolid, and quinupristin-dalfopristin against these clinical isolates and GISA.
TABLE 2.
Antibacterial activities of QI agents and reference compounds against clinical isolates of S. aureus in MHA
| Organism (no. of strains) | QI agent or antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| MSSA (32) | SEP 118843 | 0.25-2 | 1 | 1 |
| SEP 137199 | 0.5-1 | 0.5 | 1 | |
| SEP 147960 | 1-2 | 1 | 1 | |
| SEP 155342 | 2-4 | 2 | 4 | |
| SEP 155347 | 2-4 | 2 | 2 | |
| SEP 156117 | 0.25-1 | 0.5 | 0.5 | |
| SEP 156131 | 1-2 | 2 | 2 | |
| SEP 164934 | 1-4 | 1 | 4 | |
| Linezolid | 2-4 | 4 | 4 | |
| Oxacillin | 0.2-2 | 1 | 2 | |
| Synercid | 0.25-1 | 0.5 | 0.5 | |
| Vancomycin | 1-4 | 4 | 4 | |
| MRSA (31) | SEP 118843 | 0.25-2 | 1 | 1 |
| SEP 137199 | 0.5-1 | 0.5 | 1 | |
| SEP 147960 | 1-2 | 1 | 1 | |
| SEP 155342 | 1-4 | 2 | 4 | |
| SEP 155347 | 2-4 | 2 | 2 | |
| SEP 156117 | 0.25-1 | 0.5 | 0.5 | |
| SEP 156131 | 2 | 2 | 2 | |
| SEP 164934 | 2-8 | 4 | 4 | |
| Linezolid | 1-4 | 4 | 4 | |
| Oxacillin | 256-512 | 256 | 256 | |
| Synercid | 0.25-1 | 0.5 | 1 | |
| Vancomycin | 2-4 | 4 | 4 | |
| GISA (7) | SEP 118843 | 0.25-1 | ||
| SEP 137199 | 0.5-1 | |||
| SEP 147960 | 1-2 | |||
| SEP 155342 | 2-4 | |||
| SEP 155347 | 2-4 | |||
| SEP 156117 | 0.25-0.5 | |||
| SEP 156131 | 1-2 | |||
| SEP 164934 | 1-4 | |||
| Linezolid | 2-4 | |||
| Oxacillin | 256-512 | |||
| Synercid | 0.5-1 | |||
| Vancomycin | 4-13 | |||
Effects of SEP agents on growth and survival of S. aureus 8325-4.
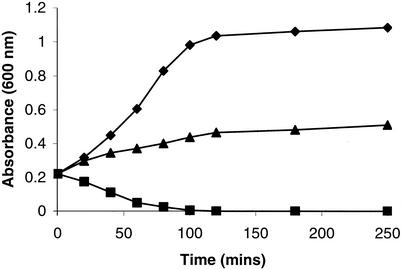
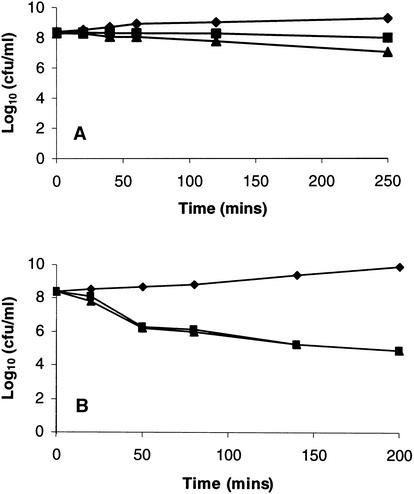
The effects of the QI agents on the growth and survival of S. aureus 8325-4 over 3- to 4-h periods were determined for liquid cultures grown in MHB. Agents were added at 10 times the MIC to early-logarithmic-phase cultures, and samples were removed to determine culture turbidity and viability. On the basis of these experiments the QI compounds could be divided into bactericidal-lytic agents and bactericidal-nonlytic agents (Table 3). Bactericidal-lytic agents (e.g., QI compound SEP 155342) promoted a loss of culture turbidity (Fig. 2) that was accompanied by a loss of viability (Fig. 3A). The bactericidal response was dose dependent since killing was greater following exposure to 20 times the MIC than to 10 times the MIC (Fig. 3A). Some QI agents, such as SEP 118843, were nonlytic (Fig. 2) but bactericidal (Fig. 3B). Furthermore, the killing response was faster than that observed with lytic QI agents. For example, at 20 times the MIC QI agent SEP 118843 decreased the viability by 90% in about 40 min, whereas a similar response with compound SEP 155342 required 4 h. However, in contrast to the bactericidal-lytic QI agents, a dose dependency for bacterial killing was not demonstrated by the bactericidal-nonlytic agents since there was no difference in the killing responses at 10 and 20 times the MIC (e.g., see data for QI agent SEP 118843 in Fig. 3B).
TABLE 3.
Effects of QI agentsa on integrity of S. aureus 8325-4 grown in MHB
| QI agent (SEP no.) | MIC (μg/ml) | Type |
|---|---|---|
| 127802 | 1 | Lytic |
| 132433 | 2 | Lytic |
| 132617 | 4 | Lytic |
| 137199 | 0.5 | Lytic |
| 147960 | 1 | Lytic |
| 155342 | 2 | Lytic |
| 155347 | 2 | Lytic |
| 155409 | 1 | Lytic |
| 156117 | 1 | Lytic |
| 156131 | 2 | Lytic |
| 160394 | 2 | Lytic |
| 164934 | 1 | Lytic |
| 173333 | 0.5 | Lytic |
| 173352 | 2 | Lytic |
| 173360 | 0.5 | Lytic |
| 173390 | 0.5 | Lytic |
| 118843 | 0.5 | Nonlytic |
| 164741 | 8 | Nonlytic |
| 164752 | 4 | Nonlytic |
| 164759 | 2 | Nonlytic |
| 173391 | 0.25 | Nonlytic |
| 173446 | 1 | Nonlytic |
The QI agents were used at 10 times the MICs.
FIG. 2.
Effects of QI agents SEP 155342 (▪) and SEP 118843 (▴) at 10 times the MIC on S. aureus 8325-4 growth. Inhibitors were added to early-logarithmic-phase cultures in MHB at time zero. ♦, control.
FIG. 3.
Effects of QI agents SEP 155342 at 10 (▪) and 20 (▴) times the MIC (A) and SEP 118843 at 10 (▪) and 20 (▴) times the MIC (B) on survival of S. aureus 8325-4. Inhibitors were added to early-logarithmic-phase cultures in MHB at time zero. ♦, control.
Control experiments for culture turbidity and viability were performed with the bactericidal-nonlytic agents ciprofloxacin and rifampin and the bactericidal-lytic agents ampicillin and d-cycloserine. These control agents behaved as predicted from earlier studies (42) (data not shown).
Activities of QI agents against antibiotic-hypersensitive E. coli mutants.
QI agents are reported to have poor activities against E. coli (19). To determine whether this might result from exclusion by the outer membrane of the gram-negative organism, the activities of a representative bactericidal-lytic QI agent (SEP 155342) and a bactericidal-nonlytic QI agent (SEP 118843) against a number of E. coli permeability mutants were determined. Compared to wild-type organisms, QI agents SEP 155342 and SEP 118843 exhibited twofold increases in activity against some permeability mutants (data not shown). However, in other cases enhanced activities against permeability mutants were not demonstrated, even though these strains were more susceptible to fusidic acid and/or mupirocin, i.e., antibiotics normally excluded by the outer membrane (43).
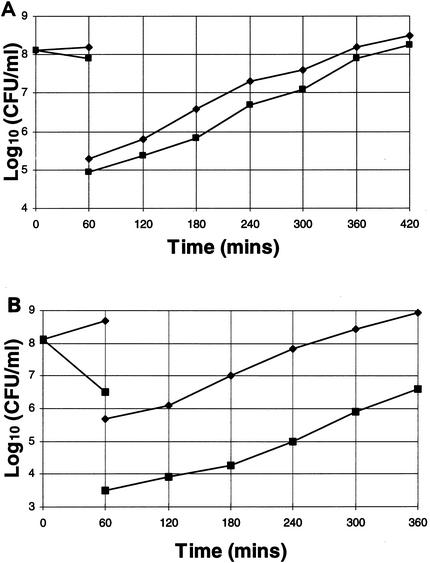
PAEs of QI agents against S. aureus.
PAEs were determined for a representative bactericidal-lytic QI agent (SEP 155342; Fig. 4A), a representative bactericidal nonlytic QI agent (SEP 118843; Fig. 4B), and rifampin as a control agent (data not shown). Cultures of S. aureus 8325-4 were exposed to 5 times the MIC for 60 min before washout, and PAEs were calculated as described in Materials and Methods. The PAE of QI agent SEP 155342 was 40 min, that of QI agent SEP 118843 was 35 min, and that of rifampin was 180 min. The PAE of rifampin is in agreement with the values for S. aureus from the literature (10).
FIG. 4.
PAEs in MHB induced by 60 min of exposure of S. aureus 8325-4 to five times the MICs of QI agents SEP 155342 (▪) (A) and SEP 118843 (▪) (B). Inhibitors were removed by dilution (10−3) at 60 min. ♦, controls.
Selection of S. aureus isolates resistant to QI agents and partial characterization of mutants resistant to QI agent SEP 11843.
Spontaneous mutants of S. aureus 8325-4 resistant to QI agents SEP 155342 and SEP 118843 were recovered by direct plating of organisms onto Iso-Sensitest agar containing the agents at four times the MIC. Mutants arose at frequencies between 10−8 (QI agent SEP 118843) and 10−9 (QI agent SEP 155342) (Table 4). The frequencies of mutational resistance to a number of reference antibiotics were determined under identical conditions. Mutation frequencies were fusidic acid > rifampin > norfloxacin > mupirocin > SEP 118843 = ciprofloxacin > SEP 155342. No mutants were recovered (frequencies <10−11) when the bacteria were selected on vancomycin or penicillin at four times the MIC.
TABLE 4.
Spontaneous mutation frequencies of S. aureus 8325-4 to QI agents SEP 155342 and SEP 118843 and control agents using selecting concentrations of four times the MICs in Iso-Sensitest agar
| QI agent or antibiotic | Mutation frequency |
|---|---|
| SEP 155342 | (1.4 ± 1.1) × 10−9 |
| SEP 118843 | (1.2 ± 0.1) × 10−8 |
| Fusidic acid | (7.6 ± 1.3) × 10−7 |
| Rifampin | (1.5 ± 0.4) × 10−7 |
| Norfloxacin | (9.2 ± 1.6) × 10−8 |
| Mupirocin | (7.2 ± 0.9) × 10−8 |
| Ciprofloxacin | (1.4 ± 0.3) × 10−8 |
| Benzyl penicillin | <10−11 |
| Vancomycin | <10−11 |
Mutants resistant to QI agent SEP 155342 were unstable and could not be analyzed further. However, it was possible to characterize mutants resistant to QI agent SEP 118843 since these were stable. The MICs of QI agent SEP 118843 for mutants selected at four times the MIC were determined to examine the distribution of resistance levels. Twenty mutants were picked at random from colonies appearing on selection plates with the drug at four times the MIC. The individual MICs of QI agent SEP 118843 were determined for these 20 resistant mutants. Resistance levels ranged from 4 times the MIC (i.e., equal to the concentration at which the mutant was selected) to 64 times the MIC for parental strain S. aureus 8325-4.
Two mutants that were resistant to QI agent SEP 118843 and that exhibited 64-fold increases in resistance were selected for determination of their susceptibilities to a range of other inhibitors. These included QI agent SEP 155342 and inhibitors of peptidoglycan synthesis (ampicillin, vancomycin, d-cycloserine, bacitracin), protein synthesis (chloramphenicol, erythromycin, streptomycin, tetracycline, fusidic acid, mupirocin), RNA synthesis (rifampin), and DNA synthesis (ciprofloxacin). The mutants resistant to QI agent SEP 118843 were not cross resistant to any of these agents, including the QI compound SEP 155342 (data not shown).
Kinetics and nature of lysis mediated by bactericidal-lytic QI agents.
The bacteriolytic activity of QI agents such as SEP 155342 might result from inhibition of peptidoglycan synthesis since induction of lysis is characteristic of agents that specifically inhibit this biosynthetic pathway. We explored this possibility by comparing the kinetics and nature of QI agent-mediated lysis with those of known inhibitors of peptidoglycan synthesis including ampicillin, imipenem, meropenem, d-cycloserine, fosfomycin, and vancomycin, all of which promoted lysis of staphylococci when they were added to growing cultures.
The rates of lysis induced by QI agent SEP 155342 and the other antibiotics were calculated from culture absorbance data, as described in Materials and Methods. At four times the MIC the control inhibitors of peptidoglycan synthesis promoted lysis with first-order rate constants ranging from 1.2 × 10−3 units/min (vancomycin) to 14.5 × 10−3 units/min (meropenem) (Table 5). The rates of lysis for the bactericidal-lytic QI agents were concentration dependent (data not shown). However, QI agent SEP 155342 at 10 times the MIC achieved a lysis rate (12.3 × 10−3 units/min) comparable to the most rapid rate achieved by a control peptidoglycan biosynthetic inhibitor (meropenem at four times the MIC) (Table 5).
TABLE 5.
Lysis of S. aureus 8325-4 in MHB induced by various inhibitors and effect of preexposure to mupirocin
| Antimicrobial agent | MIC (μg/ml) | Rate of bacterial lysis (k [10−3/min])a
|
|
|---|---|---|---|
| Absence of mupirocin | Presence of mupirocinb | ||
| Ampicillin | 2 | 1.5 ± 0.1 | 0 |
| Imipenem | 0.008 | 8.4 ± 0.7 | 0 |
| Meropenem | 0.125 | 14.5 ± 0.4 | 0 |
| d-Cycloserine | 32 | 2.4 ± 0.5 | 0 |
| Fosfomycin | 16 | 1.4 ± 0.1 | 0 |
| Vancomycin | 2 | 1.2 ± 0.3 | 0 |
| SEP 155342 | 2 | 12.3 ± 2.1 | 5.1 ± 0.6 |
First-order rate constants for lysis (k) were calculated as described in Materials and Methods. Following addition of peptidoglycan biosynthesis inhibitors (ampicillin through vancomycin) at 4 times the respective MICs or following addition of SEP 155342 at 20 μg/ml (10 times the MIC).
Pretreatment with mupirocin at 1 μg/ml (four times the MIC) for 30 min.
Although the rate of lysis induced by QI agent SEP 155342 was comparable to those induced by control inhibitors of peptidoglycan synthesis, the nature of the lysis induced by this agent differed from that induced by the controls. Lysis mediated by the known peptidoglycan inhibitors was evident only 1 to 2 h after their addition (at four times the MIC) to growing staphylococcal cultures (data not shown). In contrast, addition of QI agent SEP 155342 and other bactericidal-lytic agents at concentrations ranging from 4 to 20 times the MIC led to an immediate drop in culture turbidity (see Fig. 2 for the results for QI agent SEP 155342 at 10 times the MIC).
The nature of the lytic responses mediated by QI agent SEP 155342 and the control antibiotics could also be distinguished from each other on the basis of their actions upon staphylococci expressing the stringent response. It is well known that E. coli becomes tolerant to inhibitors of peptidoglycan synthesis following induction of the stringent response (37, 47). This was confirmed for S. aureus by demonstrating that the control inhibitors of peptidoglycan synthesis completely failed to lyse cultures preexposed to mupirocin, a strong inducer of the stringent response (11, 48) (Table 5). In contrast, preexposure to mupirocin reduced the rate of lysis mediated by QI agent SEP 155342 to only about 50% of that observed in cells not treated with mupirocin (Table 5).
Macromolecular synthesis in cells exposed to QI agent SEP 155342.
Since the lysis mediated by QI agent SEP 155342 and other lytic QI agents does not appear to result from specific inhibition of peptidoglycan synthesis, it might result from direct solubilization of the cytoplasmic membrane by detergent-like action. Prior to lysis, such agents usually cause simultaneous, nonspecific inhibition of several macromolecular biosynthetic pathways as a consequence of structural disorganization of the cytoplasmic membrane (18). To examine this possibility, QI agent SEP 155342 (at four times the MIC) was tested in five macromolecular synthesis assays involving the incorporation of radioactive precursors into DNA, RNA, protein, peptidoglycan, and lipid fractions. In each case a specific inhibitor (at four times the MIC) with a known mechanism of action was included as a positive control. The times taken to completely arrest the synthesis of the individual macromolecules following addition of inhibitors were determined from the radioactive incorporation data.
QI agent SEP 155342 completely prevented synthesis of all macromolecules within 8 min of its addition to staphylococcal cultures, and there was no evidence for specific inhibition of a single macromolecular pathway (data not shown).
Membrane damage following exposure to QI agent SEP 155342.
The effects of QI agent SEP 155342 on macromolecular synthesis are consistent with a membrane-damaging activity for this compound. Bacterial membrane damage was initially examined by using the BacLight assay (Table 6). Exposure of cells to QI agent SEP 155342 (at four times the MIC for 10 min) altered the permeability of the cytoplasmic membrane, resulting in a normalized dye fluorescence ratio <20% of the control value obtained for cells not treated with antibiotics. Polymyxin B and protegrin IB-367 are both known bacterial membrane-disrupting agents that possess detergent-like activities (18, 41). As expected, these compounds caused severe membrane damage, in both cases decreasing the fluorescence ratio to about 10% of that for the untreated controls. A variety of other agents, including peptidoglycan biosynthesis inhibitors, mupirocin, tetracycline, and ciprofloxacin had little effect on membrane integrity since at most they reduced the fluorescence to about 85% of the control value for untreated cells (Table 6). Our results with positive and negative control agents agree with previously published data from studies in which the BacLight assay was used with S. aureus (18), but they further demonstrate that inhibitors of peptidoglycan synthesis (at four times the MIC) do not elicit membrane damage within 10 min of addition to staphylococcal cultures.
TABLE 6.
Effects of various agents on membrane integrity in S. aureus 8325-4 and hemolysis of murine erythrocytes
| Antimicrobial agent | MIC (μg/ml) | BacLight assay reading (% of that for untreated control)a | Hemolysis (% of that for SDS-treated control)b |
|---|---|---|---|
| Ampicillin | 2 | 93.1 ± 5.4 | ND |
| Imipenem | 0.008 | 84.2 ± 11.4 | ND |
| Meropenem | 0.125 | 91.7 ± 1.9 | ND |
| d-Cycloserine | 32 | 102.2 ± 8.0 | ND |
| Fosfomycin | 16 | 94.1 ± 9.5 | ND |
| Vancomycin | 2 | 99.0 ± 17.0 | ND |
| Tetracycline | 0.25 | 104.6 ± 13.4 | 0.9 ± 0.7 |
| Mupirocin | 0.25 | 97.8 ± 13.4 | ND |
| Ciprofloxacin | 2 | 104.0 ± 12.8 | 0.6 ± 0.4 |
| Protegrin IB-367 | 2 | 11.6 ± 3.5 | ND |
| Polymyxin B | 128 | 10.7 ± 2.4 | 0.8 ± 0.6 |
| SEP 155342 | 2 | 19.6 ± 4.3 | 23.4 ± 0.6 |
| SEP 118843 | 0.5 | 28.4 ± 5.3 | 0 ± 0 |
For S. aureus the agents were added (at four times the MIC) to early-logarithmic-phase cultures, and 10 min later samples were taken to determine membrane damage by using the BacLight kit. Values are expressed as a percentage of those obtained with control cultures not exposed to antimicrobial agents.
For hemolysis, murine erythrocytes were exposed for 60 min to inhibitor concentrations equivalent to four times the MIC, and the release of hemoglobin into the supernatant was measured at 540 nm following centrifugation of erythrocytes at 4,000 × g for 5 min. Hemolysis effected by 5% (wt/vol) SDS was defined as 100% hemolysis. ND, not determined.
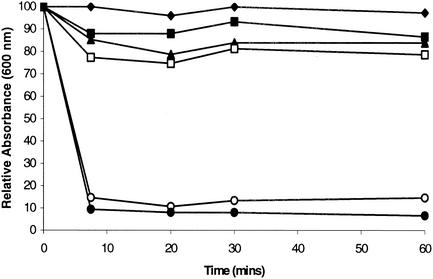
The possibility that QI agent SEP 155342 possessed detergent-like activity was examined by determining the stability of lysostaphin-induced protoplasts in the presence of the QI inhibitor and control agents (Fig. 5). Exposure of protoplasts, stabilized by resuspension in 50% (wt/vol) sucrose, to QI agent SEP 155342 (at 10 times the MIC for 60 min) failed to promote their lysis (<10% decline in turbidity at 600 nm) (Fig. 5). In contrast, addition of protegrin IB-367 (at 10 times the MIC) or SDS (5% [wt/vol]) led to rapid lysis of protoplast suspensions (>80% decline in turbidity). The control nonlytic antibiotic rifampin (at 10 times the MIC) caused only a 20% decline in the absorbances of the protoplast suspensions over 60 min (Fig. 5).
FIG. 5.
Effects of QI agent SEP 155342 at 10 times the MIC (8 μg/ml) (▪), QI agent SEP 118843 at 10 times the MIC (2 μg/ml) (▴), protegrin IB-367 at 10 times the MIC (8 μg/ml) (○), rifampin at 10 times the MIC (0.5 μg/ml) (□), and SDS (5% [wt/vol]) (•) on protoplasts of S. aureus 8325-4. ♦, control
Macromolecular synthesis in cells exposed to QI agent SEP 118843.
Rates of macromolecular synthesis were also determined for staphylococci exposed to the nonlytic QI agent SEP 118843 at four times the MIC. Nonspecific inhibition of macromolecular synthesis was also observed in cells exposed to QI agent SEP 118843. However, in contrast to QI agent SEP 155342, inhibition of the biosynthetic pathways by compound SEP 118843 was less rapid, requiring up to 20 min for complete inhibition (data not shown).
Bacterial membrane damage following exposure to QI agent SEP 118843.
Although the kinetics of macromolecular synthesis inhibition exhibited by QI agent SEP 118843 following exposure of staphylococci to that QI agent differed from the kinetics exhibited by QI agent SEP 155342, no evidence for preferential inhibition of a single macromolecular synthetic pathway by compound SEP 118843 was observed. Therefore, QI agent SEP 118843 might also be a membrane-damaging agent. This was confirmed by the BacLight assay, which demonstrated that exposure of S. aureus 8325-4 to QI agent SEP 118843 (at four times the MIC for 10 min) led to a fluorescence value <30% of that for the control (Table 6). However, QI agent SEP 118843 (at 10 times the MIC) did not cause complete lysis of S. aureus protoplasts since its addition to protoplast suspensions caused only a 15% reduction in absorbance over 60 min (Fig. 5).
Hemolytic activities of QI agents.
On the basis of the results of the experiments described above, QI agents SEP 155342 and SEP 118843 both appear to interfere with bacterial membrane integrity without causing complete disruption of the prokaryotic membrane. The effects of these QI agents on murine erythrocytes at concentrations equivalent to four times the MIC for S. aureus 8325-4 were also examined. Compared to the hemolysis caused by SDS (5% [wt/vol]), QI agent SEP 155342 caused <25% hemolysis during a 60-min incubation and QI agent SEP 118843 was nonhemolytic (Table 6). Three agents, including polymyxin B, which are not reported to possess eukaryotic membrane-damaging activity (20, 39), were included as controls. As predicted, these agents failed to cause hemolysis of murine erythrocytes (Table 6).
DISCUSSION
Antibiotic resistance in S. aureus has been a problem since the beginning of the chemotherapeutic era, requiring the successive introduction of agents such as methicillin, vancomycin, and linezolid to meet the challenges imposed by resistance (27, 45). Unfortunately, the ability of S. aureus to adapt to antimicrobial selection pressure appears set to continue, as illustrated by the emergence of GISA (27, 45) and, more recently, linezolid-resistant MRSA (46). In view of these developments, new antistaphylococcal agents with the potential to treat MRSA and GISA infections are required (24, 40).
QI agents may have a role in the chemotherapy of staphylococcal infections since they demonstrated excellent antistaphylococcal activities, encompassing MSSA, MRSA, and GISA. Furthermore, these agents were bactericidal against S. aureus. The killing activity of QI agent SEP 118843 was particularly good, as it achieved a 1,000-fold reduction in viable organisms following exposure to the drug at 10 times the MIC for 2 h. On the other hand, the modest PAEs exhibited by the two representative QI agents studied here suggest that continuous dosing with these agents would probably be necessary (10). When new agents are being considered for development, it is important to establish their potential for resistance generation (29, 36). High frequencies of mutations for resistance to rifampin and fusidic acid as single agents have resulted in their use as combined agents for the treatment of staphylococcal infections to prevent the emergence of resistance to either agent (36). The frequencies of mutations for resistance to QI agents were lower than those for fusidic acid and rifampin and comparable to those for ciprofloxacin (Table 4). Since ciprofloxacin is used as a single agent, QI agents clearly have the potential to be used alone rather than in combination with other agents.
It is clear that QI agents can be divided into two subclasses, i.e., those responsible for a bactericidal-lytic response and those that mediate nonlytic killing. Further support for the existence of two subclasses is provided by the observation that mutants resistant to QI agent SEP 118843 (bactericidal-nonlytic) were not cross resistant to QI agent SEP 155342 (bactericidal-lytic). Lysis of bacteria results from one of the following mechanisms: (i) direct interference with peptidoglycan synthesis followed by autolysis (37), (ii) direct solubilization of the cytoplasmic membrane by a detergent-like action (13), or (iii) membrane deenergization leading to autolysis (22).
Lytic QI agents like SEP 155342 are unlikely to mediate their effects by the first mechanism since there was no evidence for specific inhibition of peptidoglycan synthesis by this agent. Moreover, lytic QI agents promoted the rapid lysis of S. aureus, in contrast to the delayed lytic response observed with known inhibitors of peptidoglycan synthesis. Finally, the rate of bacterial lysis induced by QI agent SEP 155342 was only partially affected by prior exposure to mupirocin, whereas lysis mediated by known peptidoglycan biosynthesis inhibitors was completely blocked by mupirocin, an agent that induces the stringent response through production of ppGpp (11, 48). Our results on the effects of peptidoglycan biosynthesis inhibitors on staphylococci are consistent with those from earlier reports demonstrating tolerance to these agents during stringency in E. coli (37, 47) and further illustrate that the mechanism of QI agent-mediated lysis differs from that promoted by classical inhibitors of peptidoglycan synthesis.
The rapid lysis of bacterial protoplasts by an antimicrobial agent provides good evidence that it solubilizes the cytoplasmic membrane directly by a detergent-like activity (13, 22). However, QI agent-mediated lysis by the second mechanism described above, i.e., the result of detergent-like activity, is unlikely since SEP 155342 failed to lyse staphylococcal protoplasts under conditions in which SDS and protegrin IB-367 destroyed their integrity. The activity of SDS as a detergent is well known, and bacterial membrane disruption appears to be the primary mode of action of protegrin IB-367 (41).
Although we do not have direct evidence that lytic QI agents cause membrane deenergization, we nevertheless favor the view that these QI agents probably mediate their effect by the third mechanism described above, i.e., membrane deenergization leading to autolysis. Membrane-depolarizing agents cause rapid, nonspecific inhibition of macromolecular synthesis in bacteria (14, 18, 34) and lead to membrane damage detectable by the BacLight assay, which involves the differential uptake of the fluorescent stains SYTO-9 and propidium iodide (18). QI agent SEP 155342 exhibited both properties described above; i.e., it causes rapid nonspecific inhibition of macromolecular synthesis and promotes membrane damage detectable by the BacLight assay within 10 min of addition to staphylococci. The molecular target for QI agent SEP 155342 and other bactericidal-lytic QI agents is unknown. However, the ability to recover mutants resistant to QI agent SEP 155342 at frequencies of 10−9 is consistent with inhibitor interaction at a single target site (30) that is presumably located on the membrane.
The mode of action of nonlytic bactericidal QI agents such as SEP 118843 is intriguing. Although the pattern of inhibition of macromolecular synthesis differed from that exhibited by QI agent SEP 155342, no evidence for specific inhibition of an individual biosynthetic pathway was obtained, suggesting the possibility that antimicrobial activity resulted from membrane damage. Indeed, the membrane damage induced by QI agent SEP 118843 was detected by the BacLight assay but not the protoplast lysis assay. Taken together, these data suggest that the activities of nonlytic QI agents also result from interference with bacterial membrane function. However, the reason that they are unable to promote bacterial lysis is unclear. Nevertheless, the existence of two separate classes of QI agents suggests the possibility that the different responses reflect an interaction with separate membrane targets. This is consistent with the observation that mutants resistant to QI agent SEP 118843 were not cross resistant to QI agent SEP 155342.
Since early members of the QI series demonstrated poor activities against gram-negative bacteria (19), it could be postulated that the molecular targets for the actions of QI agents reside only in gram-positive bacteria. However, we noted that a representative bactericidal-lytic QI agent (SEP 155342) possessed weak activity against wild-type E. coli which was further enhanced in some permeability mutants. Furthermore, even though representative bactericidal-nonlytic QI agent SEP 118843 had no activity against wild-type E. coli, some permeability mutants also displayed enhanced susceptibility to this agent. Thus, it can be concluded that the poor activities of QI agents against gram-negative bacteria probably results from poor uptake across the cell envelope of gram-negative bacteria rather than an absence of molecular targets in gram-negative bacterial species.
The structure-activity relationships that divide QI agents into the two subclasses are complex, and precise structure-activity relationships have not been established. The structures of QI agents SEP 155342 and SEP 118843 are illustrated in Fig. 1, from which it can be seen that the nature of the substituents at positions 2, 4, 6, and 7 of the quinoline nucleus differ for the two molecules. The activities of SEP 155342 and other lytic QI agents were usually associated with substitution of a basic amine at position 4 of the quinoline nucleus, with or without a propargyl amine in the 6 position (Fig. 1). Neither the nature of the halogen (Cl, Br) on the indole moiety nor the presence or absence of a halogen atom at position 7 of the quinoline nucleus had any influence on lytic activity. In contrast, the nonlytic QI agents usually contained an aromatic ring, with or without a methoxy substituent, at position 4 of the quinoline nucleus (Fig. 1). The nonlytic QI agents possessed either a Cl or a Br in the indole moiety and either possessed or did not possess a propargyl amine in the 6 position of the quinoline nucleus (Fig. 1).
The QI agents described in this paper appear to interact with targets located in the staphylococcal membrane. Although very few antibiotic classes with membrane-active properties have been developed for clinical use, this does not necessarily preclude the development of QI agents as clinical candidates. This applies especially to nonlytic QI agents which, on the basis of the hemolysis data reported here, may interact with a bacterial target not found in mammalian membranes. There is growing interest in the development of membrane-active agents for application as antibiotics (12, 32, 44). QI agents may be further examples of bacterial membrane-active agents worthy of consideration for development.
Acknowledgments
This work was supported by grants to I. Chopra from Sepracor.
We thank W. Stubbings, A. Hoyle, and J. Hurdle for technical assistance.
REFERENCES
- 1.Boman, H. G., and D. A. Monner. 1975. Characterization of lipopolysaccharides from Escherichia coli K-12 mutants. J. Bacteriol. 121:455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.British Society for Antimicrobial Chemotherapy. 1991. A guide to sensitivity testing. Report of the working party on antibiotic sensitivity testing of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 27(Suppl. D):1-50. [PubMed] [Google Scholar]
- 3.Chopra, I., J. Hodgson, B. Metcalf, and G. Poste. 1997. The search for antimicrobial agents effective against bacteria resistant to multiple antibiotics. Antimicrob. Agents Chemother. 41:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chopra, I., R. W. Lacey, and J. Conolly. 1974. Biochemical and genetic basis of tetracycline resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 6:397-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, D. 1984. Novel antibiotic hypersensitive mutants of Escherichia coli: genetic mapping and chemical characterization. FEMS Microbiol. Lett. 21:189-195. [Google Scholar]
- 7.Clark, D. P., and J. P. Beard. 1979. Altered phospholipid composition in mutants of Escherichia coli sensitive or resistant to organic solvents. J. Gen. Microbiol. 113:267-274. [DOI] [PubMed] [Google Scholar]
- 8.Climo, M. W., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, M. L. 2000. Changing patterns of infectious disease. Nature 406:762-767. [DOI] [PubMed] [Google Scholar]
- 10.Craig, W. A., and S. Gudmundson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Philadelphia, Pa.
- 11.Crosse, A. M., D. L. A. Greenway, and R. R. England. 2000. Accumulation of ppGpp and ppGp in Staphylococcus aureus 8325-4 following nutrient starvation. Lett. Appl. Microbiol. 31:332-337. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Lopez, S., H. S. Kim, E. C. Choi, M. Delgado, J. R. Granja, A. Khasanov, K. Kraehenbuehl, G. Long, D. A. Weinberger, K. M. Wilcoxen, and M. R. Ghadiri. 2001. Antibacterial agents based on the cyclic d,l-alpha-peptide architecture. Nature 412:452-455. [DOI] [PubMed] [Google Scholar]
- 13.Gale, E. F., E. Cundliffe, P. E. Reynolds, M. H. Richmond, and M. J. Waring. 1981. The molecular basis of antibiotic action, 2nd ed. John Wiley & Sons, London, United Kingdom.
- 14.Harrington, C. R., and J. Baddiley. 1984. Synthesis of peptidoglycan and teichoic acid in Bacillus subtilis: role of the electrochemical proton gradient. J. Bacteriol. 159:925-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. P. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecker, S. J., I. S. Cho, T. W. Glinka, Z. J. Zhang, M. E. Price, V. J. Lee, B. G. Christensen, A. Boggs, S. Chamberland, F. Malouin, T. R. Parr, T. Annamalai, J. Blais, E. L. Bond, L. Case, C. Chan, J. Crase, R. Frith, D. Griffith, L. Harford, N. Liu, M. Ludwikow, K. Mathias, D. Rea, and R. Williams. 1998. Discovery of MC-02,331, a new cephalosporin exhibiting potent activity against methicillin-resistant Staphylococcus aureus. J. Antibiot. 51:722-734. [DOI] [PubMed] [Google Scholar]
- 17.Heisler, L. M., H. Suzuki, R. Landick, and C. A. Gross. 1993. Four contiguous amino acids define the target for streptolydigin resistance in the beta subunit of Escherichia coli RNA polymerase. J. Biol. Chem. 268:25369-25375. [PubMed] [Google Scholar]
- 18.Hilliard, J. J., R. M. Goldschmidt, L. Licata, E. Z. Baum, and K. Bush. 1999. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob. Agents Chemother. 43:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoemann, M. Z., G. Kumaravel, R. L. Xie, R. F. Rossi, S. Meyer, A. Sidhu, G. D. Cuny, and J. R. Hauske. 2000. Potent in vitro methicillin-resistant Staphylococcus aureus activity of 2-(1H-indol-3-yl) quinoline derivatives. Bioorg. Med. Chem. Lett. 10:2675-2678. [DOI] [PubMed] [Google Scholar]
- 20.Hsu Chen, C. C., and D. S. Feingold. 1973. The mechanism of polymyxin B action and selectivity toward biologic membranes. Biochemistry 12:2105-2111. [DOI] [PubMed] [Google Scholar]
- 21.Jacoby, G. A. 1996. Antimicrobial-resistant pathogens in the 1990s. Annu. Rev. Med. 47:169-179. [DOI] [PubMed] [Google Scholar]
- 22.Jolliffe, L. K., R. J. Doyle, and U. N. Streips. 1981. The energized membrane and cellular autolysis in Bacillus subtilis. Cell 25:753-763. [DOI] [PubMed] [Google Scholar]
- 23.Kohsaka, M., and A. L. Demain. 1976. Conversion of penicillin N to cephalosporin(s) by cell-free extracts of Cephalosporium acremonium. Biochem. Biophys. Res. Commun. 70:465-473. [DOI] [PubMed] [Google Scholar]
- 24.Labischinski, H., K. Ehlert, and B. Wieland. 1998. Novel antistaphylococcal targets and compounds. Expert Opin. Investig. Drugs 7:1245-1256. [DOI] [PubMed] [Google Scholar]
- 25.Leduc, M., R. Kasra, and J. Vanheijenoort. 1982. Induction and control of the autolytic system of Escherichia coli. J. Bacteriol. 152:26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, K. H., and J. E. Oh. 2000. Design and synthesis of novel antimicrobial pseudopeptides with selective membrane-perturbation activity. Bioorg. Med. Chem. 8:833-839. [DOI] [PubMed] [Google Scholar]
- 27.Livermore, D. M. 2000. Antibiotic resistance in staphylococci. Int. J. Antimicrob. Agents 16:S3-S10. [DOI] [PubMed] [Google Scholar]
- 28.Maki, H., K. Miura, and Y. Yamano. 2001. Katanosin B and plusbacin A(3), inhibitors of peptidoglycan synthesis in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1823-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez, J. L., and F. Baquero. 2000. Mutation frequencies and antibiotic resistance. Antimicrob. Agents Chemother. 44:1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, K., A. J. O'Neill, and I. Chopra. 2002. Response of Escherichia coli hypermutators to selection pressure with antimicrobial agents from different classes. J. Antimicrob. Chemother. 49:925-934. [DOI] [PubMed] [Google Scholar]
- 31.Moellering, R. C. 1998. Antibiotic resistance: lessons for the future. Clin. Infect. Dis. 27:S135-S140. [DOI] [PubMed] [Google Scholar]
- 32.Navon-Venezia, S., R. Feder, L. Gaidukov, Y. Carmeli, and A. Mor. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 34.Nyberg, G. K., and J. Carlsson. 1981. Metabolic inhibition of Peptostreptococcus anaerobius decreases the bactericidal effect of hydrogen peroxide. Antimicrob. Agents Chemother. 20:726-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliva, B., W. M. Maiese, M. Greenstein, D. B. Borders, and I. Chopra. 1993. Mode of action of the cyclic depsipeptide antibiotic LL-AO341-beta(1) and partial characterization of a Staphylococcus aureus mutant resistant to the antibiotic. J. Antimicrob. Chemother. 32:817-830. [DOI] [PubMed] [Google Scholar]
- 36.O'Neill, A. J., J. H. Cove, and I. Chopra. 2001. Mutation frequencies for resistance to fusidic acid and rifampicin in Staphylococcus aureus. J. Antimicrob. Chemother. 47:647-650. [DOI] [PubMed] [Google Scholar]
- 37.Rodionov, D. G., and E. E. Ishiguro. 1995. Direct correlation between overproduction of guanosine 3′,5′-bispyrophosphate (ppgpp) and penicillin tolerance in Escherichia coli. J. Bacteriol. 177:4224-4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Sanders, W. E., Jr., and C. C. Sanders. 1979. Toxicity of antibacterial agents: mechanism of action on mammalian cells. Annu. Rev. Pharmacol. Toxicol. 19:53-83. [DOI] [PubMed] [Google Scholar]
- 40.Silverman, J. A., N. Oliver, T. Andrew, and T. Li. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. C. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stratton, C. W. 1996. Mechanisms of action for antimicrobial agents: general priniciples and mechanisms for selected classes of antibiotics, p. 579-603. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Philadelphia, Pa.
- 43.Sukupolvi, S., and M. Vaara. 1989. Salmonella typhimurium and Escherichia coli mutants with increased outer-membrane permeability to hydrophobic compounds. Biochim. Biophys. Acta 988:377-387. [DOI] [PubMed] [Google Scholar]
- 44.Tally, F. P., M. Zeckel, M. W. Wasilewski, C. Carini, C. L. Berman, G. L. Drusano, and F. B. Oleson. 1999. Daptomycin: a novel agent for gram-positive infections. Expert Opin. Investig. Drugs 8:1223-1238. [DOI] [PubMed] [Google Scholar]
- 45.Tenover, F. C., J. W. Biddle, and M. V. Lancaster. 2001. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerg. Infect. Dis 7:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 47.Tuomanen, E., and A. Tomasz. 1986. Induction of autolysis in nongrowing Escherichia coli. J. Bacteriol. 167:1077-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson, M., B. Oliva, R. Cassels, P. J. O’Hanlon, and I. Chopra. 1995. SB-205952, a novel semisynthetic monic acid analog with at least two modes of action. Antimicrob. Agents Chemother. 39:1925-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao, W. H., Z. Q. Hu, S. Okubo, Y. Hara, and T. Shimamura. 2001. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]