Abstract
Forty-nine protease inhibitor (PI)-experienced but amprenavir (APV)-naïve patients experiencing virological failure were treated with ritonavir (RTV) (100 mg twice a day [b.i.d.]) plus APV (600 mg b.i.d.). Patients responded to therapy with a median viral load decrease of −1.32 log10 by week 12. The addition of low-dose RTV enhanced the minimal APV concentration in plasma (APV Cmin) up to 10-fold compared with that obtained with APV (1,200 mg b.i.d.) without RTV. Baseline PI resistance mutations (L10F/I/V, K20M/R, E35D, R41K, I54V, L63P, V82A/F/T/S, I84V) identified by univariate analysis and included in a genotypic score and APV Cmin at week 8 were predictive of the virological response at week 12. The response to APV plus RTV was significantly reduced in patients with six or more of the resistance mutations among the ones defined above. The genotypic inhibitory quotient, calculated as the ratio of the APV Cmin to the number of human immunodeficiency virus type 1 protease mutations, was a better predictor than the virological or pharmacological variables used alone. This genotypic inhibitory quotient could be used in therapeutic drug monitoring to define the concentrations in plasma needed to control replication of viruses with different levels of PI resistance, as measured by the number of PI resistance mutations.
The introduction of protease inhibitors (PIs) to antiretroviral therapy led to a profound and sustained suppression of viral loads, slower rates of disease progression, and prolonged survival in the majority of human immunodeficiency virus (HIV) type 1 (HIV-1)-infected patients (12). The success of antiretroviral therapy, however, may be impaired by the emergence of drug-resistant viral strains. Due to the high degree of structural similarity of PIs, resistant viruses may exhibit various degrees of cross-resistance even to a PI to which the patient has not yet been exposed. This makes the management of second- or third-line therapies complex in most cases.
Recently, the new PI amprenavir (APV) (VX478, 141W94) has been approved for antiretroviral treatment in several countries. The cytochrome P450 3A4 (CYP 3A4) isoenzyme is primarily responsible for the production of APV oxidative metabolites in humans for which the Kis are similar to those for indinavir and nelfinavir. Drugs that affect or that are affected by CYP3A4 have a potential interaction with APV. At present, there is a treatment paradigm shift in which PIs such as ritonavir (RTV) are used as pharmacokinetic enhancers in PI therapy. For drugs with reasonable bioavailabilities but short half-lives, such as APV, the effects of RTV are predominantly on the half-life, the minimal concentration in plasma (Cmin) at steady state (Cmin,ss), and the area under the curve (AUC). This interaction increases the level of exposure to APV by inhibiting its metabolism, and the resulting decreases in the number of daily doses and the total daily pill count have the potential to improve adherence (8, 16). Coadministration of APV and RTV (r/APV; 450 and 100 mg, respectively) twice daily (b.i.d.) resulted in an approximately 3- to 4-fold increase in the steady-state AUC (AUC1-12 h,ss) for APV in plasma, a 10- to 14-fold increase in the APV Cmin,ss, and a 1- to 1.7-fold increase in the steady-state maximal concentration of APV in plasma compared to those achieved with APV administered alone at 450 mg. The pharmacokinetic parameters for RTV in plasma did not significantly change when RTV was coadministered with APV (4, 5, 14).
The relationship between antiviral efficacy in vivo and the drug concentration in drug-naïve patients has been determined by estimation of the in vivo APV Cmin needed to provide 90% of the maximum antiviral effect for APV over 4 weeks in a pharmacodynamic sigmoid maximum-effect model, which was found to be 228 ng/ml, which is below the median Cmin achieved with the standard dose of APV without the addition of RTV (14). The increase in the APV Cmin obtained by the addition of RTV should have the potential to overcome PI resistance, which is crucial in salvage therapy.
In addition to the development of the I50V mutation, three alternative pathways leading to the development of APV resistance have been identified: the V32I mutation plus the I47V mutation, the I54L/M mutation, or, less commonly, the I84V mutation (18). A study in which a phenotype-genotype correlation was used was conducted with strains obtained from PI-experienced but APV-naïve patients. The developers of that system proposed the use of a scoring system in which 84V and/or any two of a number of mutations (10I/R/V/F, 46I/L, 54L/V, and 90M) were used to predict APV resistance (17). However, this scoring system has not been validated in vivo in relation to the virological response. On the other hand, a genotypic scoring system for APV based on a direct correlation between genotypes and the virological response was recently proposed on the basis of data from the Narval ANRS 088 trial (D. Descamps, B. Masquelier, J. P. Mamet, V. Calvez, A. Ruffault, F. Telles, A. Goetschel, P. M. Girard, F. Brun-Vézinet, and D. Costagliola, Abstr. 5th Int. Workshop HIV Drug Resist. Treat. Strat., abstr. 136, 2001). In this scoring system, at least four mutations among the L10I, V32I, M46I/L, I47V, I54V, G73S, V82A/F/T/S, I84V, and L90M mutations were associated with a poor virological response in highly pretreated patients receiving APV but not as part of a boosted regimen.
Recent studies have evaluated the combination of virological and pharmacological parameters as a predictor of the virological response in a model named the inhibitory quotient (IQ) model. This approach tries to combine the PI Cmin and the resistance levels measured by phenotype or virtual phenotype to predict the magnitude of virological response (3; D. Kempf, A. Hsu, P. Jiang, R. Rode, K. Hertogs, B. Larder, A. Zolopa, N. Shulman, D. Havlir, J. Gallant, E. Race, S. Boller, J. Swerdlow, O. Jasinsky, C. Renz, and E. Sun, Abstr. 8th Conf. Retrovir. Opport. Infect., abstr. 523, 2001; J. Hellinger, A. B. Morris, S. Piscitelli, D. Gordon, K. Foy, L. Jackson-Pope, D. Cordeiro, M. Peeters, R. Hoetelmans, P. J. de Caprariis, and C. J. Cohen, Abstr. 9th Conf. Retrovir. Opport. Infect., abstr. 451W, 2002). At this time, few data on the efficacy, the resistance, and the pharmacological determinants of the viral response to an r/APV-containing regimen in PI-experienced patients are available. The ideal ratio of Cmin to the resistance index for optimal antiviral efficacy with tolerable plasma drug concentrations remains to be determined.
The aims of this study were to investigate the efficacy of an r/APV (100 and 600 mg, respectively, b.i.d.)-containing regimen and to determine the virological and pharmacological determinants of the virological response to this combination in PI-experienced but APV-naïve patients.
(This work was presented during the 3rd International Workshop on Clinical Pharmacology of HIV Therapy, 11 to 13 April 2002, Washington, D.C. [G. Peytavin, C. Lamotte, A. G. Marcelin, C. Delaugerre, H. Ait Mohand, P. Bossi, D. Costagliola, M. Bonmarchand, A. Simon, R. Tubiana, F. Bricaire, C. Katlama, and V. Calvez, 3rd Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 7.6, 2002] and during the XI International HIV Drug Resistance Workshop, 2 to 5 July 2002, Seville, Spain [A. G. Marcelin, C. Lamotte, C. Delaugerre, N. Ktorza, H. Ait Mohand, M. Wirden, A. Simon, P. Bossi, F. Bricaire, D. Costagliola, C. Katlama, G. Peytavin, and V. Calvez, XI Int. HIV Drug Resist. Workshop, abstr. 106, 2002].)
MATERIALS AND METHODS
Patients and antiretroviral regimens.
Forty-nine PI-experienced but APV-naïve patients who were experiencing virological failure and whose plasma viral loads were >1,000 copies/ml were treated at day 0 with two or three nucleoside reverse transcriptase (RT) inhibitors (NRTIs) and r/APV (100 and 600 mg, respectively, b.i.d.). No non-NRTIs or PIs other than APV and RTV were used in the antiretroviral combinations. The choice of the NRTI was driven by genotypic testing, performed at screening (4 weeks before the start of the regimen) according to the algorithm in the French ANRS AC11 guidelines (2).
The patients were monitored prospectively at day 0, week 8, and week 12, with the monitoring including a clinical examination, measurement of the plasma HIV-1 load, CD4 cell count, and the APV Cmin. The characteristics of the patients at the baseline are presented in Table 1. The antiretroviral drugs used in combination with r/APV are summarized in Table 2.
TABLE 1.
Baseline patients characteristics
| Characteristic | Value | Range |
|---|---|---|
| No. of patients | 49 | |
| Gender (no. of patients) | ||
| Men | 44 | |
| Women | 5 | |
| Median age (yr) | 45 | 28-57 |
| Median HIV-1 RNA viral load (log10 no. of copies/ml) | 4.13 | 3-5.2 |
| Median CD4 cell count (no. of cells/mm3) | 286 | 48-700 |
| Median no. of PIs previously receiveda | 2 | 1-6 |
| RTV | 15 | |
| IDV | 43 | |
| SQV | 6 | |
| NFV | 30 | |
| r/SQV | 24 | |
| r/IDV | 6 | |
| Median no. of PI resistance mutationsb | ||
| Total (major + minor) | 6 | 1-10 |
| Major | 2 | 0-4 |
| Minor | 4 | 1-7 |
RTV, ritonavir; IDV, indinavir; SQV, saquinavir; NFV, nelfinavir; r/SQV, ritonavir plus saquinavir; r/IDV, ritonavir plus indinavir.
From the 2002 International AIDS Society table of resistance mutations (http://www.iasusa.org/resistance mutations/index.html).
TABLE 2.
Antiretroviral regimen received in combination with r/APV
| Associated antiretroviral regimena | No. of patients |
|---|---|
| ABC + 3TC | 2 |
| ABC + ddI | 3 |
| ddI + 3TC | 5 |
| ddI + d4T | 6 |
| 3TC + d4T | 5 |
| ZDV + 3TC + ABC | 2 |
| ABC + ddI + 3TC | 10 |
| ABC + d4T + 3TC | 2 |
| ddI + d4T + 3TC | 10 |
| ddI + 3TC + HU | 2 |
| ABC + ddI + 3TC + HU | 1 |
| ddI + d4T + 3TC + HU | 1 |
ABC, abacavir; 3TC, lamivudine; ddI, didanosine; d4T, stavudine; ZDV, zidovudine; HU, hydroxyurea.
HIV-1 RNA quantification and CD4 cell count measurement.
Quantification of plasma HIV-1 RNA was performed by the Amplicor Monitor assay (Roche Diagnostics, Basel, Switzerland), which has a detection limit of 400 copies/ml. The numbers of CD4 T cells in fresh blood samples were counted as described previously (10).
Genotypic resistance testing.
Plasma HIV-1 RNA taken at screening was used for sequence analysis of the RT gene (codons 1 to 240) and the protease gene (codons 1 to 99). HIV-1 RNA was purified from 1 ml of plasma that had been ultracentrifuged (19,300 × g for 60 min) by using the High Pure Viral purification kit (Boehringer Mannheim, Mannheim, Germany). Plasma HIV-1 RNA was amplified by a one-step reverse transcription-PCR with the TITAN One Tube Reverse Transcription PCR kit (Boehringer Mannheim), followed by a nested PCR with AmpliTaq Gold (Applied Biosystems, Foster City, Calif.). All primers used were described previously (7, 9, 13). Direct sequencing of the PCR product was performed with the d-Rhodamine Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, Calif.). Sequencing reaction products were analyzed on an ABI 377 Genetic Analyzer (Applied Biosystems). The sequences were analyzed with Sequence Navigator software (Applied Biosystems) by comparing the sequences of the sense and antisense strands of each fragment with the sequence of wild-type virus HXB2.
Determination of PI concentrations in plasma.
Blood samples were collected to determine plasma APV and RTV concentrations at steady state (at weeks 8 and 12). The intervals between the time of the last drug intake and the time of sampling were recorded. APV Cmins were determined by reversed-phase high-performance liquid chromatography coupled with fluorescence detection after solid-liquid-phase extraction, as described previously (15). The RTV Cmins were determined with the same samples by reversed-phase high-performance liquid chromatography coupled with detection under UV illumination after liquid-liquid-phase extraction, as described previously (11). The methods used to determine the APV and RTV concentrations in plasma were validated over ranges of concentrations in plasma of 5 to 1,000 and 30 to 15,000 ng/ml, respectively, with quantification limits of 5 and 30 ng/ml, respectively. For all assays, the between-assay bias for APV and RTV were below 6 and 10%, respectively. APV and RTV were kindly provided by GlaxoSmithKline and Abbott, respectively.
Identification of genotypic changes in HIV-1 protease at the baseline reducing in vivo virological response to r/APV.
The association between categorical variables (mutations in the HIV-1 protease gene at the baseline) and the magnitude of the decrease in the HIV-1 RNA load in plasma between day 0 and week 12 were studied by nonparametric Mann-Whitney tests. The significance level (P value) for each of the amino acid substitutions previously described to be involved in the decrease in PI efficacy was calculated (6). All other positions were also analyzed if the HIV-1 protease sequences of isolates from at least two patients harbored a difference from the sequence in HXB2. The virological cutoff, which marks the point at which the response to a given drug is a decrease in the HIV-1 RNA load in plasma of at least 1 log10 between day 0 and week 12, was determined by taking account the mutations that had a significance level with a P value of ≤0.2, as used previously in other clinical trials (1; Descamps et al., Abstr. 5th Int. Workshop HIV Drug Resist. Treat. Strat., abstr. 136, 2001).
Identification of APV Cmin as a correlate of reduced efficacy of r/APV.
Spearman's rank tests were used to evaluate if the APV Cmins at week 8 and/or week 12 were associated with the magnitude of the viral load decrease between day 0 and week 12. APV Cmins were divided into quartiles to search for a pharmacological cutoff that marks the point at which the response to a given drug is a decrease in the HIV-1 RNA load in plasma of at least 1 log10 between day 0 and week 12.
Calculation of GIQs.
The genotypic IQs (GIQs) were calculated as the ratio of the APV concentration measured at week 8 to the number of HIV-1 protease mutations measured at the initial screening. For example, the GIQ for patients who harbored isolates that had four PI resistance mutations at the initial screening and for which the APV Cmin was 1,000 ng/ml at week 8 can be calculated to be 250. One unit of GIQ was defined by the following ratio: the drug concentration associated with a ≥1 log10 decrease in the viral load between day 0 and week 12 divided by the number of mutations associated with a ≥1 log10 decrease in the viral load between day 0 and week 12.
Statistical methods.
Statview software (Abacus Concepts, Berkeley, Calif.) was used to performed statistical analyses. All statistical analyses were performed by nonparametric tests. The between-group characteristics were compared by the Mann-Whitney and Wilcoxon tests. Correlations were analyzed by Spearman's rank test.
RESULTS
Subject demographics and accountability.
All patients included in this study were enrolled between November 1999 and June 2001 in one medical center (Pitié-Salpêtrière Hospital, Paris, France). The 49 patients were treated from day 0 to week 12 with r/APV (100 and 600 mg, respectively, b.i.d.); viral load testing was done for all of them at day 0, week 8, and week 12; and determination of plasma APV and RTV concentrations was done at week 8 and week 12. No irregularities in plasma sampling (i.e., deviations in sampling time) or dosing were observed, and the intervals between the time of the last drug intake and the time of sampling at weeks 8 and 12 were 11.45 ± 1.00 and 11.42 ± 1.33 h, respectively. APV and RTV Cmins are presented in Table 3.
TABLE 3.
APV and RTV Cmin at week 8 and week 12
| Drug and time of evaluation |
Cmin (ng/ml)
|
No. of patients | |||
|---|---|---|---|---|---|
| Mean | Standard deviation | Range | Median | ||
| APV | |||||
| Wk 8 | 1,606 | 772 | 466-4,682 | 1,503 | 49 |
| Wk 12 | 1,509 | 746 | 5-4,679 | 1,241 | 49 |
| RTV | |||||
| Wk 8 | 226 | 215 | 30-815 | 139 | 49 |
| Wk 12 | 283 | 235 | 30-833 | 239 | 48 |
Virological response to r/APV-containing regimen.
By intent-to-treat analysis, the median decreases in the viral loads were −1.26 log10 (minimum, −0.97; maximum, −2.43) and −1.32 log10 (minimum, −0.62; maximum, −2.85) 8 and 12 weeks after the initiation of the r/APV regimen, respectively. Virological responses, defined as a minimum viral load of <400 copies/ml and/or a decrease in the viral load from that at the baseline of at least 1 log10 copies/ml, were observed in 65 and 70% of patients at week 8 and week 12, respectively.
Genotypic changes in HIV-1 protease correlating with reduced susceptibility to r/APV.
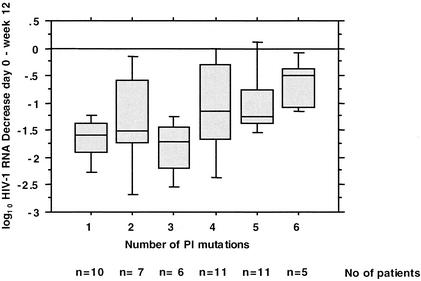
The mutations in HIV protease associated with a reduced virological response to r/APV with a P value ≤0.2 were L10F/I/V, K20M/R, E35D, R41K, I54V, L63P, V82A/F/T/S, and I84V. The P values are presented in Table 4. Some of the mutations previously associated with decreases in susceptibility to APV (i.e., I50V and I54M/L) were not analyzed because of their low prevalences at the baseline. There was a correlation between the number of mutations among the mutations listed above and the decrease in the viral load at week 12 (R = 0.47; P = 0.001). A genotypic cutoff for r/APV that marks the point at which the response was a decrease in the HIV RNA load of less than 1 log10 was determined to be six mutations (Fig. 1).
TABLE 4.
Amino acid substitution in HIV-1 protease associated with reduced virological response to r/APV with a P value of ≤0.2
| Codon no. | Median VLa decrease from day 0 to wk 12
|
P valueb | |
|---|---|---|---|
| Wild type | Mutated | ||
| L10F/I/V | −1.46 | −1.22 | 0.06 |
| K20M/R | −1.36 | −1.06 | 0.15 |
| E35D | −1.37 | −1.21 | 0.2 |
| R41K | −1.37 | −1.06 | 0.2 |
| I54V | −1.44 | −1.20 | 0.07 |
| L63P | −2.0 | −1.32 | 0.17 |
| V82A/F/T/S | −1.49 | −1.11 | 0.03 |
| I84V | −1.37 | −0.95 | 0.06 |
VL, viral load.
P values were determined by the Mann-Whitney test.
FIG. 1.
Relationship between number of mutations in HIV-1 protease and increase in HIV-1 RNA load in plasma between day 0 and week 12. A genotypic cutoff for r/APV that marks the point at which the response was than 1 log10 HIV RNA copies can be determined to be five mutations.
APV Cmin as a correlate of reduced efficacy of r/APV.
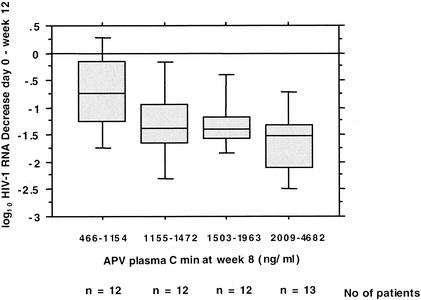
There was a correlation between the decrease in the HIV-1 RNA load in plasma between day 0 and week 12 and the APV Cmin at week 8 (R = 0.37; P = 0.009) but not the APV Cmin at week 12 (R = 0.14; P = 0.3). When the APV Cmins at week 8 were divided into quartiles, a statistical difference was observed between the groups (P = 0.03 by the Kruskal-Wallis test), and a pharmacological cutoff for r/APV that marked the point at which the response was a decrease in the HIV RNA load of at least 1 log10 could be determined to be 1,250 ng/ml (median value for the second APV Cmin quartile) (Fig. 2).
FIG. 2.
Ability of the APV Cmin measured at week 8 to predict a decrease in the HIV-1 RNA load in plasma between day 0 and week 12. The APV Cmin at week 8 was divided into quartiles. A pharmacological cutoff for r/APV that marks the point at which the response was at least 1 log10 of the decrease in the HIV RNA load could be determined to be 1,250 ng/ml (median value for the second APV Cmin quartile).
GIQ.
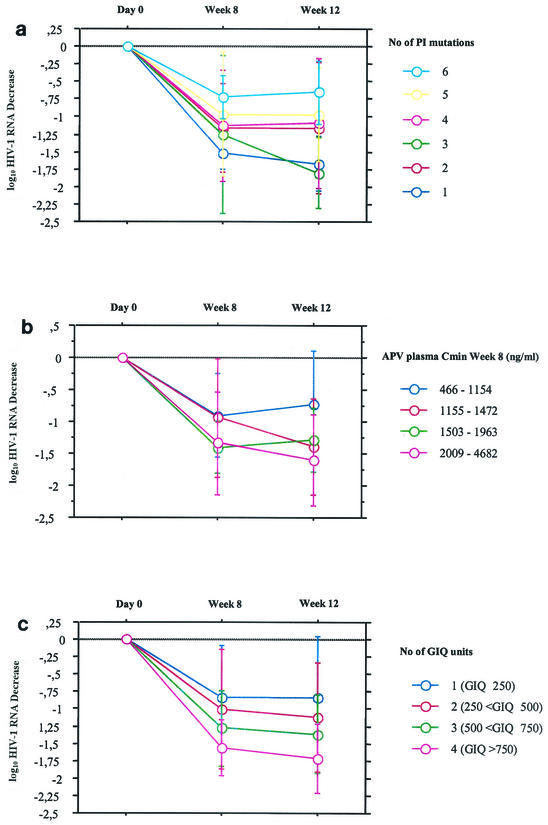
There was a correlation between the decrease in the plasma HIV-1 RNA load between day 0 and week 12 and the GIQ (R = 0.49; P = 0.001). One unit of GIQ was defined as 250, which was the ratio of the APV Cmin at week 8 associated with a ≥1 log10 decrease in the viral load (1,250 ng/ml)/number of mutations associated with a ≥1 log10 decrease in the viral load (five mutations). Patients were classified as having 1 (GIQ ≤ 250), 2 (250 < GIQ ≤ 500), 3 (500 < GIQ ≤ 750), or 4 (GIQ > 750) units of GIQ. The evolution of the decrease in the median viral load between day 0, week 8, and week 12 that takes into account the number of PI resistance mutations, the APV Cmin at week 8, and the GIQ value are presented in Fig. 3a, b, and c, respectively. By use of only the virological approach or the pharmacological approach separately, a relative good discrimination of virological responses was evidenced, but some crossovers were observed between groups. However, by use of the GIQ approach, there was a trend toward achieving a better discrimination between groups, as shown in Fig. 3.
FIG. 3.
Evolution of decrease in viral load between day 0, week 8, and week 12 taking into account the number of PI resistance mutations (a), the APV Cmin at week 8 (b), and the number of GIQ units (c). Error bars represent standard deviations.
DISCUSSION
A low dose of RTV (100 to 200 mg b.i.d.) is adequate to enhance saquinavir or indinavir concentrations in plasma (8, 19; R. Landman, G. Peytavin, J. Leibowitch, M. Crivat, E. Dohin, and J. F. Bergmann, Abstr. 12th World AIDS Conf., abstr. 42257, 1998). In this study, as with other HIV PIs, we confirmed that the addition of a low dose of RTV (100 mg b.i.d.) is adequate to enhance the APV Cmin so that it is up to 10-fold higher than the Cmins obtained with APV (1,200 mg b.i.d.) without RTV (C. Lamotte, X. Duval, G. Peytavin, and R. Farinotti, Abstr. 1st Int. Workshop Clin. Pharmacol. HIV Ther., abstr 2.7, 2000; S. Piscitelli, C. Bechtel, B. Sadler, and J. Falloon, Abstr. 7th Conf. Retrovir. Opport. Infect., abstr. 78, 2000).
These high Cmins obtained with r/APV resulted in good efficacy in terms of the virological response at week 12 (−1.32 log10) in PI-experienced patients whose isolates harbored PI resistance mutations in the protease gene. Statistical analysis allowed the identification of amino acid substitutions that had an impact on the virological response to r/APV. In the isolates from the patient population evaluated in this study, the mutations in HIV protease associated with reduced in vivo virological responses to r/APV were L10F/I/V, K20M/R, E35D, R41K, I54V, L63P, V82A/F/T/S, and I84V. The number of mutations in the protease gene was inversely associated with the virological response, and a clinical cutoff at which the response was significantly reduced was determined to be six mutations among the ones identified above (a good response was observed in patients whose isolates had up to five mutations). A clinical cutoff was determined in a previous clinical trial in which APV was used alone (Descamps et al., Abstr. 5th Int. Workshop HIV Drug Resist. Treat. Strat., abstr. 136, 2001) to be three mutations. Thus, in the present study, the addition of RTV increased the APV Cmin and led to an increased genotypic cutoff. By the same approach, a pharmacological cutoff could be determined to be 1,250 ng of APV per ml. Interestingly, the APV Cmins at week 8, but not the Cmins at week 12, were predictive of the virological response at week 12. This result suggests that Cmin measurements could be used as a factor that predicts the viral response in clinical practice, as was demonstrated for the use of APV alone (G. L. Drusano, B. M. Sadler, J. Millard, W. Symonds, M. Tisdale, C. Rawls, A. Bye, and the 141W94 International Product Development Team, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-16, 1997). We cannot exclude the possibility that APV Cmin measurements obtained earlier (i.e., week 2 or week 4) could also be predictive of the viral load evolution at an earlier time (i.e., week 8).
There was a trend for use of the GIQ, which takes into account the ratio of the APV Cmin at week 8/number of PI mutations at the baseline, to have better efficacy in discriminating the virological response at week 12 than use of the virological and pharmacological approaches independently. This model can probably be optimized to give greater differences in discrimination between the GIQ approach and the two other approaches used separately, for example, by using a larger population of study patients and earlier measurements of the APV Cmin (i.e., at weeks 2 and 4). Thus, this approach could be used in therapeutic drug monitoring to define the concentration in plasma needed to control the replication of viruses at different stages of PI resistance, as measured by the number of PI resistance mutations in the HIV-1 protease gene.
Therefore, the development of algorithms that combine virological mutations and the PI Cmin measured (for example, after several weeks) may be relevant. This strategy must be validated with a larger number of patients and in prospective clinical trials.
REFERENCES
- 1.Calvez, V., D. Costagliola, D. Descamps, A. Yvon, G. Collin, A. Cécile, C. Delaugerre, F. Damond, A. G. Marcelin, S. Matheron, A. Simon, M. A. Valantin, C. Katlama, and F. Brun-Vezinet. 2002. Impact of stavudine phenotype and thymidine analog mutations on viral response to stavudine plus lamivudine in ALTIS 2 ANRS trial. Antivir. Ther. 7:211-218. [PubMed] [Google Scholar]
- 2.Delfraissy, J. F. 2000. Prise en charge thérapeutique des personnes infectées par le VIH. Médecine-Sciences, Paris, France. [PubMed]
- 3.Duval, X., C. Lamotte, E. Race, D. Descamps, F. Damond, F. Clavel, C. Leport, G. Peytavin, and J. L. Vilde. 2002. Amprenavir inhibitory quotient and virological response in human immunodeficiency virus-infected patients on an amprenavir-containing salvage regimen without or with ritonavir. Antimicrob. Agents Chemother. 46:570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duval, X., V. Le Moing, C. Longuet, C. Leport, J. L. Vilde, C. Lamotte, G. Peytavin, and R. Farinotti. 2000. Efavirenz-induced decrease in plasma amprenavir levels in human immunodeficiency virus-infected patients and correction by ritonavir. Antimicrob. Agents Chemother. 44:2593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falloon, J., S. Piscitelli, S. Vogel, B. Sadler, H. Mitsuya, M. F. Kavlick, K. Yoshimura, M. Rogers, S. LaFon, D. J. Manion, H. C. Lane, and H. Masur. 2000. Combination therapy with amprenavir, abacavir, and efavirenz in human immunodeficiency virus (HIV)-infected patients failing a protease-inhibitor regimen: pharmacokinetic drug interactions and antiviral activity. Clin. Infect. Dis. 30:313-318. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch, M. S., F. Brun-Vezinet, R. T. D'Aquila, S. M. Hammer, V. A. Johnson, D. R. Kuritzkes, C. Loveday, J. W. Mellors, B. Clotet, B. Conway, L. M. Demeter, S. Vella, D. M. Jacobsen, and D. D. Richman. 2000. Antiretroviral drug resistance testing in adult HIV-1 infection: recommendations of an International AIDS Society—USA Panel. JAMA 283:2417-2426. [DOI] [PubMed] [Google Scholar]
- 7.Jung, M., H. Agut, D. Candotti, D. Ingrand, C. Katlama, and J. Huraux. 1992. Susceptibility of HIV-1 isolates to zidovudine: correlation between widely applicable culture test and PCR analysis. J. Acquir. Immune Defic. Syndr. 5:359-364. [PubMed] [Google Scholar]
- 8.Kempf, D. J., K. C. Marsh, G. Kumar, A. D. Rodrigues, J. F. Denissen, E. McDonald, M. J. Kukulka, A. Hsu, G. R. Granneman, P. A. Baroldi, E. Sun, D. Pizzuti, J. J. Plattner, D. W. Norbeck, and J. M. Leonard. 1997. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob. Agents Chemother. 41:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larder, B. A., P. Kellam, and S. D. Kemp. 1991. Zidovudine resistance predicted by direct detection of mutations in DNA from HIV-infected lymphocytes. AIDS 5:137-144. [DOI] [PubMed] [Google Scholar]
- 10.Li, T. S., R. Tubiana, C. Katlama, V. Calvez, H. Ait Mohand, and B. Autran. 1998. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 351:1682-1686. [DOI] [PubMed] [Google Scholar]
- 11.Marsh, K. C., E. Eiden, and E. McDonald. 1997. Determination of ritonavir, a new HIV protease inhibitor, in biological samples using reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 704:307-313. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft, A., S. Vella, T. L. Benfield, A. Chiesi, V. Miller, P. Gargalianos, A. d'Arminio Monforte, I. Yust, J. N. Bruun, A. N. Phillips, J. D. Lundgren, et al. 1998. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet 352:1725-1730. [DOI] [PubMed] [Google Scholar]
- 13.Nijhuis, M., C. A. Boucher, P. Schipper, T. Leitner, R. Schuurman, and J. Albert. 1998. Stochastic processes strongly influence HIV-1 evolution during suboptimal protease-inhibitor therapy. Proc. Natl. Acad. Sci. USA 95:14441-14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadler, B. M., C. Gillotin, Y. Lou, and D. S. Stein. 2001. Pharmacokinetic and pharmacodynamic study of the human immunodeficiency virus protease inhibitor amprenavir after multiple oral dosing. Antimicrob. Agents Chemother. 45:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadler, B. M., C. D. Hanson, G. E. Chittick, W. T. Symonds, and N. S. Roskell. 1999. Safety and pharmacokinetics of amprenavir (141W94), a human immunodeficiency virus (HIV) type 1 protease inhibitor, following oral administration of single doses to HIV-infected adults. Antimicrob. Agents Chemother. 43:1686-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadler, B. M., P. J. Piliero, S. L. Preston, P. P. Lloyd, Y. Lou, and D. S. Stein. 2001. Pharmacokinetics and safety of amprenavir and ritonavir following multiple-dose, co-administration to healthy volunteers. AIDS 15:1009-1018. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt, B., K. Korn, B. Moschik, C. Paatz, K. Uberla, and H. Walter. 2000. Low level of cross-resistance to amprenavir (141W94) in samples from patients pretreated with other protease inhibitors. Antimicrob. Agents Chemother. 44:3213-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tisdale, M., R. Myers, S. Randall, M. Maguire, M. Ait-Khaled, R. Elston, and W. Snowden. 2000. Evolution of resistance to the protease inhibitor amprenavir in vitro and in clinical studies. Clin. Drug Investig. 20:267-285. [Google Scholar]
- 19.van Heeswijk, R. P., A. I. Veldkamp, R. M. Hoetelmans, J. W. Mulder, G. Schreij, A. Hsu, J. M. Lange, J. H. Beijnen, and P. L. Meenhorst. 1999. The steady-state plasma pharmacokinetics of indinavir alone and in combination with a low dose of ritonavir in twice daily dosing regimens in HIV-1-infected individuals. AIDS 13:F95-F99. [DOI] [PubMed] [Google Scholar]