Abstract
Efflux-related multidrug resistance (MDR) is a significant means by which bacteria can evade the effects of selected antimicrobial agents. Genome sequencing data suggest that Staphylococcus aureus may possess numerous chromosomally encoded MDR efflux pumps, most of which have not been characterized. Inhibition of these pumps, which may restore clinically relevant activity of antimicrobial agents that are substrates for them, may be an effective alternative to the search for new antimicrobial agents that are not substrates. The inhibitory effects of selected phenothiazines and two geometric stereoisomers of the thioxanthene flupentixol were studied using strains of S. aureus possessing unique efflux-related MDR phenotypes. These compounds had some intrinsic antimicrobial activity and, when combined with common MDR efflux pump substrates, resulted in additive or synergistic interactions. For S. aureus SA-1199B, which overexpresses the NorA MDR efflux pump, and for two additional strains of S. aureus having non-NorA-mediated MDR phenotypes, the 50% inhibitory concentration (IC50) for ethidium efflux for all tested compounds was between 4 and 15% of their respective MICs. Transport of other substrates was less susceptible to inhibition; the prochlorperazine IC50 for acriflavine and pyronin Y efflux by SA-1199B was more than 60% of its MIC. Prochlorperazine and trans(E)-flupentixol were found to reduce the proton motive force (PMF) of S. aureus by way of a reduction in the transmembrane potential. We conclude that the mechanism by which phenothiazines and thioxanthenes inhibit efflux by PMF-dependent pumps is multifactorial and, because of the unbalanced effect of these compounds on the MICs and the efflux of different substrates, may involve an interaction with the pump itself and, to a lesser extent, a reduction in the transmembrane potential.
Efflux-related multidrug resistance (MDR) has become appreciated as a significant complicating factor in the chemotherapy of bacterial infections. This is most evident for Pseudomonas aeruginosa, an organism that possesses several three-component efflux systems consisting of an integral cytoplasmic membrane drug-proton antiporter of the resistance-nodulation-division family, a channel-forming outer membrane protein, and a periplasmic protein that links the previous two proteins (34). The most extensively studied of these efflux systems is MexAB-OprM, which along with MexXY-OprM is constitutively expressed and is involved in the intrinsic resistance to multiple drugs observed for wild-type strains (23, 34).
Gram-positive bacteria also possess efflux pumps that are capable of conferring clinically relevant resistance to antimicrobial agents such as macrolides, tetracyclines, fluoroquinolones, and selected dyes and disinfectants (39). Most of the pumps that transport drugs in gram-positive bacteria are members of the major facilitator superfamily (MFS), which are membrane-based polypeptides with 12 or 14 transmembrane helices. MFS proteins employ the proton motive force (PMF) as the energy source for drug translocation. These pumps can transport a single class of drug (TetK of Staphylococcus aureus) or multiple drugs (QacA and NorA of S. aureus, Bmr of Bacillus subtilis) (32). The NorA protein is capable of translocating hydrophilic fluoroquinolones and monocationic dyes and disinfectants. One study has shown that it may be responsible for at least 10% of antiseptic resistance in methicillin-resistant strains of S. aureus (30).
Bacterial genome sequencing projects have revealed that as genome size increases, so does the number of putative drug efflux transporters. S. aureus has a genome size of 2.8 Mb and possesses approximately 253 open reading frames encoding putative transport pumps (22). Of these, perhaps 17 may be MDR efflux pumps. In addition to NorA, our laboratory has identified two unique and non-NorA-related MDR efflux phenotypes in S. aureus that support the existence of multiple genes encoding such pumps in the genome of this organism (14, 15). These pumps could complicate therapy of S. aureus infections.
Inhibition of MDR efflux pumps can restore the activities of antimicrobial agents that are substrates for these proteins. Inhibitors of the MexAB-OprM efflux system have been identified and examined in vitro and in animal models (24; D. Griffith, O. Lomovskaya, V. Lee, and M. Dudley, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1268, p. 327, 1999). Novel inhibitors of the NorA pump have also been identified but have only been evaluated in vitro (1, 11, 25). Verapamil and reserpine are capable of inhibiting the NorA pump, but the concentrations required are too high to be clinically relevant. The identification and development of safe and effective inhibitors of bacterial efflux pumps are needed.
Phenothiazine and thioxanthene compounds, which are dopamine receptor antagonists and calmodulin inhibitors and are used clinically as neuroleptic and antiemetic agents, have been shown to have modest but broad antimicrobial activities (18-20). MICs are generally above clinically relevant concentrations, but tissue levels can be severalfold higher than those found in the serum, and inhibitory concentrations may be achieved at the site of infection (3).
The thioxanthenes demonstrate geometric stereoisomerism; in the clinical setting the cis form possesses neuroleptic activity, but both the cis and trans forms have roughly equal antibacterial potency (17, 18, 33). The combination of subinhibitory concentrations of these compounds or phenothiazines with multiple standard antimicrobial agents commonly results in a synergistic effect against many species of bacteria (19, 20, 40). Phenothiazines and thioxanthenes also have been shown to inhibit the function of eukaryotic MDR efflux pumps such as p-glycoprotein and may be useful as resistance-modulating agents for the treatment of some drug-resistant tumors (8, 9).
The mechanism(s) by which phenothiazines and thioxanthenes exert their antimicrobial effect and/or potentiate the activity of such a vast array of other antimicrobial agents against bacteria is not completely understood. It has been suggested that some of the potentiation of activity may be the result of inhibition of efflux pumps. Chlorpromazine has been shown to affect potassium flux across the membrane in both S. aureus and the yeast Saccharomyces cerevisiae and to alter the transmembrane potential in Leishmania donovani (6, 21, 42). Either of these actions may affect the function of transport proteins that depend upon the PMF to energize substrate translocation.
In this study, the activities of several phenothiazine and thioxanthene compounds against S. aureus were investigated. Of particular interest was their potential inhibitory effects on efflux-related resistance phenotypes, particularly that conferred by NorA. We found that all tested compounds had modest intrinsic antistaphylococcal activity, augmented the potency of common efflux pump substrates against S. aureus strains possessing different MDR efflux-related resistance mechanisms, and blocked NorA function and non-NorA-related efflux phenotypes in a concentration-dependent manner.
MATERIALS AND METHODS
Media and reagents.
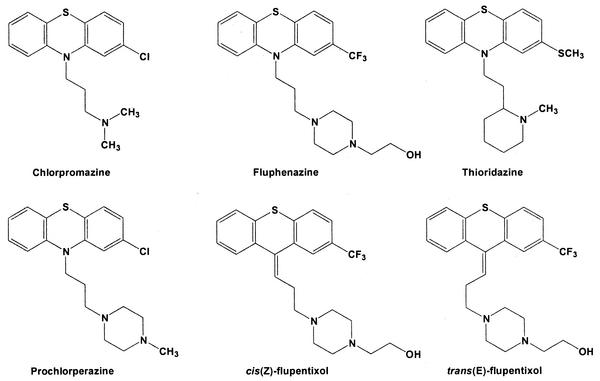
The structures of the phenothiazine and thioxanthene compounds investigated are shown in Fig. 1. Unless otherwise noted, all reagents were the highest grade available and were obtained from Sigma Chemical Co., St. Louis, Mo. cis(Z)-flupentixol and trans(E)-flupentixol were obtained from H. Lundbeck A/S, Copenhagen, Denmark. Cation-adjusted Mueller-Hinton broth (CAMHB) was used as a growth medium (Mueller-Hinton II broth; Becton Dickinson and Co., Cockeysville, Md.).
FIG. 1.
Structures of phenothiazine and thioxanthene derivatives.
Bacterial strains.
The strains of S. aureus employed are listed in Table 1. SA-1199, SA-K1712, and SA 8325-4 are the parent strains of SA-1199B, SA-K1748, and SA-K2068, respectively.
TABLE 1.
Study strains
| Strain | Relevant characteristic(s) | Reference(s) |
|---|---|---|
| SA-1199 | Clinical isolate, methicillin susceptible | 12 |
| SA-1199B | NorA-overproducing derivative of SA-1199; also has A116E GrlA substitution | 12, 13 |
| SA-K1712 | SA 8325-4 having norA insertionally inactivated by tetK | 14 |
| SA-K1748 | Non-NorA organic cation efflux mutant of SA-K1712; also has S80F GrlA substitution | 14 |
| SA 8325-4 | NCTC 8325 cured of prophages | 31 |
| SA-K2068 | Non-NorA multidrug (organic cations, moxifloxacin) efflux mutant of SA 8325-4 | 15 |
Determination of antimicrobial susceptibilities.
MICs were determined by microdilution techniques according to NCCLS guidelines (28). For SA-1199B, SA-K1748, and SA-K2068, the effect of combining 20 μg of reserpine/ml or one-fourth the determined MIC of each phenothiazine and flupentixol stereoisomer on the MICs of the antimicrobial agents tested also was determined. All susceptibility tests were performed at least in duplicate.
Checkerboard combination studies were performed as described previously using strains SA-1199B, SA-K1748, and SA-K2068 (7). Combinations tested included norfloxacin, ethidium bromide (EtBr), or tetraphenylphosphonium bromide (TPP) plus either prochlorperazine or trans(E)-flupentixol. Fractional inhibitory concentrations (FICs) were calculated as follows: (MIC of drug A or B in combination)/(MIC of drug A or B alone). The FIC index was calculated by adding the FIC values. FIC indices were interpreted as follows: ≤0.5, synergy; 1.0, additivity; 2.0, indifferent; and ≥4.0, antagonism.
Ethidium, acriflavine, and pyronin Y efflux.
The loss of ethidium cation from S. aureus strains loaded with EtBr was determined fluorometrically as previously described (14). Experiments were performed in duplicate, and the results were expressed as the mean total efflux over a 5-min time course. Acriflavine and pyronin Y efflux was determined in exactly the same fashion by using excitation and emission wavelengths appropriate for each compound. The effect of increasing concentrations of reserpine, each of the phenothiazines, and each geometric stereoisomer of flupentixol on the EtBr efflux of SA-1199B was determined. We also determined the effect of increasing concentrations of prochlorperazine and reserpine on the EtBr efflux of SA-K1748 and SA-K2068, as well as on the acriflavine and pyronin Y efflux of SA-1199B.
ΔpH determination.
A modification of the method of Zhang et al. was employed to measure the transmembrane pH gradient (41). CAMHB (pH adjusted to 7.0 [CAMHB7]) was used throughout the procedure. SA-1199B was grown in CAMHB7 to an optical density at 660 nm of 0.8. Cells were recovered by centrifugation, washed, and then concentrated 20-fold in ice-cold CAMHB7. Aliquots (1 ml) were placed on ice until used. Cells were warmed to 37°C in a shaking water bath for 10 min, and at zero time 1 μCi of [14C]salicylic acid, used as a pH probe, was added (12 mCi/mmol; Sigma). Aliquots (50 μl) were removed at frequent intervals over a 20-min time period and filtered through 0.45-μm-pore-size nitrocellulose membranes, and the membranes were washed once with 2 ml of phosphate-buffered saline (pH 7.0). Additional aliquots were removed at various times for the determination of protein content (Bio-Rad protein assay kit; Bio-Rad Laboratories, Hercules, Calif.). Radioactivity retained on filters was measured in a scintillation counter, and nonspecific binding of salicylic acid to the filters was subtracted. The experiment was repeated in duplicate, and results were expressed as mean micrograms of salicylic acid accumulated per milligram of cell protein. The effects of prochlorperazine (30 μM), reserpine (20 μg/ml [33 μM]), and carbonyl cyanide m-chlorophenyl hydrazone (CCCP; 100 μM), each added 10 min prior to the addition of salicylic acid, on salicylic acid accumulation were also determined. A cell volume of 4.2 μl/mg of cell protein was used (determined as previously described [26]), and change in pH (ΔpH) was calculated according to the method of Rottenberg (35).
Δψ determination.
Accumulation of the membrane permeant cation TPP was used to measure Δψ, the transmembrane electrical potential, by employing a modification of the procedure of Silverman et al. (36). To determine if TPP efflux by SA-1199B had a confounding effect on these experiments (TPP is known to be a good substrate for the NorA MDR efflux pump [13]), SA-K1712 (in which norA is insertionally inactivated; see Table 1) was also analyzed. Organisms were grown overnight in CAMHB and then diluted 1:1,000 into fresh prewarmed CAMHB and grown with shaking for an additional 2 h at 37°C. Cultures then were shifted to room temperature and grown with shaking for another 2 h, followed by removal of several 350-μl portions to Eppendorf tubes embedded in ice. These samples were warmed for 5 min to room temperature in a shaking water bath prior to use. The protein content of one aliquot was determined as described previously. One aliquot was treated with 8% butanol (vol/vol) for 10 min prior to labeling to obtain a background value. Cells were labeled with 1 μM [3H]TPP (27 Ci/mmol; Amersham Biosciences Corp., Piscataway, N.J.) for 10 min in the presence or absence of prochlorperazine (30 μM), trans(E)-flupentixol (50 μM; SA-K1712 only), reserpine (33 μM), or CCCP (100 μM). Samples then were filtered through GF/C filters (Whatman, Maidstone, United Kingdom), which were washed once with 2 ml of 0.15 M NaCl. Radioactivity retained on filters was measured in a scintillation counter, and background counts were subtracted. Internal cell volume was estimated as described previously, and the Nernst equation, Δψ = −(RT/F) · ln([TPP+]in/[TPP+]out), was used to calculate Δψ. The experiment was repeated three times, and results were expressed as the mean ± standard deviation Δψ (in millivolts).
RESULTS
Antimicrobial susceptibilities and effects of inhibitors.
For the sake of simplicity, reserpine, the phenothiazines, and both flupentixol stereoisomers are referred to as inhibitors. MICs for the study strains are shown in Table 2. Within the concentration range employed, some intrinsic antimicrobial activity was measurable for all inhibitors except reserpine. Thioridazine was more potent than the other phenothiazines against all strains except SA-1199 and SA-1199B. The remaining four strains all are derived from SA 8325-4, so it is not surprising that the increased susceptibility to thioridazine was consistent among them. Against these same strains, the trans isomer of flupentixol was consistently twofold more potent than the cis form. Also of note is the fact that susceptibilities to phenothiazines and thioxanthenes essentially were unaffected by the presence of an efflux-related MDR phenotype, as MICs for parent and mutant strains differed by no more than twofold.
TABLE 2.
Susceptibilities of study strains
| Drug | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| SA-1199 | SA-1199B | SA-K1712 | SA-K1748 | SA 8325-4 | SA-K2068 | |
| Chlorpromazine | 100 | 100 | 100 | 100 | 100 | 100 |
| Fluphenazine | 100 | 100 | 100 | 100 | 100 | 100 |
| Thioridazine | 100 | 100 | 25 | 50 | 50 | 50 |
| Prochlorperazine | 100 | 100 | 100 | 100 | 100 | 100 |
| cis(Z)-flupentixol | 100 | 100 | 50 | 50 | 50 | 100 |
| trans(E)-flupentixol | 100 | 100 | 25 | 25 | 25 | 50 |
| Reserpine | >100 | >100 | >100 | >100 | >100 | >100 |
| Norfloxacin | 1.25 | 50 | 1.25 | 6.25 | 2.5 | 12.5 |
| Acriflavine | 12.5 | 50 | 3.13 | 6.25 | 6.25 | 25 |
| EtBr | 6.25 | 50 | 1.56 | 12.5 | 6.25 | 25 |
| Pyronin Y | 3.13 | 12.5 | 1.56 | 12.5 | 3.13 | 6.25 |
| TPP | 25 | 125 | 6.25 | 50 | 12.5 | 125 |
| Gentamicin | 1.25 | 1.25 | 0.16 | 0.16 | 0.16 | 0.31 |
| Linezolid | 6.25 | 6.25 | 3.13 | 1.56 | 3.13 | 1.56 |
| Nafcillin | 0.31 | 0.31 | 0.16 | 0.16 | 0.16 | 0.16 |
| Tetracycline | 1.56 | 1.56 | 6.25 | 6.25 | 0.31 | 0.63 |
These data illustrate the effect that overexpression of NorA has on norfloxacin, acriflavine, EtBr, pyronin Y, and TPP MICs (Table 2, compare SA-1199 with SA-1199B data), although some of the increase in the norfloxacin MIC for SA-1199B is related to its GrlA substitution (A116E). NorA overexpression had no effect on the MICs of gentamicin, linezolid, nafcillin, or tetracycline, which are not substrates of NorA. SA-K1712 is an SA 8325-4 derivative with norA insertionally inactivated by tetK, which encodes a 14-transmembrane segment MFS transporter (10). The effect of a single chromosomal copy of tetK can be seen by comparing the tetracycline MICs for SA-K1712 and its parent, SA 8325-4.
The SA-K1712 derivative SA-K1748 possesses a non-NorA-mediated inducible MDR phenotype (14). The resistance profile of this strain includes organic cations such as EtBr and TPP, but it does not include fluoroquinolones. The raised norfloxacin MIC in this strain is the result of a GrlA substitution (S80F) and not drug efflux. SA-K2068 is an SA 8325-4 derivative selected by exposure of the organism to gradually increasing concentrations of both EtBr and moxifloxacin. It demonstrates a non-NorA-mediated MDR phenotype that includes efflux-related resistance to selected fluoroquinolones, including the C8-methoxy fluoroquinolones moxifloxacin and gatifloxacin, as well as EtBr and TPP (15). As for the NorA-overexpressing strain (SA-1199B), neither SA-K1748 nor SA-K2068 showed any significant change in susceptibility to gentamicin, linezolid, nafcillin or, in the case of SA-K2068, tetracycline.
The effects of subinhibitory concentrations of each inhibitor on MICs of selected pump substrates for strains expressing efflux-related MDR phenotypes are shown in Table 3. In general, all inhibitors reduced MICs by fourfold or more with the exception of SA-K1748, where norfloxacin susceptibility was minimally affected. As stated previously, norfloxacin resistance in this strain is not related to efflux but rather to a GrlA substitution (14). Differential effects were noted for some of the inhibitors; for example, with respect to the phenothiazines, fluphenazine, thioridazine, and prochlorperazine appeared most efficient in reducing the MICs of EtBr and TPP for SA-K1748 and SA-K2068.
TABLE 3.
Effects of inhibitors on susceptibilities of strains with MDR phenotypesa
| MIC (μg/ml)
|
|||
|---|---|---|---|
| SA-1199B | SA-K1748 | SA-K2068 | |
| Norfloxacin plus: | |||
| RESb | 12.5 (4) | 6.3 (1) | 1.6 (8) |
| CPZ | 12.5 (4) | 3.1 (2) | 1.6 (8) |
| FLU | 12.5 (4) | 3.1 (2) | 1.6 (8) |
| THIO | 12.5 (4) | 3.1 (2) | 1.6 (8) |
| PCPZ | 12.5 (4) | 3.1 (2) | 3.1 (4) |
| CFP | 12.5 (4) | 6.3 (1) | 1.6 (8) |
| TFP | 25 (2) | 6.3 (1) | 1.6 (8) |
| EtBr plus: | |||
| RES | 6.3 (8) | 3.1 (4) | 3.1 (8) |
| CPZ | 6.3 (8) | 3.1 (4) | 3.1 (8) |
| FLU | 6.3 (8) | ≤0.19 (>64) | 0.4 (64) |
| THIO | 6.3 (8) | ≤0.19 (>64) | 0.4 (64) |
| PCPZ | 6.3 (8) | ≤0.19 (>64) | 0.4 (64) |
| CFP | 12.5 (4) | 3.1 (4) | 3.1 (8) |
| TFP | 6.3 (8) | 3.1 (4) | 3.1 (8) |
| TPP plus: | |||
| RES | 25 (5) | 6.3 (8) | 3.1 (40) |
| CPZ | 25 (5) | 6.3 (8) | 12.5 (10) |
| FLU | 6.3 (20) | 1.6 (32) | 3.1 (40) |
| THIO | 12.5 (10) | 1.6 (32) | 3.1 (40) |
| PCPZ | 12.5 (10) | 1.6 (32) | 3.1 (40) |
| CFP | 25 (5) | 12.5 (4) | 12.5 (10) |
| TFP | 12.5 (10) | 12.5 (4) | 12.5 (10) |
Values in parentheses are fold reductions in MICs. Concentrations of inhibitors used were one-fourth their respective MICs.
RES, reserpine; CPZ, chlorpromazine; FLU, fluphenazine; THIO, thioridazine; PCPZ, prochlorperazine; CFP, cis(Z)-flupentixol; TFP, trans(E)-flupentixol.
Inhibitors generally reduced the MICs of gentamicin, nafcillin, linezolid, and tetracycline no more than twofold (data not shown). An exception to this was observed for SA-K1748, where reserpine, chlorpromazine, and both flupentixol stereoisomers (the only inhibitors tested against this strain) reduced tetracycline MICs by four- to eightfold. It must be remembered that this strain possesses the tetK determinant, and it appears as though these inhibitors may have some effect on the function of the TetK transporter.
Checkerboard combination study results are shown in Table 4. The combination of norfloxacin, EtBr, or TPP with either prochlorperazine or trans(E)-flupentixol was either additive or synergistic against S. aureus strains with efflux-related MDR phenotypes.
TABLE 4.
Combination studies
| Strain | Effect of combinationc
|
|||||
|---|---|---|---|---|---|---|
| Norfloxacin plus:
|
EtBr plus:
|
TPP plus:
|
||||
| PCPZa | TFPb | PCPZ | TFP | PCPZ | TFP | |
| SA-1199B | A | S | A | S | S | S |
| SA-K1748 | A | A | S | S | S | A |
| SA-K2068 | S | S | S | S | S | S |
PCPZ, prochlorperazine.
TFP, trans(E)-flupentixol.
A, additive; S, synergistic.
Effect of inhibitors on efflux.
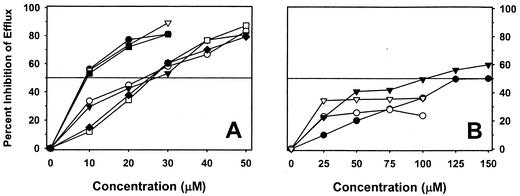
Figure 2 illustrates the effects of increasing concentrations of inhibitors on EtBr efflux (Fig. 2A) and on acriflavine and pyronin Y efflux (Fig. 2B) of SA-1199B. In almost every case a concentration-dependent effect was observed, with the exception of reserpine-mediated inhibition of acriflavine and pyronin Y efflux. The concentrations required to cause at least a 50% inhibition (IC50) of EtBr efflux were 10 μM for reserpine, thioridazine, and prochlorperazine, whereas the remainder of the tested compounds did not result in this degree of inhibition until a concentration of 30 μM was used. For both SA-K1748 and SA-K2068, the IC50 of reserpine and prochlorperazine for EtBr efflux was 10 μM (data not shown). With respect to acriflavine and pyronin Y efflux of SA-1199B, the IC50s for prochlorperazine were 125 and 100 μM, respectively, whereas reserpine did not achieve this degree of inhibition at the highest concentration tested (100 μM, near the limit of solubility).
FIG. 2.
Effects of inhibitors on substrate efflux of SA-1199B (data are means of duplicate experiments). The horizontal line indicates the concentration necessary to inhibit efflux by 50%. (A) Inhibition of ethidium efflux. •, reserpine; ○, chlorpromazine; ▾, fluphenazine; ▿, thioridazine; ▪, prochlorperazine; □, cis(Z)-flupentixol; ♦, trans(E)-flupentixol. (B) Inhibition of acriflavine and pyronin Y efflux. • and ○, inhibition of acriflavine by prochlorperazine and reserpine, respectively; ▾ and ▿, inhibition of pyronin Y by prochlorperazine and reserpine, respectively.
The concentrations of inhibitors required to produce ≥50% inhibition of EtBr efflux were significantly lower than their respective MICs. The ratio of the IC50 for each compound to its MIC for SA-1199B ranged from a low of 0.04 (for thioridazine) to a high of 0.15 (for fluphenazine). Thus, for all phenothiazines and both flupentixol stereoisomers concentrations of significantly less than one-fourth the MIC reduced NorA-mediated EtBr efflux by at least half. Similar results were observed for the inhibition of EtBr efflux by prochlorperazine versus SA-K1748 and SA-K2068; the IC50-to-MIC ratio for this compound was 0.06 for both strains. However, the effect of prochlorperazine on acriflavine and pyronin Y efflux of SA-1199B was much less, with IC50-to-MIC ratios of 0.76 and 0.61, respectively.
Effects of inhibitors on ΔpH and Δψ.
At pH 7.0, the ΔpH of SA-1199B in CAMHB was +0.6 to +0.8 over the entire 20-min time course of the experiment (data not shown). The addition of reserpine or prochlorperazine had no effect, but the ΔpH was completely abolished by CCCP. The Δψ of SA-1199B in CAMHB was −148 ± 2 mV, and in the presence of prochlorperazine, reserpine, and CCCP Δψ was −105 ± 6, −158 ± 1, and 0 mV, respectively. For SA-K1712 the results were −146 ± 3 mV for CAMHB and −164 ± 1 mV for reserpine. At the concentrations employed, prochlorperazine, trans(E)-flupentixol, and CCCP reduced Δψ of SA-K1712 to zero.
DISCUSSION
Bacterial efflux pumps clearly contribute to the increasing problem of MDR. Identification of inhibitors of efflux pumps for which antimicrobial agents are substrates is an active area of research in both the pharmaceutical and academic sectors (1, 11, 24, 25). The study of inhibitors of the MexAB-OprM efflux system is perhaps the most advanced, but to our knowledge only limited in vivo testing has been done and no human toxicology of promising candidates has been performed. Compounds that inhibit the activity of NorA also have been described, including the proton pump inhibitor omeprazole, but only in vitro testing has been done. In addition, the concentration of omeprazole required was above what is clinically attainable (1). Obviously, necessary characteristics of a pump inhibitor are that it be active at an achievable serum drug concentration and that it has minimal toxicity.
The search for candidate efflux pump inhibitors can be costly and time consuming. It makes reasonable sense to examine selected drugs already in clinical use as potential inhibitors. The pharmacokinetics and the toxicities of these compounds generally are known, obviating the need to determine these parameters. The choice of candidates may be difficult, but perhaps can be simplified by examining drugs for which some data already exist regarding additive or synergistic antibacterial effects when combined with compounds known to be efflux pump substrates, such as fluoroquinolones, tetracyclines, macrolides, or quaternary amine-containing antiseptic agents (39). Such data were available for the phenothiazines and thioxanthenes and prompted the present study (17-20, 40).
We found that phenothiazines and two geometric stereoisomers of the related thioxanthene class possessed some intrinsic antistaphylococcal activities, albeit at concentrations above those which are clinically achievable. This activity was unaffected by the presence of a variety of efflux-related phenotypes, indicating that these compounds are not substrates for the involved pumps themselves. We found some evidence of stereoselectivity, which was strain related, in that trans(E)-flupentixol was twofold more potent than the cis isomer against SA 8325-4 and its derivatives. There were no differences in the potencies of the flupentixol compounds versus SA-1199 or SA-1199B, and each was equipotent in inhibition of EtBr efflux in SA-1199B. The dissociation between the antibacterial and the anti-EtBr efflux effect (trans isomer equipotent or superior) and the neuroleptic effect (only present in the cis isomer) has been observed previously and is an important consideration in the possible design of more-potent inhibitors of the thioxanthene class (17).
More interesting than intrinsic antistaphylococcal activity was the fact that when combined at one-fourth their MICs with common efflux pump substrates all inhibitors augmented the antimicrobial activity of those substrates. The greatest effects in this regard were observed for EtBr and TPP, and the most potent inhibitors were fluphenazine, thioridazine, and prochlorperazine, especially against strains SA-K1748 and SA-K2068. Checkerboard testing verified the above observations in that an additive or synergistic effect was observed against all strains tested expressing efflux-related MDR phenotypes by combining prochlorperazine or trans(E)-flupentixol with pump substrates.
Thioridazine and prochlorperazine were the most potent of the tested compounds with respect to inhibition of NorA-mediated EtBr efflux, having IC50-to-MIC ratios of 0.04 and 0.06, respectively, indicating significant NorA inhibition at concentrations well below their respective MICs. Prochlorperazine was also an efficient inhibitor of EtBr efflux against SA-K1748 and SA-K2068, where its IC50-to-MIC ratio was also 0.06. However, a differential effect was observed when the effects of prochlorperazine on NorA-mediated efflux of acriflavine and pyronin Y were examined. IC50 values which were more than 10-fold higher than those determined for EtBr efflux were observed. This observation, when combined with the differential effect of some inhibitors on MICs of selected pump substrates (see above), suggests the possibility that these compounds may physically interact with the pumps themselves. There is evidence to support such a contention with respect to reserpine for the B. subtilis MDR efflux pump Bmr (2). Such an interaction may affect pump affinity for one substrate more than another, perhaps by altering a substrate recognition site, such that export proceeds with variable efficiency for different substrates. Clearly, more work needs to be done to determine if such a physical interaction actually occurs.
Unfortunately, the IC50 values of inhibitors for EtBr, acriflavine, and pyronin Y efflux are above those employed in clinical practice. Even so, prochlorperazine is of particular interest because of its reduced central nervous system toxicity compared to other phenothiazines and its current widespread clinical use as an antiemetic agent. Additionally, this drug has been shown to increase the retention of doxorubicin in tumor cells recovered from patients receiving both drugs concurrently (37, 38). However, peak prochlorperazine concentrations found in those patients were approximately half the determined IC50 for EtBr efflux mediated by NorA and the SA-K1748 and SA-K2068 efflux systems, and about 5% of the determined IC50 for NorA-mediated acriflavine and pyronin Y efflux. It is possible that for bacteria maximal pump inhibition is not required to achieve a beneficial effect in vivo. Animal studies examining this issue would seem to be in order. It is also possible that chemical modification of the prochlorperazine molecule could result in a compound with even less central nervous system toxicity than it currently has as well as improved inhibitory activity towards bacterial efflux pumps.
The equivalence of the Δψ in the absence of NorA (SA-K1712, −146 ± 3 mV) and in the presence of its overexpression (SA-1199B, −148 ± 2 mV) indicate that the function of this MDR pump does not interfere with the methodology employed for the determination of Δψ, despite TPP being a good NorA substrate. Our Δψ data suggest that, in addition to a possible direct interaction with pumps, depolarization of the cytoplasmic membrane by phenothiazines and thioxanthenes may also play a role in efflux pump inhibition. This activity can result in a decrease in the PMF, upon which most bacterial drug efflux pumps are dependent for their function. The degree of depolarization appears strain dependent, as demonstrated by the differences observed between SA-1199B and SA-K1712. The PMF is related to both Δψ and ΔpH in the following manner: PMF (in millivolts) = Δψ − 59ΔpH (at room temperature) (35). If ΔpH remains constant, and our determination in the presence of prochlorperazine and reserpine suggests that it does, the PMF of SA-1199B in the absence of prochlorperazine was −183 mV, and in its presence it was −140 mV. This modest (23%) PMF change could reduce the efficiency of NorA and other PMF-dependent pumps. The PMF calculation cannot be done for SA-K1712 with the available data, as ΔpH was not determined for that organism, but the total collapse of its Δψ in the presence of both prochlorperazine and trans(E)-flupentixol would result in a greater decrease in the PMF than that observed for SA-1199B.
If membrane depolarization were the main means of pump inhibition, it would be expected that the same, or at least similar, concentrations of prochlorperazine would inhibit the PMF-driven efflux of all substrates to a similar degree. This was not observed for SA-1199B, in which the IC50 of prochlorperazine for acriflavine and pyronin Y efflux was at least 10 times that observed for EtBr efflux. In addition, the fact that inhibitors had minimal effects on gentamicin MICs, which is dependent on Δψ for its transport, suggests that the depolarization effects, which are strain dependent, may be of secondary importance to another means of pump inhibition, such as a direct interaction of inhibitor and pump (5).
Our data indicate that reserpine does not affect pump function by decreasing the PMF. In fact, the Δψ of SA-1199B and SA-K1712 was increased in its presence while ΔpH remained constant, changes that would result in an increase in the PMF. The explanation for this observation is not clear but may be related, at least in part, to a broad inhibition of many pumps for which TPP is a substrate resulting in somewhat higher intracellular concentrations of the cation in the presence of the inhibitor and thus an increase in Δψ. The facts that prochlorperazine was equipotent to reserpine in the inhibition of EtBr efflux (for all efflux-related MDR strains) and more potent in the inhibition of acriflavine and pyronin Y efflux (for SA-1199B), combined with the observed opposite effects of these compounds on Δψ, are more evidence in favor of a direct interaction with the pumps being the main mechanism of action of these inhibitors.
Indirect effects on multiple cytoplasmic membrane-based processes could result from perturbations in Δψ. The phenothiazines and thioxanthene derivatives we investigated did have small effects on the MICs of antibiotics that are not pump substrates, which may be due to the changes in Δψ we observed. In fact, the Δψ effect may be the explanation, at least in part, for an earlier observation of a remarkable lowering of the methicillin MIC in a strain of methicillin-resistant S. aureus in the presence of subinhibitory concentrations of selected phenothiazines (19). It also has been shown that in the presence of chlorpromazine S. aureus cells undergo ultrastructural changes including abnormal cell walls, asymmetrical cell divisions, and the appearance of large mesosome-like structures intracellularly (16, 20). It is conceivable that processes associated with these structural changes could affect susceptibility to antibiotics that act either intracellularly or at bacterial surfaces. The test strains employed in this study were all susceptible to the nonpump substrates that we investigated. Had we employed additional strains for which starting MICs to these compounds were higher, a greater effect may have been observed. Further work along these lines is warranted.
It is clear that the mechanism by which phenothiazines and thioxanthenes inhibit the function of MDR efflux pumps is multifactorial, including perturbation of membrane energetics and possibly a direct interaction with the transporters themselves. It has been shown that a single amino acid change in human p-glycoprotein affects the inhibitory activity of both cis(Z)- and trans(E)-flupentixol, suggesting that these stereoisomers directly interact with that pump (4). Further work would be necessary to establish direct evidence that this occurs with bacterial efflux pumps. It also would be of great interest to determine whether or not these compounds can inhibit the activity of other bacterial drug transporters, such as the MexAB-OprM system of P. aeruginosa, or even ATP-dependent pumps, such as the macrolide efflux pump MsrA of Staphylococcus epidermidis and S. aureus. If so, structure-activity relationships for phenothiazines and thioxanthene derivatives, especially trans isomers of the latter compounds, would be a logical step toward finding a potent, and ultimately minimally toxic, broad-spectrum bacterial MDR efflux pump inhibitor. The evaluation of other drugs currently in clinical practice, such as selective serotonin reuptake inhibitors, which have been shown to possess some antimicrobial activity, as potential efflux pump inhibitors also seems reasonable (27, 29).
Acknowledgments
This study was supported by VA Research Funds.
REFERENCES
- 1.Aeshlimann, J. R., L. D. Dresser, G. W. Kaatz, and M. J. Rybak. 1999. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, M., C. M. Borsch, A. A. Neyfakh, and S. Schuldiner. 1993. Mutants of the Bacillus subtilis multidrug transporter Bmr with altered susceptibility to the antihypertensive alkaloid reserpine. J. Biol. Chem. 268:11086-11089. [PubMed] [Google Scholar]
- 3.Crowle, A. J., G. S. Douvas, and M. H. May. 1992. Chlorpromazine: a drug potentially useful for treating mycobacterial infections. Chemotherapy 38:410-419. [DOI] [PubMed] [Google Scholar]
- 4.Dey, S., P. Hafkemeyer, I. Pastan, and M. M. Gottesman. 1999. A single amino acid residue contributes to distinct mechanisms of inhibition of the human multidrug transporter by stereoisomers of the dopamine receptor antagonist flupentixol. Biochemistry 38:6630-6639. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg, E. S., L. J. Mandell, H. R. Kaback, and M. H. Miller. 1984. Quantitative association between electrical potential across the cytoplasmic membrane and early gentamicin uptake and killing in Staphylococcus aureus. J. Bacteriol. 157:863-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliam, Y. 1983. Membrane effects of phenothiazines in yeasts. I. Stimulation of calcium and potassium fluxes. Biochim. Biophys. Acta 733:242-248. [DOI] [PubMed] [Google Scholar]
- 7.Eliopoulos, G. M., and R. C. Moellering, Jr. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams and Wilkins, Baltimore, Md.
- 8.Ford, J. M., W. C. Prozialeck, and W. N. Hait. 1989. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol. Pharmacol. 35:105-115. [PubMed] [Google Scholar]
- 9.Ford, J. M., E. P. Bruggemann, I. Pastan, M. M. Gottesman, and W. N. Hait. 1990. Cellular and biochemical characterization of thioxanthenes for reversal of multidrug resistance in human and murine cell lines. Cancer Res. 50:1748-1756. [PubMed] [Google Scholar]
- 10.Ginn, S. L., M. H. Brown, and R. A. Skurray. 1997. Membrane topology of the metal-tetracycline/H+ antiporter TetA(K) from Staphylococcus aureus. J. Bacteriol. 179:3786-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guz, N. R., F. R. Stermitz, J. B. Johnson, T. D. Beeson, S. Willen, J.-F. Hsiang, and K. Lewis. 2001. Flavonolignan and flavone inhibitors of a Staphylococcus aureus multidrug resistance pump: structure-activity relationships. J. Med. Chem. 44:261-268. [DOI] [PubMed] [Google Scholar]
- 12.Kaatz, G. W., S. L. Barriere, D. R. Schaberg, and R. Fekety. 1987. The emergence of resistance to ciprofloxacin during therapy of experimental methicillin-susceptible Staphylococcus aureus endocarditis. J. Antimicrob. Chemother. 20:753-758. [DOI] [PubMed] [Google Scholar]
- 13.Kaatz, G. W., S. M. Seo, and C. A. Ruble. 1993. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 37:1086-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaatz, G. W., S. M. Seo, L. O'Brien, M. Wahiduzzaman, and T. J. Foster. 2000. Evidence for the existence of a multidrug efflux transporter distinct from norA in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaatz, G. W., V. V. Moudgal, and S. M. Seo. 2002. Identification and characterization of a novel efflux-related multidrug resistance phenotype in Staphylococcus aureus. J. Antimicrob. Chemother. 50:833-838. [DOI] [PubMed]
- 16.Kristiansen, J. E., and J. Blom. 1981. Effect of chlorpromazine on the ultrastructure of Staphylococcus aureus. Acta Pathol. Microbiol. Scand. B 89:399-405. [PubMed] [Google Scholar]
- 17.Kristiansen, J. E., and I. Mortensen. 1981. Stereo-isomeric dissociation of the antibacterial and the neuroleptic effect of clopenthixol. Acta Pathol. Microbiol. Scand. B 89:437-438. [PubMed] [Google Scholar]
- 18.Kristiansen, J. E. 1990. The antimicrobial activity of psychotherapeutic drugs and stereo-isomeric analogues. Dan. Med. Bull. 37:165-182. [PubMed] [Google Scholar]
- 19.Kristiansen, J. E. 1993. Chlorpromazine: non-antibiotics with antimicrobial activity—new insights in managing resistance? Curr. Opin. Investig. Drugs 2:587-591. [Google Scholar]
- 20.Kristiansen, J. E., and L. Amaral. 1997. The potential management of resistant infections with non-antibiotics. J. Antimicrob. Chemother. 40:319-327. [DOI] [PubMed] [Google Scholar]
- 21.Kristiansen, J. E., I. Mortensen, and B. Nissen. 1982. Membrane stabilizers inhibit potassium efflux from Staphylococcus aureus strain U2275. Biochim. Biophys. Acta 685:379-382. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumara, A., Maruyama, H. Murakami, A., Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 23.Li, X.-Z., H. Nikaido, and K. Poole. 1995. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1948-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markham, P. N., E. Westhaus, K. Klyachko, M. E. Johnson, and A. A. Neyfakh. 1999. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mates, S. M., E. S. Eisenberg, L. J. Mandel, L. Patel, H. R. Kaback, and M. H. Miller. 1982. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 79:6693-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Bellido, J. L., S. Munoz-Criado, and J. A. Garcia. 2000. Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int. J. Antimicrob. Agents 14:177-180. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 1999. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 29.Ni, Y. G., and R. Miledi. 1997. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac). Proc. Natl. Acad. Sci. USA 94:2036-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noguchi, N., M. Hase, M. Kitta, M. Sasatsu, K. Deguchi, and M. Kono. 1999. Antiseptic susceptibility and distribution of antiseptic-resistance genes in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 172:247-253. [DOI] [PubMed] [Google Scholar]
- 31.Novick, R. P. 1963. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen, P. V. 1977. The thioxanthenes, p. 827-867. In E. Usdin and I. Forrest (ed.), Psychotherapeutic drugs. Marcel Dekker, New York, N.Y.
- 34.Poole, K., and R. Srikumar. 2001. Multidrug efflux in Pseudomonas aeruginosa: components, mechanisms and clinical significance. Curr. Top. Med. Chem. 1:59-71. [DOI] [PubMed] [Google Scholar]
- 35.Rottenberg, H. 1979. The measurement of membrane potential and ΔpH in cells, organelles, and vesicles. Methods Enzymol. 55:547-569. [DOI] [PubMed] [Google Scholar]
- 36.Silverman, J. A., N. Oliver, T. Andrew, and T. Li. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sridhar, K. S., A. Krishnan, T. S. Samy, A. Sauerteig, L. L. Wellam, G. V. McPhee, R. C. Duncan, S. Y. Anac, B. Ardalan, and P. W. Benedetto. 1993. Prochlorperazine as a doxorubicin-efflux blocker: phase I clinical and pharmacokinetic studies. Cancer Chemother. Pharmacol. 31:423-430. [DOI] [PubMed] [Google Scholar]
- 38.Sridhar, K. S., A. Krishnan, T. S. Samy, R. C. Duncan, A. Sauerteig, G. V. McPhee, M. E. Auguste, and P. W. Benedetto. 1994. Phase I and pharmacokinetics studies of prochlorperazine 2-h i.v. infusion as a doxorubicin-efflux blocker. Cancer Chemother. Pharmacol. 34:377-384. [DOI] [PubMed] [Google Scholar]
- 39.Van Bambeke, F., E. Balzi, and P. M. Tulkens. 2000. Antibiotic efflux pumps. Biochem. Pharmacol. 60:457-470. [DOI] [PubMed] [Google Scholar]
- 40.Viveiros, M., and L. Amaral. 2001. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int. J. Antimicrob. Agents 17:225-228. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, Y., A. Scorpio, H. Nikaido, and Z. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 181:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zilberstein, D., V. Liveanu, and A. Gepstein. 1990. Tricyclic drugs reduce the proton motive force in Leishmania donovani promastigotes. Biochem. Pharmacol. 39:935-940. [DOI] [PubMed] [Google Scholar]