Abstract
We have studied the aminoglycoside resistance gene, which confers high levels of resistance to both amikacin and gentamicin, that is carried by plasmid pSTI1 in the PER-1 β-lactamase-producing strain of Salmonella enterica serovar Typhimurium previously isolated in Turkey. This gene, called aac(6′)-Ib11, was found in a class 1 integron and codes for a protein of 188 amino acids, a fusion product between the N-terminal moiety (8 amino acids) of the signal peptide of the β-lactamase OXA-1 and the acetyltransferase. The gene lacked a plausible Shine-Dalgarno (SD) sequence and was located 45 nucleotides downstream from a small open reading frame, ORF-18, with a coding capacity of 18 amino acids and a properly spaced SD sequence likely to direct the initiation of aac(6′)-Ib11 translation. AAC(6′)-Ib11 had Leu118 and Ser119 as opposed to Gln and Leu or Gln and Ser, respectively, which were observed in all previously described enzymes of this type. We have evaluated the effect of Leu or Gln at position 118 by site-directed mutagenesis of aac(6′)-Ib11 and two other acetyltransferase gene variants, aac(6′)-Ib7 and -Ib8, which naturally encode Gln118. Our results show that the combination of Leu118 and Ser119 confers an extended-spectrum aminoglycoside resistance, with the MICs of all aminoglycosides in clinical use, including gentamicin, being two to eight times higher for strains with Leu118 and Ser119 than for those with Gln118 and Ser119.
Resistance to the aminoglycoside group of antibiotics is an important clinical problem since these antibiotics are widely used in the treatment of severe infections. The production of aminoglycoside-modifying enzymes, such as aminoglycoside phosphotransferases, nucleotidyltransferases, and acetyltransferases (AAC), is the most frequent mechanism of bacterial resistance to these antibiotics. Two 6′-N-acetyltransferase isoenzymes have been reported, AAC(6′)-I and AAC(6′)-II, which modify the same amino group but differ in their substrate specificities (27, 34). Both acetylate kanamycin, tobramycin, netilmicin, and sisomicin but not gentamicin C1. However, the type I enzyme also modifies amikacin whereas the type II enzyme acetylates gentamicin but not amikacin. Recently it was demonstrated that different modifications of the amino acid sequences of the AAC(6′) proteins may influence their enzymatic activities (4, 27, 30) or the levels of resistance (11). Using site-directed mutagenesis of the genes encoding AAC(6′)-Ib and AAC(6′)-IIa proteins, Rather et al. (27) identified the amino acid at position 119 as the one responsible for influencing the substrate specificity, where leucine was correlated with amikacin resistance and serine was correlated with gentamicin resistance. In a previous study, two naturally occurring AAC(6′)-Ib variants [AAC(6′)-Ib7 and -Ib8] in which serine instead of leucine was present at position 119 were observed to be responsible for reduced susceptibility to gentamicin and susceptibility to amikacin (4).
Almost all aac(6′)-Ib genes (synonymously called aacA4) described to date exist as gene cassettes carried by class 1 integrons (9). As a consequence, the acetyltransferase genes are transcribed from the integron-borne promoters Pant or Pant/P2 (14, 28), and the enzymes are subject to N-terminal sequence variations due to sequence rearrangements that may occur during integration at the attI1 site (4, 5, 8, 16).
A Salmonella enterica serovar Typhimurium strain isolated in Turkey and producing the extended-spectrum β-lactamase PER-1 was resistant to multiple antibiotics, including gentamicin and amikacin (35). Preliminary resistance profile analysis suggested the presence of a single 6′-N-acetyltransferase. Besides being encoded by an integron-borne cassette and fused to the signal peptide of the OXA-1 β-lactamase, this acetyltransferase, called AAC(6′)-Ib11, was found to carry the novel amino acid combination of Leu118 and Ser119. All previously described acetyltransferases of type Ib carry Gln118, while several natural or in vitro-generated variants with Ser119 have been reported (4, 13, 17, 22). The present study was undertaken to evaluate the impact on the resistance profile of Leu at position 118.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All strains and plasmids used in this study are listed in Table 1. The multiple-antibiotic-resistant Salmonella serovar Typhimurium(pSTI1), producing the extended-spectrum β-lactamase PER-1, was isolated from a patient in Turkey with a nosocomial infection (35). Enterobacter cloacae strain ECl563(pLMM563) and Citrobacter freundii strain CFr564(pLMM564) have been described previously (4). Strains were grown in Luria-Bertani (LB) broth or LB agar, which was supplemented, when required, with ampicillin (100 μg/ml) or kanamycin (50 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Phenotype, genotype, or relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli BHM 71-18 mutS | thi supE Δ(lac-proAB) [mutS::Tn10] [F′ proAB laqIqZΔM15] | Promega |
| E. coli JM109 | endA1 recA1 gyrA96 thi hsdR17 (rK− mK+) relA1 supE44 Δ(lac-proAB)[F′ traD36 proAB laq1qZΔM15] | Promega |
| E. coli DH5α | deoR endA1 gyrA96 hsdR17 (rK−mK+) recA1 relA1 supE44 thi-1 Δ(lacZYA-argFV169) φ80lacZΔM15 F− | Clontech |
| Citrobacter freundii CFr564 (pLMM564) | Netr Tobr Genr [AAC(6′)-Ib7 (Gln118, Ser119)] Strr Spcr Sulr | 4 |
| Enterobacter cloacae EC1563(pLMM563) | Netr Tobr Genr [AAC(6′)-Ib8 (Gln118, Ser119)] Strr Spcr Sulr CmprTetr | 4 |
| Salmonella serovar Typhimurium(pSTI1) | Netr Tobr Genr [AAC(6′)-Ib11(Leu118, Ser119)] Strr Spcr Sulr | 35 |
| Plasmids | ||
| pAZ505 | pBR322 containing a 1.5-kb fragment [AAC(6′)-Ib (Gln118, Leu119)] Ampr Netr Tobr Amir | 34 |
| pGEM | Ampr, cloning vector | Promega |
| pGEMM564 | 1.06-bp EaeI fragment [AAC(6′)-Ib7] of pLMM564 cloned into pGEM | 11 |
| pGEMM563 | 1.09-bp EaeI fragment [AAC(6′)-Ib8] of pLMM563 cloned into pGEM | This study |
| pGEMMST | 1.14-bp EaeI fragment [AAC(6′)-Ib11] of pST11 cloned into pGEM | This study |
| pGEMM564M | Derivative of pGEMM564 [AAC(6′)-Ib7 (Gln118 replaced by Leu)] | This study |
| pGEMM563M | Derivative of pGEMM563 [AAC(6′)-Ib8 (Gln118 replaced by Leu)] | This study |
| pGEMMSTM | Derivative of pGEMMST [AAC(6′)-Ib11 (Leu118 replaced by Gln)] | This study |
| pGEMMSTP1 | Derivative of pGEMMST [AAC(6′)-Ib11 (A325TG replaced by AGG)] | This study |
| pGEMMSTP2 | Derivative of pGEMMST [AAC(6′)-Ib11 (A325TG replaced by GGG)] | This study |
Ami, amikacin; Amp, ampicillin; Cmp, chloramphenicol; Gen, gentamicin; Net, netilmicin; Spc, spectinomycin; Str, streptomycin; Sul, sulfonamide; Tet, tetracycline; Tob, tobramycin. The amino acids are numbered as for AAC(6′)-Ib (34).
Antibiotic susceptibility testing.
Susceptibility to 14 aminoglycosides was determined by disk diffusion with disks provided by the Schering-Plough Research Institute. MICs were determined by serial twofold dilution of antibiotics in Mueller-Hinton agar. Gentamicin, isepamicin, and netilmicin were from Schering-Plough, amikacin and kanamycin were from Bristol-Myers Squibb, and tobramycin was from Eli Lilly and Company.
Immunoblot analyses and determination of N-terminal amino acid sequences of AAC(6′)-Ib variants.
Cell extracts were electrophoresed on sodium dodecyl sulfate-containing polyacrylamide gels. After electrotransfer onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore), the acetyltransferases were revealed with an anti-AAC(6′)-Ib rabbit antiserum and peroxidase-labeled anti-immunoglobulin G as previously described (33). The AAC(6′)-Ib variants produced by Escherichia coli DH5α(pGEMM563) and Salmonella serovar Typhimurium(pSTI1) were purified as previously described for the variant produced by DH5α(pGEMM564) (11). Briefly, bacteria were grown to an optical density of ca. 0.6 at 650 nm and the cells were lysed by sonication. The lysates were passed over a gel filtration column (Ultrogel AcA54; Réactifs IBF) after centrifugation at 12,000 × g for 90 min at 4°C. The fractions containing AAC(6′)-Ib were detected by measuring acetyltransferase activity (6) with [1-14C]acetyl coenzyme A (0.6 GBq/mmol, Amersham International) as the cofactor. AAC-containing fractions were loaded onto a column of immobilized neomycin (34). The bound protein was eluted, concentrated, and visualized by Rouge Ponceau S staining after sodium dodecyl sulfate gel electrophoresis and transfer to an Immobilon-P membrane. The AAC(6′)-Ib-containing areas were excised, and the N-terminal amino acid sequences of the AAC(6′)-Ib variants were determined. The sequence analysis was carried out with a model 494 Procise sequencer (Applied Biosystems, Foster City, Calif.; CNRS UMR 7637, Paris, France).
DNA procedures.
Plasmids were isolated (2) and introduced into E. coli DH5α by electroporation as described previously (1). Digestion with restriction enzymes and ligation were carried out by using New England Biolabs reagents and following standard methods (1). Sequencing of the PCR products from Salmonella serovar Typhimurium was carried out by using an automated sequencer (ABE 50, model 373; Génome Express, Montreuil, France) and dye nucleotides. Nucleotide homology searches were carried out with the alignment program BioEdit version 5.09 (10). All DNA constructs obtained after site-directed mutagenesis were verified by DNA sequence analysis. The nucleotide sequence of the region between the 5′ and the 3′ conserved integron segments was sequenced after amplification by PCR with the respective specific primers (15) and an additional primer annealing to nucleotides 936 to 955 (3) upstream from Pant.
Plasmids pGEMM563 and pGEMMST were constructed as follows: a 1.1-kb EaeI fragment of pLMM563 (4) and pSTI1 (35) containing the complete aac(6′)-Ib gene and ca. 0.5 kb of its upstream sequence was subcloned into the ApaI and NotI sites of pGEM to yield pGEMM563 and pGEMMST, respectively; pGEMM564 was constructed similarly (11).
Site-directed mutagenesis.
Site-directed mutagenesis was used to change the putative translation initiation codon of aac(6′)-Ib11 from A325TG (Fig. 1) to AGG or GGG; the amino acids at position 118 were changed from Gln118 to Leu in the AAC(6′)-Ib variants encoded by pGEMM563 and pGEMM564 and from Leu118 to Gln in the variant encoded by pGEMMST. Site-directed mutagenesis was performed with the GeneEditor in vitro site-directed mutagenesis system from Promega (Charbonnières, France). The procedure was applied according to the manufacturer's instructions. All plasmids were denatured by alkaline treatment. Annealing was performed with 20 μl of a mixture containing 1 pmol of the primer Bottom Strand, 1.25 pmol of 5′-phosphorylated primers 11PSP1 (P-GATATGTATTGTGTTTTTCCTAATAGTATTGGTTTGG), 11PSP2 (P-GATATGTATTGTGTTTTTCCCAATAAGTATTGGTTTGG), AC11DP2 (P-TGCATTCG-CCAGTGACAGGTCTATTCCGCG), or AC11CP2 (P-TGCATTCGCGAGTGACTGGTCTATTCCGCG), 2 μl of T4 annealing buffer, and 200 ng of denatured plasmid template (pGEMM564 and pGEMM563 for primer AC11DP2 and pGEMMST for primers AC11CP2, 11PSP1, and 11PSP2). The mixture was heated to 75°C for 5 min, followed by controlled cooling (1.5°C per min). Once the temperature had reached 37°C, mutant strand synthesis and ligation were performed in 30 μl containing T4 DNA polymerase (5 to 10 U) and T4 DNA ligase (1 to 3 U) for 90 min. The mixture was used to transform E. coli BHM 71-18 mutS-competent cells, which were incubated at 37°C in 4 ml of LB medium containing 100 μl of the GeneEditor antibiotic selection mixture. DNA from the overnight culture was purified by using QIAGEN-tip20 and transferred into E. coli JM109 by transformation. Resistant clones were selected on plates containing amoxicillin and GeneEditor antibiotic selection mixture. After purification as described above, the plasmids were transferred into DH5α by electroporation. After verification of the nucleotide sequence, the mutant plasmids were called pGEMMSTP1, pGEMMSTP2, pGEMM563M, pGEMM564M, and pGEMMSTM, respectively.
FIG. 1.
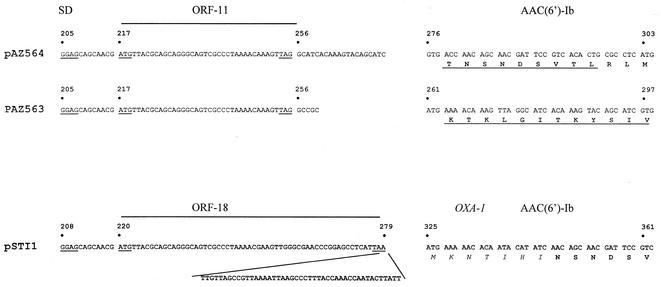
Alignment of TIRs and N-terminal sequences for AAC(6′)-Ib variants. Underlined nucleotides indicate the SD sequences and start and stop codons; underlined amino acids were determined by Edman degradation. The N-terminal sequence of the signal peptide of OXA-1 is shown in italics. The nucleotides are numbered with respect to the first base of the −35 box of the promoter P1 (position 1035 in the sequence for GenBank accession number M73819) in the 5′ conserved sequence of integron In0 (3).
Nucleotide sequence accession number.
The nucleotide sequence for aac(6′)-Ib11 has been deposited in GenBank under accession number AY136758.
RESULTS
Aminoglycoside resistance phenotype of Salmonella serovar Typhimurium(pSTI1).
Salmonella serovar Typhimurium (pSTI1) was previously reported to be resistant to gentamicin, netilmicin, tobramycin, kanamycin, and amikacin (35). An extended antibiogram produced by the disk diffusion method with 14 compounds confirmed the strain's resistance to the above-mentioned antibiotics and to some additional compounds, with inhibition zone diameters of ≤6 mm for kanamycin and tobramycin, 10 mm for netilmicin and gentamicin, and 12 mm for 2′-N-ethylnetilmicin, 5′-episisomicin, and amikacin, while the strain was susceptible to isepamicin (zone diameter, 22 mm) and to apramycin, 6′-N-ethylnetilmicin, fortimicin, neomycin, streptomycin, and spectinomycin (≥25 mm). This profile was evocative of AAC(6′)-Ib production, except with respect to gentamicin. The observed resistance to gentamicin was unlikely to be due to AAC(3) production [except maybe the production of a rare AAC(3)-III], since the diameters for 6′-N-ethylnetilmicin, fortimicin, and apramycin were not notably reduced, or to ANT(2′)-I production, since its gene could not be amplified with the corresponding primers (data not shown). The MICs of several aminoglycosides were also determined (Table 2), confirming that the susceptibilities to both gentamicin and amikacin (but not isepamicin) were similarly reduced.
TABLE 2.
Resistance profiles of AAC(6′)-Ib7, AAC(6′)-Ib8, and AAC(6′)-Ib11 and correlation between resistance phenotypes and amino acids at positions 118 and 119a
| Strain (plasmid) | Amino acid at position:
|
MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| 118 | 119 | Gen | Ami | Net | Tob | Ise | |
| E. coli | |||||||
| DH5α | 0.125 | 0.25 | 0.125 | 0.125 | 0.125 | ||
| BM694(pAZ505) | Q | L | 0.5 | 16 | 32 | 128 | 4 |
| DH5α(pLMM563) | Q | S | 4 | 2 | 8 | 8 | 0.5 |
| DH5α(pGEMM563) | Q | S | 4 | 2 | 8 | 8 | 0.5 |
| DH5α(pGEMM563 M) | L | S | 16 | 16 | 32 | 32 | 2 |
| DH5α(pLMM564) | Q | S | 2 | 1 | 4 | 8 | 0.25 |
| DH5α(pGEMM564) | Q | S | 2 | 2 | 8 | 8 | 0.25 |
| DH5α(pGEMM564 M) | L | S | 4 | 8 | 16 | 16 | 0.5 |
| DH5α(pSTI1) | L | S | 8 | 16 | 32 | 16 | 2 |
| DH5α(pGEMMST) | L | S | 8 | 16 | 32 | 16 | 2 |
| DH5α(pGEMMSTM) | Q | S | 4 | 4 | 8 | 16 | 0.5 |
| Salmonella serovar Typhimurium (pSTI1) | L | S | 16 | 32 | 64 | 32 | 4 |
Aminoglycoside abbreviations are as given in Table 1, footnote a. Ise, isepamicin; Kan, kanamycin.
Characterization of the pSTI1-borne aac(6′)-Ib gene.
Plasmid pSTI1 (about 81 MDa) was transferred by electroporation into E. coli DH5α. Recipient cells were selected on plates containing tobramycin (10 μg/ml). The cells were resistant to gentamicin, tobramycin, netilmicin, and amikacin. They were also resistant to chloramphenicol, sulfonamide, and extended-spectrum cephalosporins as a consequence of PER-1 β-lactamase production.
The resistance of Salmonella serovar Typhimurium(pSTI1) to mercuric chloride and sulfonamides evoked the possibility that an aac(6′)-Ib gene was carried by a class 1 integron. PCR amplification with primers specific for the 5′ and 3′ conserved integron segments was carried out and yielded a ca. 1,400-bp fragment, which was subsequently sequenced (accession number AY136758). Two open reading frames (ORFs), of 567 and 288 nucleotides, were identified as gene cassettes within a typical integron structure upstream from qacEΔ1 and sulI in the 3′ conserved segment. From the larger, 5′ conserved segment-proximal ORF, which was not preceded by a plausible ribosome binding site, a protein of 188 amino acids could be deduced, with a calculated molecular mass of 21 kDa and a theoretical isoelectric point of 4.7. Comparison of the predicted amino acid sequence with those from the GenBank database revealed identity with sections of two proteins, the 8 N-terminal amino acids of the signal peptide of OXA-1 (21) and the 180 C-terminal amino acids of AAC(6′)-Ib (34) except for three amino acid changes, Gln118 to Leu, Ser119 to Leu, and Val200 to Asp.
At the nucleotide level, the homology with the oxa-1 gene cassette extended across the attI1 site. The nucleotide sequence of the oxa-1 core site is GTTGGGC, instead of GTTAGRY or GTTAARY as in most resistance gene cassettes (7). The fused, Shine-Dalgarno (SD) sequence-deficient oxa-aac(6′)-Ib gene was located 45 nucleotides downstream from a small ORF with a coding capacity of 18 amino acids. The fused gene, just as oxa-1 (21), was apparently transcribed by the weak promoter variant of Pant coupled with the active form of promoter P2, resulting from the insertion of a G triplet between the −35 and −10 boxes (14, 29).
This configuration differed notably from those of the aac(6′)-Ib variants aac(6′)-Ib7 and -Ib8 that were previously described (4) (Fig. 1). In pLMM564, the transcription of aac(6′)-Ib7 was under the control of the strong form of the Pant promoter. The translational initiation of the AAC(6′)-Ib7 protein started at a GTG codon without a preceding SD sequence that was located 20 nucleotides downstream from a small ORF coding for 11 amino acids (ORF-11) and overlapping the attI1 site. The level of aac(6′)-Ib7 translation has been found to depend primarily upon the translation of ORF-11 (11). In pLMM563, aac(6′)-Ib8 was transcribed also from the strong variant of Pant and translated from an ATG codon located in a 21-bp sequence duplication overlapping the attI1 site. An ORF-11 identical to that found upstream from aac(6′)-Ib7 ended 5 nucleotides upstream from its translation start codon (Fig. 1). Apart from the different N termini, the AAC(6′)-Ib7 and -Ib8 proteins had a Gln118 and a Ser119 and the AAC(6′)-Ib11 had a Leu118 and a Ser119 while the prototype AAC(6′)-Ib had Gln and Leu at positions 118 and 119, respectively.
The second gene cassette, spanning 378 nucleotides, contained an ORF of 288 nucleotides that had 100% identity with ORF D, an ORF of unknown function located downstream from the gene cassette oxa-2 in the integrons of class 1, In1 (8) and In9 (12), and also of class 2, In16, immediately downstream from dfrB3 (26).
Determination of the translation start sites of AAC(6′)-Ib11 and AAC(6′)-Ib8.
According to the deduced amino acid sequences, the fused aac(6′)-Ib11 gene specified a protein of 188 amino acids and the aac(6′)-Ib8 gene specified a protein of 196 amino acids. Immunoblot analysis with anti-AAC(6′)-Ib antibodies of cell extracts from the wild strains STI1 and ECL563 revealed proteins of estimated sizes in good agreement with those predicted from the nucleotide sequences (data not shown). To confirm the positions of the translation initiation codons of aac(6′)-Ib8 and aac(6′)-Ib11, the genes were cloned into pGEM and expressed in E. coli DH5α and the acetyltransferases were purified. N-terminal sequence analysis of the first 10 amino acids (Fig. 1) confirmed the 196-amino-acid size of AAC(6′)-Ib8, while the result for AAC(6′)-Ib11 was ambiguous, possibly due to insufficient protein purification. Therefore, in this case, the most likely initiation codon, A325TG (Fig. 1), was changed to two noninitiating codons. E. coli DH5α transformants carrying the two corresponding plasmids (pGEMMSTP1 and pGEMMSTP2 [Table 1]) became susceptible to gentamicin and amikacin, with the inhibition zone diameters increasing from 10 and 12 mm [DH5α(pGEMMST)] to 22 and 24 mm, respectively, and the diameters around tobramycin increasing somewhat less (from 8 to 15 mm).
Expression levels and resistance profiles of the AAC(6′)-Ib7, -Ib8, and -Ib11 enzymes.
We have previously observed that AAC(6′)-Ib7 and -Ib8 have modified aminoglycoside substrate profiles with respect to gentamicin and amikacin compared to that of AAC(6′)-Ib (4). These differences are presumably due to the replacement of the amino acid Leu by Ser at position 119. In this study, we have found a variant, AAC(6′)-Ib11, with two amino acid changes, at positions 118 (Gln to Leu) and 119 (Leu to Ser), conferring a broad substrate profile and a higher level of resistance to aminoglycosides than that of the AAC(6′)-Ib7 or -Ib8 variants. However, since the 5′ regions in the aac(6′)-Ib7, -Ib8, and -Ib11 genes are different, the different resistance profiles may result from differences in expression. In order to explore the possible roles of the amino acids at position 118, the three genes were subcloned into pGEM. Each of the genes, on plasmids pGEMM564, pGEMM563, and pGEMMST, respectively, was transcribed from its respective natural promoter. These promoters do not differ in strength by a factor of more than two (14, 29). The corresponding aminoglycoside resistance profiles are shown in Table 2. No obvious differences in MICs were observed for E. coli strains containing the aac(6′)-Ib variants cloned in pGEM compared to those for E. coli strains containing the native plasmids.
The MICs of gentamicin (8 μg/ml) and amikacin (16 μg/ml) for E. coli strains containing pGEMMST [aac(6′)-Ib11] appeared to be two- to eightfold higher than the MICS of these drugs for E. coli strains containing pGEMM563 or pGEMM564. This difference indicated that a Gln-to-Leu change at position 118 might be critical for shifting the resistance profile and for increasing the resistance level. Oligonucleotide-directed mutagenesis was used to make the corresponding change in plasmids pGEMM563 and pGEMM564, yielding pGEMM563M and pGEMM564M. The changes resulted in a two- to eightfold increase in the MICs of gentamicin and amikacin for strains carrying these plasmids, with strains carrying pGEMM563M but not those carrying pGEMM564M reaching the level of resistance observed for strains carrying pGEMMST. Conversely, a Leu-to-Gln change was introduced in pGEMMST, yielding pGEMMSTM. The corresponding enzyme was less efficient at acetylating gentamicin and amikacin, as shown by the two- to fourfold reduction in the MICs of these antibiotics (Table 2). These results demonstrate (i) that aac(6′)-Ib7 was less efficiently translated than the aac(6′)-Ib8 and -Ib11 variants, (ii) that the fusion of aac(6′)-Ib with 8 amino acids of the signal peptide of oxa-1 did not result in a higher level of expression, and (iii) that Leu118 increases, at least in the variants AAC(6′)-Ib7, -Ib8, and -Ib11, the intrinsic activity of the enzyme or its stability.
DISCUSSION
Amikacin resistance in Enterobacteriaceae results mainly from the dissemination of the aac(6′)-Ib gene coding for acetyltransferases capable of modifying amikacin but not gentamicin. This gene is typically found on a gene cassette in class 1 integrons. Exceptionally, on Tn1331, this cassette has been observed outside an integron and then with the gene fused to the 5′-terminal sequence of blaTEM-1 and under the control of the β-lactamase gene promoter (32, 34). Here, we have analyzed a second case of a bla-aac(6′)-Ib fusion, this time with oxa-1 and carried by a class 1 integron which provides the promoter. The original isolate was a PER-1-producing strain of serovar Typhimurium from Turkey with a high level of resistance to gentamicin, amikacin, and other aminoglycosides (35).
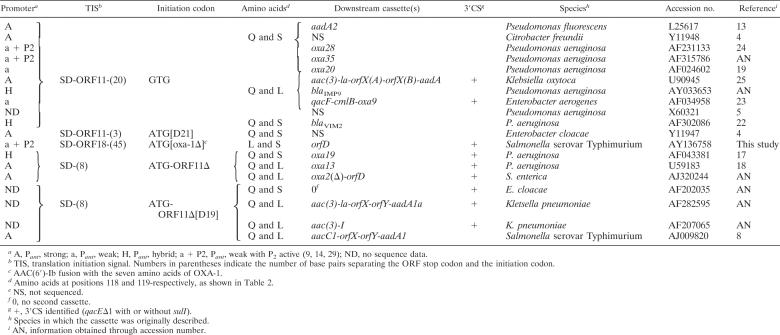
Frequently inserted at the attI1 site, the cassette-borne aac(6′)-Ib genes show great diversity at their 5′ termini in their translational initiation regions (TIR) as well as in their corresponding N-terminal amino acid sequences. With the available sequence information, schematically, five situations can be deduced (Table 3). In the most frequently occurring situation, the gene has a GTG start codon and no plausible SD sequence and is located 20 nucleotides downstream from a short ORF, ORF-11, which promotes its translation (11). Alternatively, the acetyltransferase gene may be fused in two ways with ORF-11, thus using its ATG initiation codon along with its SD sequence (8, 17). In a fourth case, an ATG start codon may be placed in frame through the duplication of 21 bp immediately downstream from ORF-11 (4). Finally, as studied here, the gene may be fused to the 5′ end of oxa-1, 45 nucleotides downstream from ORF-18, an extension of ORF-11 by 21 nucleotides. Based on the strong effect of the abolition of A325TG on the resistance level (see Results) and with the TIR organization upstream from this codon being similar to that of aac(6′)-Ib7 (11), we conclude that the translation of aac(6′)-Ib11 is coupled with that of ORF-18.
TABLE 3.
Diversity of TIRs in aac(6′)-Ib cassettes inserted at the attI1 site and in amino acids encoded at positions 118 and 119
The fusion of AAC(6′)-Ib to a short segment of the signal peptide of TEM-1 has been speculated to alter the cellular localization of the enzyme to the periplasm, thereby enhancing its effectiveness (27). With respect to the AAC(6′)-Ib11 variant, such an effect of the seven N-terminal amino acids of the signal peptide of OXA-1 is not obvious if one compares the MICs of gentamicin and amikacin for E. coli strains containing pGEMM563M to those for E. coli strains containing pGEMMST (Table 2). They are close to identical, with the enzymes differing only in their N termini.
Aside from the differences observed at their 5′ termini, the naturally occurring aac(6′)-Ib variants differ with respect to the codons specifying the amino acids at positions 118 and 119. The amino acids at positions 118 through 121 have been previously altered by site-directed mutagenesis (27). A change of Gln118 to Ser, or Ala121 to Ser, had no effect on the MICs of gentamicin or amikacin, while a change of Leu119 to Ser increased the MIC of gentamicin by a factor of eight and decreased that of amikacin in the same proportion. This situation is frequently observed with clinical isolates, as with the AAC(6′)-Ib7 and -Ib8 variants (Table 3), and was also found in the acetyltransferase gene encoded on In6 of pSa (31), a plasmid originally observed in Shigella before amikacin was marketed. A change of Leu120 to Ser decreased the MIC of amikacin 48-fold but had no effect on that of gentamicin (27). The natural AAC(6′)-Ib11 variant has a Leu at position 118, the effect of which has not been evaluated previously by site-directed mutagenesis. Based on our results from back mutation of this amino acid to Gln, we infer that Leu at position 118 substantially elevates MICs of aminoglycosides, including gentamicin, when there is a Ser at position 119. In this case, the enzyme may be considered an extended-spectrum acetyltransferase. The effect of Leu at position 118 was verified in the reverse experiment with the AAC(6′)-Ib7 and -Ib8 variants after the replacement of Gln118 by Leu.
Elevated MICs of amikacin, or of both amikacin and gentamicin, may be explained by single mutations if one considers the AAC(6′)-Ib variant of the pSa type to be the original enzyme, as depicted in Table 4. In both cases, a resident amino acid, Gln or Ser, is replaced by a Leu, which increases the hydrophobicity of the region, a factor that has been considered to be important for efficient aminoglycoside acetylation (27).
TABLE 4.
Effects of amino acids at positions 118 and 119 on gentamicin and amikacin resistancea
| Gene | Amino acid encoded at position:
|
MIC (μg/ml) ofb:
|
||
|---|---|---|---|---|
| 118 | 119 | Gen | Ami | |
| aac(6′)-Ib | Q | L | 0.5 | 16 |
| aac(6′)-Ib7/8 | Q | S | 2-4 | 1-2 |
| aac(6′)-Ib11 | L | S | 8 | 16 |
The amino acids at positions 118 and 119 are located in motif A, one of the two most conserved motifs in the N-acetyltransferase superfamily (20). Site-directed mutagenesis of residues in the other motif, B, has recently been carried out, but only decreases in the MICs of amikacin resulted from the changes introduced (30). When the sequences of 6′-N-acetyltransferases of type I and type II, 24 in all, are aligned (Fig. 2), position 118 appears to be immediately adjacent to a conserved box spanning five amino acids. Only in a subgroup of four enzymes, AAC(6′)-Ib, -Ie, -IIa, and -IIb, is there a Gln in this position that is otherwise almost invariably occupied by a Gly. It would seem worthwhile to replace this residue with a Leu to verify the importance for efficient acetylation of increased hydrophobicity in this area.
FIG. 2.
Motif A of the acetyltransferases AAC(6′)-I and AAC(6′)-II (20). The amino acid position 118 is indicated; the GenBank accession numbers are given to the left.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, and J. A. Smith. 1997. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 2.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissonnette, L., and P. H. Roy. 1992. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J. Bacteriol. 174:1248-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casin, I., F. Bordon, P. Bertin, A. Coutrot, I. Podglajen, R. Brasseur, and E. Collatz. 1998. Aminoglycoside 6′-N-acetyltransferase variants of the Ib type with altered substrate profile in clinical isolates of Enterobacter cloacae and Citrobacter freundii. Antimicrob. Agents Chemother. 42:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galimand, M., T. Lambert, G. Gerbaud, and P. Courvalin. 1993. Characterization of the aac(6′)-Ib gene encoding an aminoglycoside 6′-N-acetyltransferase in Pseudomonas aeruginosa BM2656. Antimicrob. Agents Chemother. 37:1456-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas, M. J., and J. E. Dowding. 1975. Aminoglycoside-modifying enzymes. Methods Enzymol. 43:611-628. [DOI] [PubMed] [Google Scholar]
- 7.Hall, R. M., D. E. Brookes, and H. W. Stokes. 1991. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol. Microbiol. 5:1941-1959. [DOI] [PubMed] [Google Scholar]
- 8.Hall, R. M., and C. Vockler. 1987. The region of the IncN plasmid R46 coding for resistance to beta-lactam antibiotics, streptomycin/spectinomycin and sulphonamides is closely related to antibiotic resistance segments found in IncW plasmids and in Tn21-like transposons. Nucleic Acids Res. 15:7491-7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall, R. M., and C. M. Collis. 1995. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15:593-600. [DOI] [PubMed] [Google Scholar]
- 10.Hall, T. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 11.Hanau-Berçot, B., I. Podglajen, I. Casin, and E. Collatz. 2001. An intrinsic control element for translational initiation in class 1 integrons. Mol. Microbiol. 44:119-130. [DOI] [PubMed] [Google Scholar]
- 12.Kratz, J., F. Schmidt, and B. Wiedemann. 1983. Characterization of Tn2411 and Tn2410, two transposons derived from R-plasmid R1767 and related to Tn2603 and Tn21. J. Bacteriol. 155:1333-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert, T., M. C. Ploy, and P. Courvalin. 1994. A spontaneous point mutation in the aac(6′)-Ib′ gene results in altered substrate specificity of aminoglycoside 6′-N-acetyltransferase of a Pseudomonas fluorescens strain. FEMS Microbiol. Lett. 115:297-304. [DOI] [PubMed] [Google Scholar]
- 14.Levesque, C., S. Brassard, J. Lapointe, and P. H. Roy. 1994. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene 142:49-54. [DOI] [PubMed] [Google Scholar]
- 15.Lévesque, C., L. Piché, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabilat, C., J. Lourencao-Vital, S. Goussard, and P. Courvalin. 1992. A new example of physical linkage between Tn1 and Tn21: the antibiotic multiple-resistance region of plasmid pCFF04 encoding extended-spectrum beta-lactamase TEM-3. Mol. Gen. Genet. 235:113-121. [DOI] [PubMed] [Google Scholar]
- 17.Mugnier, P., I. Casin, A. T. Bouthors, and E. Collatz. 1998. Novel OXA-10-derived extended-spectrum β-lactamases selected in vivo or in vitro. Antimicrob. Agents Chemother. 42:3113-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugnier, P., I. Podglajen, F. W. Goldstein, and E. Collatz. 1998. Carbapenems as inhibitors of OXA-13, a novel, integron-encoded beta-lactamase in Pseudomonas aeruginosa. Microbiology 144:1021-1031. [DOI] [PubMed] [Google Scholar]
- 19.Naas, T., W. Sougakoff, A. Casetta, and P. Nordmann. 1998. Molecular characterization of OXA-20, a novel class D β-lactamase, and its integron from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:2074-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuwald, A., and D. Landsman. 1997. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 22:154-155. [DOI] [PubMed] [Google Scholar]
- 21.Ouellette, M., L. Bissonnette, and P. H. Roy. 1987. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 beta-lactamase gene. Proc. Natl. Acad. Sci. USA 84:7378-7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pallecchi, L., M. L. Riccio, J. D. Docquier, R. Fontana, and G. M. Rossolini. 2001. Molecular heterogeneity of bla(VIM-2)-containing integrons from Pseudomonas aeruginosa plasmids encoding the VIM-2 metallo-beta-lactamase. FEMS Microbiol. Lett. 195:145-150. [DOI] [PubMed] [Google Scholar]
- 23.Ploy, M.-C., P. Courvalin, and T. Lambert. 1998. Characterization of In40 of Enterobacter aerogenes BM2688, a class 1 integron with two new gene cassettes, cmlA2 and qacF. Antimicrob. Agents Chemother. 42:2557-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel, L., D. Girlich, T. Naas, and P. Nordmann. 2001. OXA-28, an extended-spectrum variant of OXA-10 β-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob. Agents Chemother. 45:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston, K. E., M. A. Kacica, R. J. Limberger, W. A. Archinal, and R. A. Venezia. 1997. The resistance and integrase genes of pACM1, a conjugative multiple-resistance plasmid, from Klebsiella oxytoca. Plasmid 37:105-118. [DOI] [PubMed] [Google Scholar]
- 26.Rådström, P., O. Sköld, G. Swedberg, J. Flensburg, P. H. Roy, and L. Sundström. 1994. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J. Bacteriol. 176:3257-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rather, P. N., H. Munayyer, P. A. Mann, R. S. Hare, G. H. Miller, and K. J. Shaw. 1992. Genetic analysis of bacterial acetyltransferases: identification of amino acids determining the specificities of the aminoglycoside 6′-N-acetyltransferase Ib and IIa proteins. J. Bacteriol. 174:3196-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology 141:3015-3027. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt, F. R., E. J. Nucken, and R. B. Henschke. 1988. Nucleotide sequence analysis of 2′-aminoglycoside nucleotidyl-transferase ANT(2′) from Tn4000: its relationship with AAD(3′) and impact on Tn21 evolution. Mol. Microbiol. 2:709-717. [DOI] [PubMed] [Google Scholar]
- 30.Shmara, A., N. Weinsetel, K. J. Dery, R. Chavideh, and M. E. Tolmasky. 2001. Systematic analysis of a conserved region of the aminoglycoside 6′-N-acetyltransferase type Ib. Antimicrob. Agents Chemother. 45:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tait, R. C., H. Rempel, R. L. Rodriguez, and C. I. Kado. 1985. The aminoglycoside-resistance operon of the plasmid pSa: nucleotide sequence of the streptomycin-spectinomycin resistance gene. Gene 36:97-104. [DOI] [PubMed] [Google Scholar]
- 32.Tolmasky, M. E., and J. H. Crosa. 1993. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid 29:31-40. [DOI] [PubMed] [Google Scholar]
- 33.Tran van Nhieu, G., F. Bordon, and E. Collatz. 1992. Incidence of an aminoglycoside 6′-N-acetyltransferase, ACC(6′)-1b, in amikacin-resistant clinical isolates of gram-negative bacilli, as determined by DNA-DNA hybridisation and immunoblotting. J. Med. Microbiol. 36:83-88. [DOI] [PubMed] [Google Scholar]
- 34.Tran van Nhieu, G., and E. Collatz. 1987. Primary structure of an aminoglycoside 6′-N-acetyltransferase AAC(6′)-4, fused in vivo with the signal peptide of the Tn3-encoded β-lactamase. J. Bacteriol. 169:5708-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vahaboglu, H., S. Dodanli, C. Eroglu, R. Öztürk, G. Soyletir, I. Yildirim, and V. Avkan. 1996. Characterization of multiple-antibiotic-resistant Salmonella typhimurium strains: molecular epidemiology of PER-1-producing isolates and evidence for nosocomial plasmid exchange by a clone. J. Clin. Microbiol. 34:2942-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]