Abstract
We evaluated the prophylactic and therapeutic efficacy of interferon α-2b, pegylated interferon α-2b, poly(I · C), and Ampligen against Modoc virus encephalitis in an animal model for flavivirus infections. All compounds significantly delayed virus-induced morbidity (paralysis) and mortality (due to progressive encephalitis). Viral load (as measured on day 7 postinfection) was significantly reduced by 80 to 100% in the serum, brain, and spleen in mice that had been treated with either interferon α-2b, pegylated interferon α-2b, poly(I · C), or Ampligen. We also studied whether a combination of interferon α-2b and ribavirin (presently the standard therapy for the treatment of infections with hepatitis C virus) would be more effective than treatment with interferon alone. However, ribavirin did not enhance the inhibitory effect of interferon therapy in this animal model for flavivirus infections.
The genus Flavivirus contains over 70 virus species, of which many cause disease in humans. Severe flavivirus infections are primarily characterized by encephalitic or hemorrhagic symptoms. Mortality rates vary, depending on the infecting virus species, from 1 to 2% (e.g., for the Central European encephalitis virus) up to 30 to 40% (e.g., for the Japanese encephalitis virus and the Russian spring-summer encephalitis virus) (14, 15, 27). Although an effective vaccine is available for the prevention of yellow fever virus infections, annually, over 5,000 fatal cases are reported worldwide (28). Dengue fever virus, of which there are four serotypes that do not cause a mutually protective serological response, is prevalent around the world in tropical and subtropical areas. Each year, over 500,000 cases of dengue fever are reported, of which over 25,000 are fatal due to the development of dengue hemorrhagic fever or dengue shock syndrome (9, 30). Also, West Nile virus (1, 5, 19), St. Louis encephalitis virus (17), and Murray Valley encephalitis virus (26) cause many human fatalities.
There is no specific antiviral therapy available for the treatment of infections with flaviviruses. Symptomatic treatment and intensive medical care are essential to improve the chance of survival in patients with severe disease. Early diagnosis of the infection before the onset of serious disease, together with an early start of symptomatic treatment, favors survival rates (33, 34).
During a severe dengue virus epidemic on the island of Cuba in 1981, alpha interferon (IFN-α) was administered to children and adults with dengue fever (23). Although amelioration of the disease was described, there have been no other reports of the use of IFN in patients with dengue fever virus infection. Two patients, of a group of four, with severe Japanese encephalitis, showed improved clinical signs and recovered from the infection following treatment with IFN-α. The two patients who did not receive IFN therapy died (11).
In mice, intraperitoneal administration of human-mouse hybrid recombinant IFN-α and mouse recombinant IFN-β significantly reduced mortality caused by Banzi virus, a mosquito-borne flavivirus, but only when administered shortly after infection (29). Intraperitoneal treatment of mice that had been infected with St. Louis encephalitis virus with human recombinant IFN-α resulted in a significant protective effect on virus-induced mortality (2).
Pegylation increases the relatively short half-life of IFN and helps to avoid repeated “peak-through cycling,” which is believed to contribute to drug intolerance during IFN therapy in patients with hepatitis C virus (HCV) infection (13, 16). Pegylated IFN-α-2b (PEG-IFN), either alone or in combination with ribavirin (RIB), is presently being used for the treatment of HCV infections. Combined PEG-IFN-RIB therapy resulted in a response superior to that gained by PEG-IFN monotherapy (8, 24). Only one report has been made on the use of PEG-IFN for the treatment of virus infections in an animal model. PEG-IFN protected mice from Venezuelan equine encephalitis virus-induced mortality but only when treatment was initiated 2 days before infection. Intraperitoneal (i.p.) administration of IFN as such was ineffective in this model (25). No reports have been made on the use of PEG-IFN for the treatment of infections with flaviviruses in an animal model.
Ampligen [poly(I)-poly(C12U)] is an IFN inducer that consists of poly(I · C) but which has, in contrast to poly(I · C), a U mismatch at every 12th base of the C strand. Ampligen is presently in preclinical evaluation and clinical study for the treatment of various viral diseases, including infections with the human immunodeficiency virus (7) and the hepatitis B virus (http://www.hemispherx.net/). Poly(I · C) has shown efficacy in mice against experimental infections with tick-borne encephalitis virus (18), Japanese encephalitis virus (12, 31), and West Nile virus (10).
We elaborated a convenient and easy-to-manipulate flavivirus infection model in SCID mice using the Modoc virus (MODV) with the aim of studying novel therapeutic strategies against flavivirus encephalitis. MODV, a flavivirus that is classified as a biosafety level 2 (BSL2) pathogen (yellow fever and West Nile viruses = BSL3; tick-borne encephalitis virus [TBEV] = BSL4), causes pronounced morbidity (i.e., an encephalitis that is reminiscent of flavivirus encephalitis in humans) and 100% mortality in SCID mice inoculated via the intraperitoneal, intranasal, or intracerebral route. In immunocompetent NMRI mice, morbidity and mortality are observed only when the virus is inoculated via the intranasal (55% mortality) or intracerebral (100% mortality) route but not by the intraperitoneal route (22). Since MODV, inoculated in SCID mice via the intraperitoneal route, (i) causes a uniformly lethal infection and (ii) closely resembles natural transmission of a flavivirus (i.e., a bite of a mosquito or a tick), MODV infections in SCID mice represent a convenient animal model for the study of therapy of flavivirus encephalitis.
In the present study, we describe the protective and therapeutic effects of (i) human recombinant IFN-α-2b (commercially called INTRON A), (ii) human recombinant PEG-IFN (commercially called PEG-INTRON), (iii) poly(I · C), and (iv) Ampligen on lethal flavivirus encephalitis. In addition, we also investigated whether the combination of IFN and RIB, which is now the standard therapy for the treatment of HCV infections, offers an advantage over the use of IFN alone for the treatment of flavivirus infections.
MATERIALS AND METHODS
Propagation of MODV in Vero cells.
African green monkey kidney (Vero) cells were grown in minimum essential medium (MEM; Gibco, Paisley, Scotland) supplemented with 10% inactivated fetal calf serum (Integro, Zaandam, Holland), 1% l-glutamine, and 0.3% bicarbonate. MODV was obtained from the American Type Culture Collection (ATCC VR-415). Confluent cultures of Vero cells were infected and incubated at 37°C until an extensive cytopathic effect was observed (generally 7 to 9 days postinfection). At that time, culture medium was collected, cell debris was pelleted by centrifugation, and the supernatant was aliquoted and stored at −80°C.
Plaque-forming assay.
Serial dilutions of the virus were added to confluent Vero cell cultures that were grown in six-well microtiter trays. Cultures were incubated at 37°C for 1 h, after which the virus inoculum was removed and the cells were washed twice with warm medium. Subsequently, the cells were overlaid with MEM containing 2% fetal calf serum and 2% agar. The cultures were further incubated for 7 to 9 days, after which they were stained with neutral red solution and plaques were counted under an inverted microscope.
Compounds, dilutions, and doses.
IFN-α-2b and PEG-IFN were kindly provided by Schering-Plough (Brussels, Belgium). Ampligen was a kind gift from Hemispherx Biopharma (Philadelphia, Pa.). Poly(I · C) was purchased from Sigma (Bornem, Belgium), and RIB was purchased from ICN Pharmaceuticals (Costa Mesa, Calif.). Dilutions of IFN (106 U/ml), PEG-IFN (106 U/ml), and RIB (10 mg/ml) were prepared in phosphate-buffered saline (PBS). Poly(I · C) and Ampligen were dissolved at a concentration of 0.3 mg/ml in RNase-free water (Acros Organics, Geel, Belgium) to avoid degradation by RNases. Throughout the experiments, except when mentioned otherwise in the text, animals received the compounds by i.p. injection at the following doses: IFN (100 μl, 105 U, i.p.), PEG-IFN (100 μl, 105 U = 1.724 μg, i.p.), poly(I · C) (100 μl, 1.5 mg/kg of body weight, i.p.), Ampligen (100 μl, 1.5 mg/kg, i.p.), or RIB (100 μl, 50 mg/kg, i.p.) or mock treatment with PBS (100 μl).
Animals.
SCID (severe combined immune deficiency) mice weighing 18 to 22 g were used throughout the experiments. The SCID mice were bred at the Rega Institute under germfree conditions and maintained under artificial diurnal lighting conditions with free access to food and water. The principles of good laboratory animal care were followed. The experiments were approved by the ethical committee on vertebrate animal experiments of the Katholieke Universiteit Leuven.
Animal experiments.
SCID mice were infected with 104 PFU of MODV via the intraperitoneal route. The animals were monitored daily for signs of morbidity and for mortality. Because of the limited resources of IFN, PEG-IFN, and Ampligen, a limited number of animals had to be used. Each data set, however, represents the results obtained for two independent experiments with a total of at least of six animals. The statistical significance of the results was assessed by means of the Student t test. A treatment condition was considered to be biologically significant when both the mean day of paralysis (MDP) and the mean day of death (MDD) were significantly (P ≤ 0.05) delayed compared to the untreated controls.
Effect of treatment on viral titers using quantitative RT-PCR.
SCID mice (three animals/group) received pretreatment, a combination of pre- and posttreatment, or posttreatment only with IFN, PEG-IFN, poly(I · C), or Ampligen, as described above. Treatment was continued until day 6 postinfection. On day 7 postinfection (1 day after cessation of therapy), the animals were sacrificed by ether anesthesia. A blood sample was drawn by cardiac puncture, after which the animals were perfused with 10 ml of cold PBS. The brain and spleen were removed, 10% tissue homogenates were prepared in MEM, and cell debris was pelleted by centrifugation. Total RNA was extracted from 140 μl of serum or supernatant of tissue homogenate using the QIAamp Viral RNA kit (Qiagen, Hilden, Germany) (no carrier RNA was used to extract the RNA from the tissue homogenate supernatant). Quantitation of viral RNA was performed by means of real-time quantitative reverse transcriptase (RT)-PCR as previously reported (22). Briefly, cDNA, reverse transcribed from the isolated RNA (20 min at 55°C), was used in a PCR containing a MODV-specific primer set, ModS1 (5′-CCA GGA CAA GTC ATG TGG TAG C-3′) and ModAS1 (5′-TCC CAA AGA TGT TCC TCA CCT T-3′), flanking a 5′-carboxyfluorescein (FAM)-3′-carboxytetramethylrhodamine-labeled MODV-specific probe, ModP1 (5′-CAG AAT GGG CCA AGT TGC TTC CAG T-3′). During the PCR (denaturation at 95°C for 5 min and 40 cycles of 95°C for 5 s and 60°C for 20 s), the annealed probe, equal to the amount of available complementary sequence in the reaction, is hydrolyzed, causing the release of FAM in the solution and an increase in FAM-specific fluorescence. As quantification standard for real-time PCR, a strong positive MODV stock was diluted in negative human plasma prior to RNA preparation. The amount of 1 PCR unit of MODV RNA was defined to be the lowest possible dilution step that could be amplified in five of five replicate reactions (22). All samples were analyzed in at least two replicate sets of reactions. Virus RNA load is presented as percentage of the virus control.
RESULTS
Effect of treatment with IFN and PEG-IFN on MODV-induced morbidity and mortality.
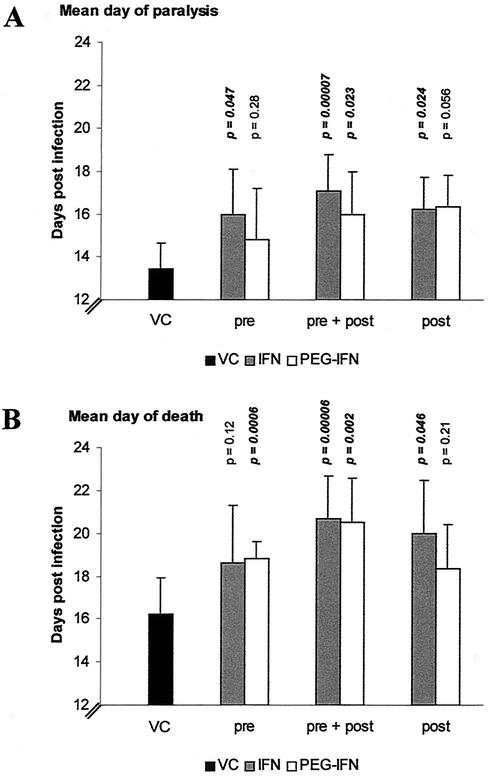
The prophylactic and/or therapeutic effect of treatment with IFN or PEG-IFN on MODV infection in SCID mice was studied using the following treatment conditions: (i) pretreatment, in which the animals received only one single dose of 105 U of the respective IFNs by i.p. injection at 24 h before inoculation with 104 PFU of MODV (i.p. injection at the opposite site of the site of treatment), (ii) pretreatment combined with posttreatment, in which the mice received pretreatment as described above, followed by inoculation with the virus, and posttreatment for the next six consecutive days, and (iii) posttreatment, in which the animals were treated for six consecutive days starting at 1.5 h postinfection. Pretreatment with IFN or PEG-IFN 24 h before infection did not result in a statistically significant delay in the progression of MODV-induced disease, although for both drugs, some delay in MDP (Fig. 1A, pre) and MDD (Fig. 1B, pre) was observed. When either IFN or PEG-IFN was administered before and following inoculation with the virus, a statistically significant delay in the onset (Fig. 1A, pre + post) and progression of the disease (Fig. 1B, pre + post) was observed. When IFN was administered following infection, an inhibitory effect was noticed, but it was less pronounced (Fig. 1A and B, post).
FIG. 1.
Effect of treatment with IFN and PEG-IFN on virus-induced morbidity and mortality. VC (virus control), virus-infected and mock-treated mice; pre, pretreatment with a single dose of 105 U of the respective IFNs (administered by the i.p. route 24 h before i.p. inoculation with 104 PFU of MODV); pre + post, pretreatment (105 U, i.p.) 24 h before infection, followed by six consecutive days of posttreatment (105 U, i.p.); post, 6 days of posttreatment (105 U, i.p.) starting at 1.5 h postinfection. P indicates statistical significance of the treatment compared to the virus control. Bars indicate the MDP (A) or MDD (B). Data represent mean values ± standard deviations for ≥6 animals/group obtained in two independent experiments.
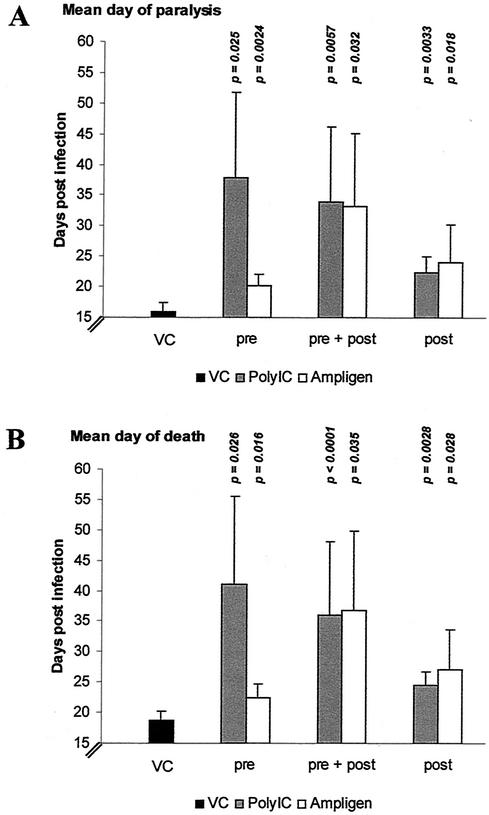
Effect of treatment with the IFN inducers poly(I · C) and Ampligen on MODV-induced morbidity and mortality.
Both poly(I · C) and Ampligen, whether given before infection (Fig. 2, pre: 15 mg/kg), before and after infection (Fig. 2, pre: 15 mg/kg + post: 1.5 mg/kg) or only after infection (Fig. 2, post: 1.5 mg/kg), significantly delayed onset of the disease (Fig. 2A) and increased the mean survival time (Fig. 2B). The effect of pretreatment with poly(I · C) on both the MDP (Fig. 2A, pre) and MDD (Fig. 2B, pre) was more pronounced than the effect of Ampligen. When either poly(I · C) or Ampligen was given before and after infection (pre + post), both molecules proved equally effective and delayed virus-induced mortality by 17 to 18 days (Fig. 2B, pre + post). Also, when treatment was first started after infection, both drugs were still able to significantly delay virus-induced morbidity (Fig. 2A, post) and mortality (Fig. 2B, post). In most experimental conditions, treatment with poly(I · C) and Ampligen caused some SCID mice to survive the infection with MODV. Pre- and posttreatment with poly(I · C) resulted in about 57% survival (four of seven; experiment was terminated at 64 days postinfection), whereas, throughout all experiments, 100% of the untreated mice succumbed. Of mice that received pretreatment with poly(I · C) or Ampligen or those that received pre- and posttreatment with Ampligen, about 29% (two of seven) survived the infection.
FIG. 2.
Effect of treatment with poly(IC) and Ampligen on virus-induced morbidity and mortality. VC (virus control), virus-infected and mock-treated mice; pre, pretreatment with a single dose of 15 mg/kg (i.p.) of the respective IFN inducers at 24 h before i.p. inoculation with 104 PFU of MODV; pre + post, pretreatment (15 mg/kg, i.p.), followed by six consecutive days of posttreatment with 1.5 mg of the respective IFN inducers per kg, i.p.; post, 6 days of posttreatment (1.5 mg/kg, i.p.) starting at 1.5 h postinfection. Bars indicate MDP (A) or MDD (B). P indicates statistical significance of the treatment compared to the virus control. Data represent mean values ± standard deviation for ≥6 animals/group obtained in two independent experiments.
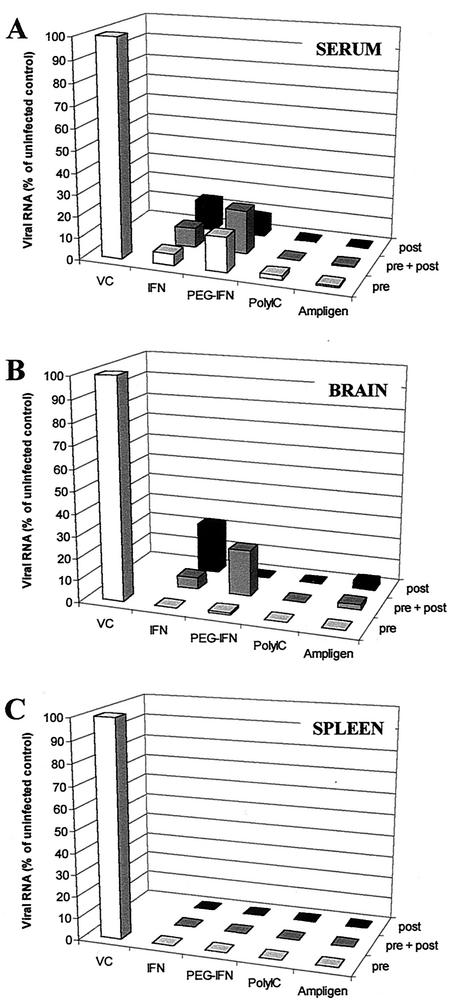
Effect of treatment on viral RNA levels.
We previously reported the detection of viral RNA in the brain and spleen of SCID mice infected with MODV (22). Viral RNA was also detectable in the serum (our unpublished results). We have now assessed by means of quantitative RT-PCR (which provided us, in addition to monitoring disease progression and mortality, with a second and independent parameter of drug efficacy) whether the treatment schedules with IFN, PEG-IFN, poly(I · C), or Ampligen that were used and that caused a (partially) inhibitory effect result in a reduction of viral RNA in the serum, spleen, and brain of infected mice. We employed quantitative RT-PCR, because the detection limit of this methodology is much lower than that of a titration for infectious virus content. Viral RNA levels were measured at day 7 postinfection. All treatment conditions described above significantly reduced the level of viral RNA in the serum, brain, and spleen (Fig. 3A to C), compared to the level in the untreated animals. A somewhat higher viral titer was measured in the serum and brain of the “IFN post” and the “PEG prepost” condition, whereas this is not immediately reflected in a more rapid progression of the disease in the parallel groups of mice. However, there was at least a time span of 7 days between (i) cessation of treatment and measuring viral RNA load at 7 days postinfection on the one hand and (ii) recording of morbidity and mortality at day 14 or later on the other hand. Mice that were sacrificed at day 7 postinfection for determination of the viral RNA load were obviously not the same animals as the parallel groups of animals that were used to record morbidity and mortality at day 14 postinfection or later, which may thus explain some variation. However, in general, inhibition of viral RNA replication by 80 to 100% corroborated the data on the protective and therapeutic activity of these compounds.
FIG. 3.
Effect of treatment with IFN, PEG-IFN, poly(I · C), and Ampligen on viral RNA titers as determined by quantitative RT-PCR. Viral RNA titers are represented as percentages of the untreated controls. Viral titers were determined in the serum (A), brain (B), and spleen (C) at day 7 postinfection (three mice/group). Treatment schedules are as described for the experiments presented in Fig. 1 and 2. The average level of viral RNA for the virus controls for the serum, brain, and spleen was, respectively, 69.897, 204, and 620.015 PCR units (PCRU). One PCR unit is defined to be the lowest template copy number detectable in five of five replicate sets of reactions, as determined by limiting dilution series.
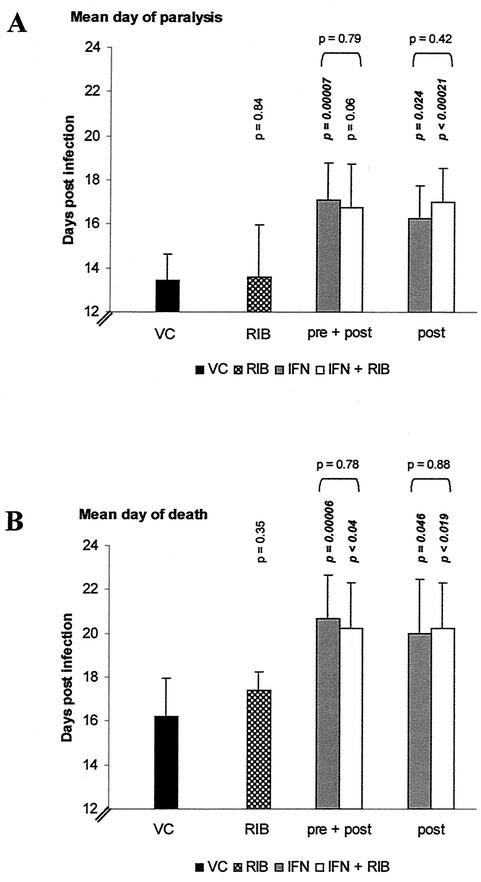
Effect of treatment with RIB or the combination of IFN and RIB on MODV-induced morbidity and mortality.
Although RIB inhibits the replication of several flaviviruses in cell culture, protective activity against flavivirus infections in animals has never been reported (reviewed in reference 21). We wanted to assess whether combination of IFN and RIB (the standard therapy for the treatment of HCV infections) would prove more effective compared to monotherapy with IFN. A daily dose of RIB (50 mg/kg) alone did not significantly delay virus-induced morbidity (Fig. 4A, RIB), nor did it increase the mean survival time (Fig. 4B, RIB). When RIB was added to the treatment schedule with IFN, no additional beneficial effect was observed over the effect seen with IFN alone (Fig. 4A and B, pre + post, post).
FIG. 4.
Effect of treatment with IFN in combination with RIB on virus-induced morbidity and mortality. VC (virus control), virus-infected and mock-treated mice; RIB, continuous treatment with RIB (50 mg/kg, two doses/day, i.p.) starting at 1.5 h following i.p. inoculation with 104 PFU of MODV; pre + post, pretreatment with IFN (105 U, i.p.) at 24 h before infection followed by six consecutive days of treatment with IFN (105 U, i.p.) and continuous treatment with RIB (50 mg/kg/day, 2 doses/day, i.p.); and post, 6 days of posttreatment with IFN (105 U, i.p.) and continuous treatment with RIB (50 mg/kg/day, two doses/day, i.p.) starting at 1.5 h postinfection. Vertically printed P indicates statistical significance of treatment compared to the virus control; horizontally printed P indicates the statistical significance between IFN treatment and IFN-RIB treatment. Data represent mean values ± standard deviations for ≥6 animals/group obtained in two independent experiments.
DISCUSSION
We here studied the effect of IFNs and of IFN inducers on a lethal flavivirus infection in mice. Heretofore, we employed the MODV/SCID mouse model (22). Since SCID mice are not able to mount a protective immune response, fulminant, fatal disease should eventually develop as long as circulating or replicating virus is present. In this model system, delay of onset of disease (paralysis) and increased survival time are critical parameters in the assessment of the antiviral efficacy of novel compounds. Therefore, treatment was only considered to be effective when both the MDP and MDD were significantly delayed compared to the untreated controls.
We chose to perform the IFN experiments with IFN-α-2b rather than with murine IFN because PEG-IFN is available for direct comparison, whereas pegylated murine IFN is not. The IFN inducer poly(I · C) and its analogue Ampligen offer the advantage of inducing species-specific IFNs.
Overall, treatment with IFN, PEG-IFN, and poly(I · C), as well as with Ampligen (pre, pre + post, or post) delayed the onset of disease and increased the mean survival time of infected mice. As a confirmatory parameter of efficacy, we quantitated the viral RNA load in the brain and spleen (organs in which MODV replicates) and serum. Virus titers were quantified at day 7 postinfection, because 1 day after cessation of treatment, a maximal effect of treatment may be expected at this time point. All treatment regimens [IFN, PEG-IFN, poly(I · C), and Ampligen] proved markedly effective in reducing viral RNA load.
Although IFN has proven to be effective for the treatment of infections with HCV (reviewed in reference 6) and although IFN inducers have been shown to elicit a clinical benefit in patients infected with human immunodeficiency virus (7) and hepatitis B virus (http://www.hemispherx.net/), the use of these drugs for the treatment of flavivirus infections is still questioned, as the pathogenesis of flavivirus-induced disease, in particular dengue hemorrhagic fever and dengue shock syndrome, is not fully understood. Dengue hemorrhagic fever and dengue shock syndrome may, by and large, be caused by immune-related mechanisms that are triggered by the viral infection (3; reviewed in reference 21). However, (i) IFN is effective in animal models for flavivirus encephalitis (2, 29) (the present study) and (ii) IFN showed clinical benefit in a small number of patients with severe Japanese encephalitis (11). Therefore, it may be worth further exploring whether IFN or IFN inducers would indeed show benefit in the pre- and postexposure prophylaxis and therapy of flavivirus encephalitis in the clinical setting.
RIB was shown to display in vitro antiviral activity against most flaviviruses but failed to protect experimentally infected animals (reviewed in reference 21). Also, monotherapy with RIB proved ineffective in the treatment of infections with HCV (20, 35). However, when combined with IFN, RIB potentiates the inhibitory effect of IFN on HCV infection by a yet-unexplained mechanism (4, 32). It was therefore appealing to study whether the combination of IFN and RIB would also prove more efficacious for the treatment of flavivirus infections than monotherapy with IFN. However, in our animal model system, combination therapy of IFN with RIB did not prove to be more effective than treatment with IFN alone. Thus, although RIB exerts selective antiviral activity against flavivirus replication in vitro, it did not offer any clinical beneficial effect against flavivirus-induced encephalitis in SCID mice, either when given alone or in combination with IFN. This does prove that the antiviral efficacy of RIB is not sufficiently pronounced to result in an additive or synergistic inhibition of viral replication, even under conditions where the dynamics of viral replication are already disturbed by IFN treatment. Because the results presented in the present study have been obtained in immunodeficient mice, this does not exclude the possibility that RIB may yield greater benefit in the immunocompetent host, particularly if its mechanism of action in vivo would be dependent on an intact immune system. Use of the SCID mouse thus allowed us to dissect a potential direct antiviral effect of RIB from an immunomodulatory activity. It will now be of interest to study the effect of IFN-RIB in immunocompetent animals that have been infected with viruses such as Japanese encephalitis virus, West Nile virus, or TBEV. If the protective effect of such a combination is greater in immunocompetent than it is in immunodeficient mice (as observed here), then this will provide evidence for an immunomodulatory activity of RIB against flavivirus infections.
In conclusion, we report here that IFN-α-2b, PEG-IFN, and the IFN inducers poly(I · C) and Ampligen significantly delayed progression of MODV encephalitis in SCID mice. IFN-RIB did not provide incremental benefit in the immunodeficient host.
Acknowledgments
This work was supported by grants from the “Flemisch Institute for the stimulation of scientific-technological research in industry” to P. Leyssen (IWT/SB/981025/Leyssen), N. Charlier (IWT/SB/991056/Charlier), and J. Paeshuyse (IWT/SB/11148/Paeshuyse) and by grants from “Geconcerteerde Onderzoeksacties-Vlaamse Gemeenschap” (GOA project no. 00/12) and the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (FWO)” (G. 0122-00). J. Neyts is a fellow of the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen” (FWO).
REFERENCES
- 1.Briese, T., X.-Y. Jia, C. Huang, L. J. Grady, and W. I. Lipkin. 1999. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet 354:1261-1262. [DOI] [PubMed] [Google Scholar]
- 2.Brooks, T. J., and R. J. Phillpotts. 1999. Interferon-alpha protects mice against lethal infection with St. Louis encephalitis virus delivered by the aerosol and subcutaneous routes. Antivir. Res. 41:57-64. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi, U. C., R. Agarwal, E. A. Elbishbishi, and A. S. Mustafa. 2000. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol. Med. Microbiol. 28:183-188. [DOI] [PubMed] [Google Scholar]
- 4.Crotty, S., C. Cameron, and R. Andino. 2002. Ribavirin's antiviral mechanism of action: lethal mutagenesis? J. Mol. Med. 80:86-95. [DOI] [PubMed] [Google Scholar]
- 5.Deubel, V., D. J. Gubler, M. Layton, and M. Malkinson. 2001. West Nile virus: a newly emergent epidemic disease. Emerg. Infect. Dis. 7:536.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Bisceglie, A. M., J. McHutchison, and C. M. Rice. 2002. New therapeutic strategies for hepatitis C. Hepatology 35:224-231. [DOI] [PubMed] [Google Scholar]
- 7.Essey, R. J., B. R. McDougall, and W. E. Robinson, Jr. 2001. Mismatched double-stranded RNA (polyI-polyC(12)U) is synergistic with multiple anti-HIV drugs and is active against drug-sensitive and drug-resistant HIV-1 in vitro. Antivir. Res. 51:189-202. [DOI] [PubMed] [Google Scholar]
- 8.Glue, P., R. Rouzier-Panis, C. Raffanel, R. Sabo, S. K. Gupta, M. Salfi, S. Jacobs, and R. P. Clement. 2000. A dose-ranging study of pegylated interferon alfa-2b and ribavirin in chronic hepatitis C. Hepatology 32:647-653. [DOI] [PubMed] [Google Scholar]
- 9.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haahr, S. 1971. The influence of poly I:C on the course of infection in mice inoculated with West Nile virus. Arch. Gesamte Virusforsch. 35:1-9. [DOI] [PubMed] [Google Scholar]
- 11.Harinasuta, C., S. Nimmanitya, and U. Titsyakorn. 1985. The effect of interferon-alpha A on two cases of Japanese encephalitis in Thailand. Southeast Asian J. Trop. Med. Public Health 16:332-336. [PubMed] [Google Scholar]
- 12.Harrington, D. G., D. E. Hilmas, M. R. Elwell, R. E. Whitmire, and E. L. Stephen. 1977. Intranasal infection of monkeys with Japanese encephalitis virus: clinical response and treatment with a nuclease-resistant derivative of poly (I).poly (C). Am. J. Trop. Med. Hyg. 26:1191-1198. [DOI] [PubMed] [Google Scholar]
- 13.Harris, J. M., N. E. Martin, and M. Modi. 2001. Pegylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 40:539-551. [DOI] [PubMed] [Google Scholar]
- 14.Heinz, F. X., and C. W. Mandl. 1993. The molecular biology of tick-borne encephalitis virus. APMIS 101:735-745. [DOI] [PubMed] [Google Scholar]
- 15.Kalita, J., and U. K. Misra. 1998. EEG in Japanese encephalitis: a clinico-radiological correlation. Electroencephalogr. Clin. Neurophysiol. 106:238-243. [DOI] [PubMed] [Google Scholar]
- 16.Kozlowski, A., S. A. Charles, and J. M. Harris. 2001. Development of pegylated interferons for the treatment of chronic hepatitis C. BioDrugs 15:419-429. [DOI] [PubMed] [Google Scholar]
- 17.Kramer, L. D., S. B. Presser, J. L. Hardy, and A. O. Jackson. 1997. Genotypic and phenotypic variation of selected Saint Louis encephalitis viral strains isolated in California. Am. J. Trop. Med. Hyg. 57:222-229. [DOI] [PubMed] [Google Scholar]
- 18.Kunz, C., and H. Hofmann. 1969. Die Beeinflussung der experimentellen Fruhsommer-Meningoenzephalitis (FSME)-Virusinfektion durch die Interferon induzierende Substanz Poly I:C. Zentbl. Bakteriol. 211:270-273. [PubMed] [Google Scholar]
- 19.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. Mackenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. H., M. von Wagner, W. K. Roth, G. Teuber, C. Sarrazin, and S. Zeuzem. 1998. Effect of ribavirin on virus load and quasispecies distribution in patients infected with hepatitis C virus. J. Hepatol. 29:29-35. [DOI] [PubMed] [Google Scholar]
- 21.Leyssen, P., N. Charlier, J. Paeshuyse, E. De Clercq, and J. Neyts. Prospects for antiviral therapy. In T. J. Chambers and T. P. Monath (ed.), The flaviviruses: current molecular aspects of evolution, biology and disease prevention, in press. Academic Press, London, United Kingdom.
- 22.Leyssen, P., A. Van Lommel, C. Drosten, H. Schmitz, E. De Clercq, and J. Neyts. 2001. A novel model for the study of the therapy of Flavivirus infections using the Modoc virus. Virology 279:27-37. [DOI] [PubMed] [Google Scholar]
- 23.Limonta, M., V. Ramirez, P. Lopez Saura, A. Aguilera, E. Penton, S. Barcelona, A. Gonzalez, R. Dujarric, C. Dotres, O. Legon, and E. Selman-Houssein. 1984. Uso del interferon leucocitario durante una epidemia de dengue hemorragico (virus tipo II) en Cuba. Interferon Biotecnol. 1(3):15-22. [Google Scholar]
- 24.Lindsay, K. L., C. Trepo, T. Heintges, M. L. Shiffman, S. C. Gordon, J. C. Hoefs, E. R. Schiff, Z. D. Goodman, M. Laughlin, R. Yao, and J. K. Albrecht. 2001. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology 34:395-403. [DOI] [PubMed] [Google Scholar]
- 25.Lukaszewski, R. A., and T. J. Brooks. 2000. Pegylated alpha interferon is an effective treatment for virulent Venezuelan equine encephalitis virus and has profound effects on the host immune response to infection. J. Virol. 74:5006-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackenzie, J. S., and A. K. Broom. 1995. Australian X disease, Murray Valley encephalitis and the French connection. Vet. Microbiol. 46:79-90. [DOI] [PubMed] [Google Scholar]
- 27.Misra, U. K., J. Kalita, and M. Srivastava. 1998. Prognosis of Japanese encephalitis: a multivariate analysis. J. Neurol. Sci. 161:143-147. [DOI] [PubMed] [Google Scholar]
- 28.Monath, T. P. 1987. Yellow fever: a medically neglected disease. Report on a seminar. Rev. Infect. Dis. 9:165-175. [DOI] [PubMed] [Google Scholar]
- 29.Pinto, A. J., P. S. Morahan, M. Brinton, D. Stewart, and E. Gavin. 1990. Comparative therapeutic efficacy of recombinant interferons-alpha, -beta, and -gamma against alphatogavirus, bunyavirus, flavivirus, and herpesvirus infections. J. Interferon Res. 10:293-298. [DOI] [PubMed] [Google Scholar]
- 30.Rice, C. M. 1996. Flaviviridae: the viruses and their replication, p. 931-959. In B. N. Fields, D. M. Knipe, and P. M. Howley, Fields virology, 3rd ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 31.Singh, B., and B. Postic. 1970. Enhanced resistance of mice to virulent Japanese B encephalitis virus following inactivated vaccine and poly I:C. J. Infect. Dis. 122:339-342. [DOI] [PubMed] [Google Scholar]
- 32.Tam, R., J. Lau, and Z. Hong. 2001. Mechanisms of action of ribavirin in antiviral therapies. Antivir. Chem. Chemother. 12:261-272. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. 1979. Technical information on Japanese encephalitis and guidelines for treatment. World Health Organization, New Delhi, India.
- 34.World Health Organization. 1982. Technical guide for diagnosis, treatment, surveillance, prevention, and control of dengue haemorrhagic fever. World Health Organization, Geneva, Switzerland.
- 35.Zoulim, F., J. Haem, S. S. Ahmed, P. Chossegros, F. Habersetzer, M. Chevallier, F. Bailly, and C. Trepo. 1998. Ribavirin monotherapy in patients with chronic hepatitis C: a retrospective study of 95 patients. J. Viral Hepatol. 5:193-198. [DOI] [PubMed] [Google Scholar]