Abstract
Skeletal muscle development is controlled by a family of muscle-specific basic helix–loop–helix (bHLH) transcription factors that activate muscle genes by binding E-boxes (CANNTG) as heterodimers with ubiquitous bHLH proteins, called E proteins. Myogenic bHLH factors are expressed in proliferating undifferentiated myoblasts, but they do not initiate myogenesis until myoblasts exit the cell cycle. We describe a bHLH protein, MyoR (for myogenic repressor), that is expressed in undifferentiated myoblasts in culture and is down-regulated during differentiation. MyoR is also expressed specifically in the skeletal muscle lineage between days 10.5 and 16.5 of mouse embryogenesis and down-regulated thereafter during the period of secondary myogenesis. MyoR forms heterodimers with E proteins that bind the same DNA sequence as myogenic bHLH/E protein heterodimers, but MyoR acts as a potent transcriptional repressor that blocks myogenesis and activation of E-box-dependent muscle genes. These results suggest a role for MyoR as a lineage-restricted transcriptional repressor of the muscle differentiation program.
Members of the basic helix–loop–helix (bHLH) family of transcription factors regulate cell fate specification, differentiation, and morphogenesis of a wide range of cell types. Skeletal muscle is one of the most thoroughly characterized developmental systems utilizing bHLH-mediated transcriptional networks (1, 2). The four skeletal muscle-specific bHLH transcription factors, MyoD, myogenin, Myf5, and MRF4, act at multiple steps in the myogenic lineage to control muscle gene expression. In the embryo, MyoD and Myf5 play overlapping roles in specification of myoblasts; in the absence of one factor or the other, myogenesis is unaffected, whereas in the absence of both, no myoblasts are formed (3). In contrast, myogenin is required for muscle differentiation; in its absence, myoblasts are specified but their ability to differentiate is impaired (4, 5). MRF4 and MyoD also have overlapping functions in the differentiation pathway, such that either factor alone is dispensable, whereas in the absence of both factors, myoblasts fail to differentiate (6).
The skeletal muscle bHLH factors heterodimerize preferentially with a ubiquitous family of bHLH proteins, called E proteins, which includes E12, E47, E2–2, and HEB (7, 8). Myogenic bHLH/E-protein heterodimers bind the E box DNA consensus sequence (CANNTG) in the control regions of muscle-specific genes (9). Myogenic bHLH proteins and E proteins contain strong transcriptional activation domains that are important for muscle gene activation (10–12).
Although myogenic bHLH proteins are expressed in proliferating, undifferentiated myoblasts, they do not activate muscle differentiation genes until myoblasts exit the cell cycle. Several mechanisms have been shown to inhibit the functions of myogenic bHLH proteins in myoblasts, including expression of the inhibitory HLH protein Id and the immediate early gene products Fos and Jun, as well as changes in phosphorylation of the myogenic factors (reviewed in ref. 13). Given the transcriptional potency of the myogenic factors and the diverse environmental influences on developing skeletal muscle cells, it is likely that multiple mechanisms are involved in modulating the activities of these factors.
Here we describe a bHLH protein, called MyoR (myogenic repressor), that is expressed at high levels in proliferating myoblasts in culture and is down-regulated during myogenesis. During mouse embryogenesis, MyoR is expressed in developing skeletal muscle between embryonic day 10.5 (E10.5) and E16.5 and is down-regulated thereafter during the period of secondary muscle development. MyoR shares high homology with the bHLH protein capsulin, which is expressed in smooth muscle cell precursors during mouse embryogenesis (14), and the Drosophila bHLH protein bHLH54F, which is expressed specifically in visceral and skeletal muscle cell precursors (15). MyoR forms heterodimers with E proteins and can bind the same DNA sequences as myogenic bHLH factors, but it acts as a transcriptional repressor and inhibitor of myogenesis. These findings suggest that MyoR functions as a lineage-restricted negative regulator of myogenesis that may delay muscle fiber maturation or modulate the timing of expression of muscle-specific genes during embryonic muscle development.
MATERIALS AND METHODS
Cloning and DNA Sequencing.
Mouse MyoR genomic clones were isolated by screening a mouse genomic library with a 32P-labeled capsulin (14) cDNA probe under conditions of low stringency. On the basis of sequence obtained from the MyoR gene, we designed primers from the 5′ and 3′ ends of the gene and performed PCR amplification using mouse E11 Marathon cDNA (CLONTECH) as template. MyoR cDNAs were sequenced by using oligonucleotide primers corresponding to sequences within the cDNA.
In Situ Hybridization and Autoradiography.
In situ hybridization on paraffin sections was performed as described previously (14).
RNA Isolation and Reverse Transcriptase–PCR.
Total RNA was isolated from C2 cells by using Trizol (GIBCO/BRL). First-strand cDNA synthesis was performed as described (16), and PCR amplification was performed with 1 μl of cDNA, 0.1 μCi (1 μCi = 37 kBq) of 32P-labeled deoxycytidine triphosphate (dCTP), and gene-specific primers under conditions of linearity for each primer set. Duplicate PCRs were also performed in the absence of reverse transcriptase to confirm the absence of contaminating genomic DNA. All PCR products corresponded to the sizes predicted from the corresponding transcripts. PCR cycles were as follows: 99°C for 2 min, then 22–30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. PCR products were separated by 6% polyacrylamide gel electrophoresis. Sequences of primers are available on request.
Gel Mobility-Shift Assays and in Vitro Translation.
MyoR, myogenin, and protein E12 were transcribed and translated in a coupled rabbit reticulocyte lysate transcription/translation system (Promega). In vitro translation products were used in gel mobility-shift assays with a 32P-labeled oligonucleotide probe corresponding to the right E-box from the muscle creatine kinase (MCK) enhancer. The sequence of the top strand of the probe was 5′-GATCCAACACCTGCTGCCTGAG-3′. DNA–protein complexes were resolved on polyacrylamide gels, followed by autoradiography, as described (17). For competition experiments, excess unlabeled oligonucleotide probe was added to the binding reaction mixtures.
Transfection Assays and DNA Constructs.
Transient transfections were performed with Fugene6 (Boehringer Mannheim) according to manufacturer’s instructions. 10T½ cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (GIBCO/BRL). Briefly, 0.3 μg of reporter [4R-tk-luc (18) or MCK4800-luc (19)] and 0.3 μg of each activator (EMSV-MyoD, EMSV-E12, pECE-FLAG-MyoR, or pECE-MyoD∼E47) was mixed with 3 μl of Fugene6 and added to cells in six-well plates. After 24 hr, the medium was changed to differentiation medium (DMEM with 2% horse serum), and 48 hr later cells were harvested for luciferase assays. The total amount of DNA added in each transfection was kept constant by addition of pECE-FLAG or EMSV.
To assay for repression, 0.3 μg of L8G5-luc (20), 0.1 μg of LexA-VP16, and 0.2 μg of pM1 or pM1-MyoR were mixed with 2 μl of Fugene6 and added to six-well plates. After 48 hr, cells were harvested for luciferase assays.
To assay for myogenic conversion, 10T½ cells were transiently transfected with 1 μg of the indicated expression vectors (see Table 1). The amount of DNA in each transfection was kept constant at 2 μg by addition of parent vectors EMSV or pECE-FLAG. Two days after transfection, cultures were transferred from growth to differentiation medium, and myosin heavy chain (MHC) expression was detected 5 days later by immunostaining with anti-MHC antibody (Sigma), as described (21).
Table 1.
Inhibition of myogenesis by MyoR
| Expression vector | No. of MHC-positive cells per well |
|---|---|
| None | 0 |
| MyoR | 0 |
| MyoR + MEF2C | 0 |
| MyoD | >50 |
| MyoD + MyoR | 0–2 |
| Myogenin | >20 |
| Myogenin + MyoR | 0 |
10T½ cells were transiently transfected with 1 μg of MyoD or myogenin expression vectors in the presence or absence of 1 μg of MyoR expression vector. The number of MHC-positive cells per well is indicated. The few MHC-positive cells observed in transfections with MyoD + MyoR did not express MyoR, as detected with anti-FLAG-antibody.
RESULTS
Structural Characteristics of MyoR.
We previously described a mouse bHLH protein, called capsulin (also known as Pod-1), that is expressed in mesenchymal cells at sites of epithelial–mesenchymal interactions, in undifferentiated vascular and visceral smooth muscle cells, and in multiple regions of the heart and epicardium (ref. 14; see also refs. 22 and 23). Using the capsulin cDNA as probe, we screened a mouse genomic library in an effort to clone capsulin-related genes. Genomic clones representing a capsulin-related gene were sequenced and PCR primers from the 5′ and 3′ ends of the gene were used for PCR amplification of mouse E11 cDNA. This resulted in cloning of the capsulin-related cDNA encompassing the entire ORF (Fig. 1). On the basis of its expression pattern in embryonic skeletal muscle and function as a repressor of myogenesis (see below), we named this factor MyoR, for myogenic repressor.
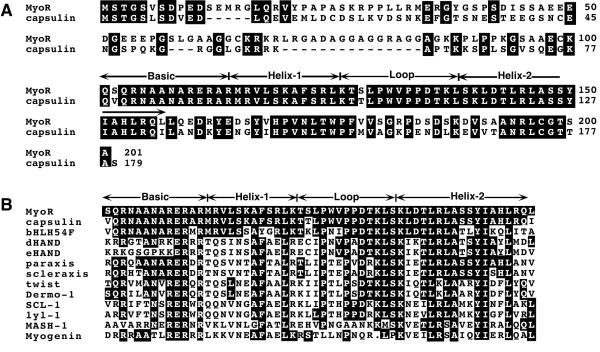
Figure 1.
Conceptual ORF of MyoR and homology with other bHLH proteins. (A) The deduced ORF of MyoR and amino acid alignment with capsulin is shown. (B) Homology between the bHLH region of MyoR and other bHLH proteins.
The ORF of MyoR predicts a 201-amino acid protein with Mr = 21,538 and pI = 9.45 (Fig. 1A). The C-terminal regions of MyoR and capsulin share high homology, whereas their N-termini are relatively divergent except for the first 11 amino acids. Of note, MyoR contains a proline-rich region between amino acids 21 and 34 and a glycine-rich insert between residues 70 and 84 that are not found in capsulin. The bHLH regions of MyoR and capsulin are closely related to the Drosophila bHLH factor bHLH54F, which is expressed in subsets of visceral and somatic muscle cells during embryogenesis (15). Outside the bHLH region, there is no significant homology between MyoR and bHLH54F. The bHLH region of MyoR also shows substantial homology to several other cell type-restricted bHLH proteins.
As this work was being completed, a human bHLH factor, called ABF-1 (activated B-cell factor 1), was reported (24). The amino acid sequence of the bHLH region of human ABF-1 is identical to that of mouse MyoR, but there are regions of divergence in the N- and C-termini of the proteins. Overall, mouse MyoR and human ABF-1 share 77% amino acid identity. Mouse and human capsulin, by contrast, share 96% identity over their entire lengths (22), suggesting either that MyoR and ABF-1 are not orthologs or that MyoR is less conserved than capsulin across species. The expression pattern of ABF-1 during embryogenesis has not been reported. However, in adult tissues, ABF-1 appears to be largely restricted to a subset of lymphoid tissues (24).
Embryonic Expression Pattern of MyoR.
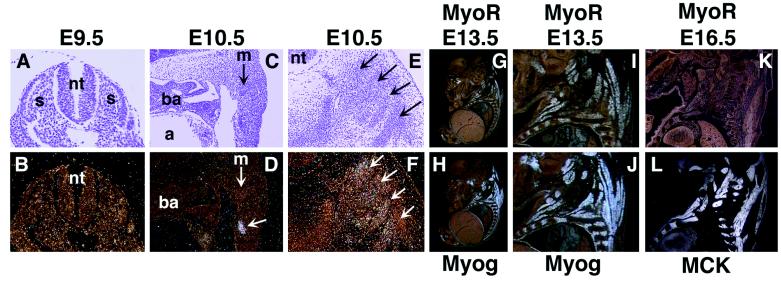
The expression pattern of MyoR during mouse embryogenesis was determined by in situ hybridization of thin sections of staged embryos from E8.5 to E16.5. Transverse sections through the somites did not reveal MyoR mRNA expression in rostral or caudal somites at E8.5 (not shown) or E9.5 (Fig. 2 A and B). We first detected MyoR expression at E10.5 at a low level in population of muscle precursor cells in the posterior body wall ventrolateral to the myotome and slightly rostral of the forelimb bud (Fig. 2 C and D). We are uncertain as to the precise muscle groups to which these muscle cells contribute. We also detected a very low level of MyoR expression in a few cells within the epaxial caudal dermamyotome at E10.5 (not shown). MyoR transcripts were also present in a subset of developing muscle fibers in the thoracic body wall at E10.5 (Fig. 2 E and F). Expression of MyoR in skeletal muscle was especially prominent at E13.5 (Fig. 2 G and I). At this stage, the MyoR expression pattern overlapped that of myogenin, which marks all skeletal muscle cells (Fig. 2 H and J). However, myogenin expression was slightly more extensive within muscle-forming regions at this stage. By E16.5, MyoR transcripts were down-regulated in most developing muscle fibers, (Fig. 2K), whereas myogenin expression was maintained (not shown). The high level expression of MyoR in developing skeletal muscle at E13.5 and down-regulation by E16.5 contrasted with the expression of MCK, which is not up-regulated until after E13.5, and continues to increase to high levels of expression at E16.5 (ref. 25; Fig. 2L).
Figure 2.
Detection of MyoR and myogenin expression in developing skeletal muscle by in situ hybridization. Transcripts for MyoR were detected in transverse sections of E9.5 (B) and E10.5 (F) and in sagittal sections of E10.5 (D), E13.5 (G and I), and E16.5 (K) embryos. Expression of myogenin at E13.5 is illustrated in sagittal sections (H and J). Transcripts for MCK are shown in sagittal section of an E16.5 embryo (L). I and J show enlarged views of G and H, respectively. At E16.5, MyoR expression is down-regulated in contrast to the abundant expression of MCK. a, Atrium; ba, branchial arch; m, myotome; nt, neural tube; s, somite. The arrow in D shows a cluster of MyoR-expressing cells in body wall ventrolateral to the myotome (see text). Arrows in E and F mark differentiating axial skeletal muscle. (A and B, ×60; C–F, ×30; G and H, ×4; I and J, ×8; and K and L, ×5.)
Northern analysis of RNA from adult tissues did not reveal MyoR expression in any tissues examined, including skeletal muscle, heart, liver, spleen, kidney, brain, and intestine. We conclude that the embryonic skeletal muscle lineage is the major site of MyoR expression.
MyoR Expression Is Down-Regulated During Myogenesis in Culture.
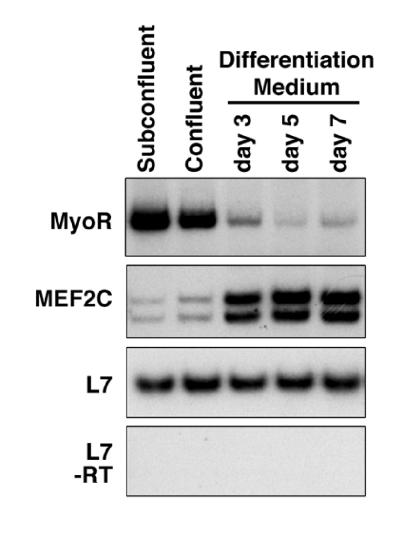
To further investigate the expression pattern of MyoR during muscle development, we examined expression of MyoR transcripts by semiquantitative reverse transcriptase–PCR during differentiation of the C2 skeletal muscle cell line. In subconfluent or confluent C2 myoblasts in growth medium, MyoR mRNA was expressed at a relatively high level, whereas transcripts were down-regulated when undifferentiated cells were induced to differentiate by transfer to differentiation medium (Fig. 3). In contrast, transcripts encoding the MADS-box transcription factor, MEF2C, a marker for differentiation, were up-regulated under conditions in which MyoR was down-regulated. L7 transcripts were measured as a control for equal loading of mRNA. These results suggested that MyoR was unlikely to be required for activation of muscle differentiation.
Figure 3.
Expression of MyoR transcripts during differentiation of C2 cells. Total RNA was isolated from C2 cells maintained at subconfluent or confluent densities in growth medium or after transfer to differentiation medium for 3, 5, and 7 days. Transcripts for MyoR, MEF2C, and L7 were measured by semiquantitative reverse transcriptase–PCR. MyoR and L7 yield single PCR products of the predicted size, and MEF2C yields two products reflecting alternative splicing. In the absence of reverse transcriptase (-RT), no products were observed for L7 (Bottom), MyoR, or MEF2C (not shown).
DNA-Binding Activity of MyoR.
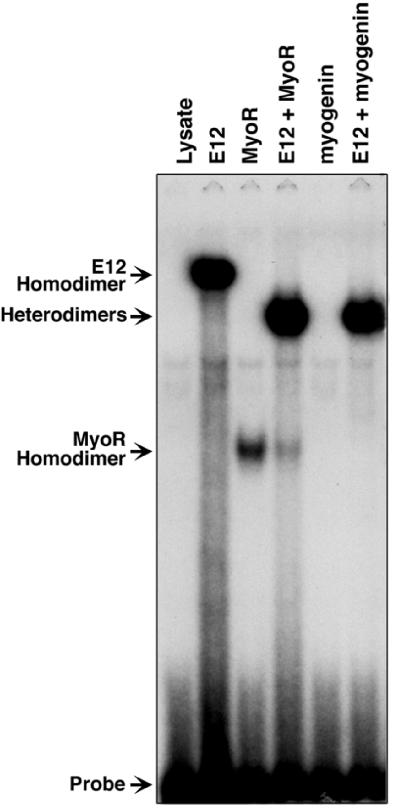
To test for DNA-binding activity, we translated MyoR in vitro in the presence and absence of E12 and performed gel mobility-shift assays using a 32P-labeled oligonucleotide probe corresponding to the right E-box from the MCK enhancer, which binds heterodimers of myogenic bHLH factors and E12 with high affinity (17, 26). MyoR and E12 each bound the MCK E-box as homodimers, yielding DNA–protein complexes with different mobilities (Fig. 4). In the presence of MyoR plus E12, a DNA–protein complex with mobility intermediate between the E12 and MyoR homodimeric complexes was observed, indicative of heterodimer function. Myogenin did not bind the E-box alone, but myogenin plus E12 yielded a prominent DNA–protein complex, reflecting the formation of a DNA-binding heterodimer. When MyoR and myogenin were cotranslated, we observed no evidence for the formation of a MyoR/myogenin heterodimer (not shown). DNA binding by MyoR homodimers and MyoR/E12 heterodimers was sequence-specific and was blocked by competition with the cognate site, but not a mutant site (data not shown). Thus, MyoR/E12 and myogenin/E12 heterodimers bind the same target sequence shown to be essential for activation of numerous muscle-specific genes.
Figure 4.
Binding of MyoR/E12 heterodimers to DNA. E12, MyoR, and/or myogenin were transcribed and translated separately or together, as indicated, using a coupled in vitro transcription/translation system. In vitro translation products were used in gel mobility-shift assays with an end-labeled double-stranded oligonucleotide probe corresponding to the MCK right E-box. In the presence of lysate alone, no binding activity was observed.
MyoR Blocks the Ability of MyoD to Activate Transcription.
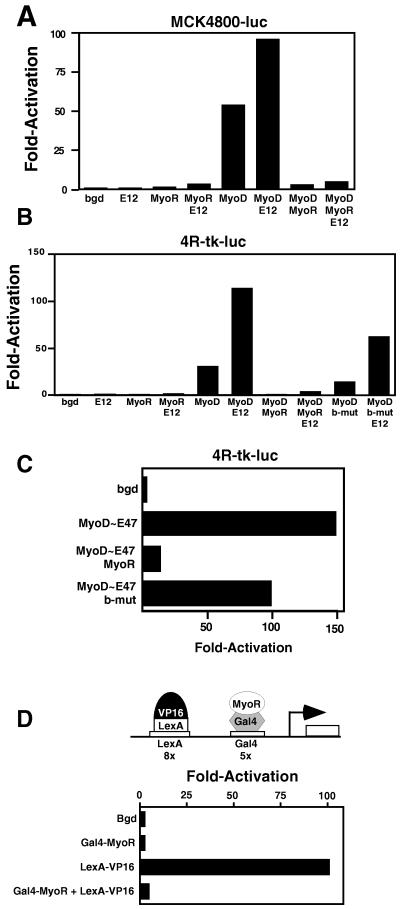
To further characterize the functions of MyoR, we performed transient transfection assays in 10T½ fibroblasts with E-box-dependent reporter genes. The reporter MCK4800-luc contains the 4,800-bp upstream region from the MCK gene (19) and has been shown to be transactivated by myogenic bHLH proteins, which bind two adjacent E-boxes in an upstream enhancer (17, 18, 26). MyoD potently activated this reporter gene, and activation was augmented in the presence of exogenous E12 (Fig. 5A). In contrast, MyoR showed no transcriptional activity on its own or with E12 and it completely prevented activation of the reporter gene by MyoD.
Figure 5.
MyoR inhibits transcription. 10T½ cells were transiently transfected with MCK4800-luc (A) or 4R-tk-luc (B and C) and expression vectors encoding the indicated bHLH proteins. The background level of expression of the reporters without cotransfected bHLH expression vectors (bgd) was assigned a value of 1. (D) COS cells were transiently transfected with L8G5-luc reporter and expression vectors encoding LexA-VP16 and GAL4-MyoR, as indicated. The amount of DNA in each transfection was kept constant by addition of parent vector pM1. GAL4-MyoR resulted in greater than a 20-fold reduction in reporter gene expression in the presence of LexA-VP16. Assays were performed three times with comparable results. Luciferase activity was normalized to activity of β-galactosidase, obtained by cotransfection of RSV-lacZ as an internal control.
To determine whether MyoR could specifically block the ability of MyoD to activate transcription through the E-box motif, we used the E-box-dependent reporter gene 4Rtk-luc, which contains four tandem E-boxes from the MCK enhancer upstream of the thymidine kinase basal promoter (18). This reporter was up-regulated by MyoD, and in the presence of E12, MyoD-dependent activation was enhanced (Fig. 5B). However, MyoR was unable to activate the reporter, despite the fact that it bound avidly to the MCK E-box. Moreover, MyoR interfered with the ability of MyoD to activate transcription (Fig. 5B). Inhibition of MyoD activity by MyoR was not relieved by addition of ectopic E proteins, suggesting that E protein sequestration is not the major mechanism for repression.
To determine whether DNA binding was required for MyoR to inhibit the activity of MyoD, we created a basic domain mutant (b-mut) of MyoR, in which amino acids 110–112 were changed from RER to LEG. This mutant lacks DNA-binding activity (data not shown). In contrast to wild-type MyoR, which inhibited MyoD activity by greater than 20-fold reduction, b-mut caused only about a 2-fold decrease in transcriptional activation by MyoD or MyoD plus E12 (Fig. 5B). These results are consistent with the idea that the major mechanism for repression by MyoR involves DNA binding.
As a further means of testing whether MyoR inhibited myogenesis by sequestering E proteins, we performd transfection assays using a tethered MyoD∼E47 heterodimer (27). MyoD∼E47 strongly activated 4Rtk-luc and this activation was inhibited approximately 10-fold by MyoR (Fig. 5C). In contrast, b-mut caused only a 2-fold decrease in transcriptional activation of 4Rtk-luc by MyoD∼E47. The slight decrease in activity of MyoD∼E47 in the presence of b-mut may result from nonspecific squelching. Together, these results demonstrate that E-protein sequestration contributes to repression by MyoR, but is not the major mechanism for repression.
MyoR Acts As a Transcriptional Repressor.
In light of the inhibitory activity of MyoR, we investigated whether it might function as a transcriptional repressor, using a luciferase reporter gene controlled by eight copies of the binding site for LexA immediately adjacent to five copies of the binding site for GAL4 (20). In the presence of a LexA-VP16 fusion coactivator, this reporter was activated to high levels of expression (Fig. 5D). By fusing MyoR to the DNA-binding domain of GAL4, we tested whether binding of a GAL4-MyoR fusion protein adjacent to LexA-VP16 was sufficient to reduce expression of the reporter. GAL4-MyoR inhibited by more than 20-fold the transcriptional activity of LexA-VP16. We conclude that MyoR acts as a powerful transcriptional repressor that can inhibit the activity of heterologous transactivators bound to adjacent DNA sequences.
MyoR Blocks the Ability of MyoD to Activate Myogenesis.
To further test the function of MyoR as a negative regulator of myogenesis, we examined its effect on the ability of MyoD and myogenin to initiate myogenesis in transiently transfected 10T½ cells. As shown in Table 1, MyoD and myogenin efficiently induced MHC expression and myotube formation in transfected 10T½ cells. MyoR alone had no myogenic activity and, when expressed together with MyoD or myogenin, it completely blocked their ability to activate myogenesis.
Previously, we showed that MEF2C can cooperate with the bHLH regions of myogenic bHLH factors to activate E-box-dependent transcription (28). We therefore tested whether MEF2C might cooperate with MyoR to activate myogenesis. However, in the presence of MEF2C and MyoR, no myogenic conversion was observed. We conclude that MyoR functions as a repressor of the myogenic program by interfering with the activity of myogenic bHLH proteins.
We confirmed that FLAG-tagged MyoR was expressed in the nuclei of transfected cells, by staining with anti-FLAG antibody, and that inhibition of MyoD-mediated myogenesis was not attributable to cell death or a decrease in number of transfected cells. MyoR also did not affect expression of cotransfected β-galactosidase reporters under control of the Rous sarcoma virus, cytomegalovirus, or heat shock promoters (not shown).
DISCUSSION
The myogenic bHLH proteins are among the most potent tissue-specific transcription factors identified, as reflected by their ability to activate muscle gene expression in a broad range of cell types from all three germ layers. Because of the dominant transcriptional activity of these factors, equally powerful mechanisms must exist to restrict their muscle-inducing activity until the appropriate stage of development. There must also be mechanisms that modulate the actions of myogenic bHLH proteins on different target genes, so as to allow for differences in the temporospatial patterns of expression of individual muscle genes during embryogenesis. Our results suggest that MyoR functions as a lineage-restricted antagonist of myogenic bHLH proteins at specific stages of muscle development.
MyoR Expression Marks a Subset of Developing Skeletal Muscle Cells.
MyoD and Myf5 are expressed in proliferating myoblasts, but they do not activate muscle differentiation genes until myoblasts exit the cell cycle. MyoR is expressed at high levels in proliferating C2 myoblasts in culture and is down-regulated at the onset of differentiation. The ability of MyoR to block transcriptional activation of E-box-dependent reporters and induction of myogenesis in transfected 10T½ fibroblasts by myogenin and MyoD suggests that MyoR can act to repress the differentiation program in myoblasts in culture. However, the expression pattern of MyoR during embryogenesis suggests a somewhat different function in vivo. Myogenesis is initiated in the somites at E8.0 in the mouse when Myf5 gene expression is activated in the dorsomedial lip of the dermamyotome (reviewed in ref. 29). By E8.5, myogenin is expressed throughout the myotome, followed by expression of downstream muscle structural genes. We did not detect expression of MyoR in the somite myotomes between E8.5 and E10.5, except at a low level in a few cells in the epaxial myotome at E9.5. Thus, MyoR does not appear to participate in the initial steps of muscle development in vivo.
Robust expression of MyoR is observed in a subset of skeletal muscle cells between E10.5 and E16.5. Expression is especially pronounced in developing muscles of the trunk. The muscle fibers in which MyoR is expressed also express the four myogenic bHLH proteins, as well as contractile protein genes. Why would an inhibitor of myogenesis be expressed in developing muscle fibers between E10.5 and 16.5 and what might its functions be at this stage? Muscle development occurs in successive waves, with primary myogenesis taking place between E10.5 and E14.5, as primitive myotubes are laid down (30). Thereafter, secondary myogenesis ensues, as primary myotubes serve as templates to recruit myoblasts, leading to growth and maturation of muscle fibers. MyoR is expressed predominantly during the stages of primary myogenesis and is down-regulated as secondary muscle fibers develop.
Based on its ability to inhibit E-box-dependent transcription in vitro, we speculate that MyoR may be important for selectively delaying expression of certain muscle-specific genes during primary myogenesis in vivo. In this regard, an intriguing aspect of muscle gene regulation that has not been explained is the unique temporospatial expression patterns of different muscle-specific genes. Some muscle genes, for example, are activated early in the differentiation program, whereas others are not activated until the period of secondary myogenesis, or even later. Of note, despite the fact that MCK is controlled by myogenic bHLH factors and MEF2, which are expressed in myogenic cells as early as E8.5, MCK does not begin to be expressed until E13.5 in the mouse (25). Our results reveal a reciprocal relationship between MyoR and MCK expression in developing skeletal muscle fibers; only after MyoR is down-regulated does MCK begin to be expressed in skeletal muscle. Thus, the ability of MyoR to inhibit the MCK enhancer in transfection assays may reflect a negative regulatory influence of MyoR on MCK expression in vivo.
Mechanisms for MyoR-Mediated Repression of Myogenesis.
MyoR forms heterodimers with E12 that bind the E-box from the MCK enhancer but fail to activate transcription. These results, and the finding that MyoR interferes with the ability of myogenin and MyoD to activate myogenesis in 10T½ fibroblasts, demonstrate that MyoR functions as a repressor of myogenesis. Consistent with this conclusion, MyoR fused to the DNA-binding domain of GAL4 can block transcriptional activity of a LexA-VP16 coactivator bound to adjacent sites. The related bHLH protein, ABF-1, also acts as a transcriptional repressor and inhibits transcriptional activity of E47 homodimers (24). Most downstream target genes of the myogenic bHLH proteins contain multiple E-boxes in conjunction with other sites for muscle-restricted and widely expressed transcription factors. Our results suggest that MyoR need not occupy every E-box within a muscle gene control region to repress expression of a gene.
While MyoR/E12 heterodimers bind the MCK right E-box with high affinity, we do not yet know whether this is the preferred binding site or whether there is a spectrum of sites to which MyoR can bind. It is interesting, in this regard, that many E-box-dependent muscle genes are coexpressed with MyoR during embryogenesis. Thus, assuming MyoR functions as an inhibitor of myogenic bHLH protein functions in vivo, as our in vitro results suggest, it must be able to discriminate between different downstream genes in the muscle differentiation pathway.
Activation of muscle-specific transcription by myogenic bHLH proteins is dependent on two conserved amino acids, alanine-threonine, in the center of their basic regions (31, 32). When these residues are mutated, the myogenic bHLH proteins can still bind DNA but cannot activate muscle gene expression. The myogenic activity of these residues has been ascribed to their involvement in formation of a transcriptionally active complex with MEF2 (28, 33). MyoR lacks these myogenic amino acids in its basic region and cannot cooperate with MEF2 to induce transcription.
Our results suggest at least three types of mechanisms whereby MyoR can block muscle gene expression. (i) It can compete with myogenic bHLH/E-protein heterodimers for E-box binding sites in muscle gene control regions. (ii) MyoR bound to E-boxes in muscle control regions can actively repress transcription through its transcriptional repression domain. (iii) MyoR can compete with myogenic bHLH proteins for limiting quantities of E protein dimerization partners. However, because excess E12 did not rescue the ability of MyoD or myogenin to activate muscle genes in the presence of MyoR, and MyoR showed strong inhibition of the MyoD∼E47 tethered heterodimer, the latter mechanism for repression appears to be of lesser importance. While one could imagine other mechanisms whereby MyoR might inhibit myogenesis—for example, by inducing cell death or stimulating cell proliferation—our results argue against these types of mechanisms.
The functions of MyoR resemble those of another bHLH protein, Mist1 (34), but what distinguishes MyoR is its unique expression pattern during a specific period of muscle development in vivo. By contrast, Mist1 shows a much broader expression pattern in multiple cell types during development (35). Members of the Twist family of bHLH proteins, which are expressed in paraxial mesoderm and nonmyotomal compartments of the somites (36), also act through multiple mechanisms to inhibit muscle gene expression (37, 38).
It is worth noting that the function of MyoR in the network of bHLH protein regulators of myogenesis is reminiscent of the function of the bHLH protein Mad, which acts as a transcriptional repressor to block activation of Myc-regulated genes (39). Myc binds the E-box consensus sequence as a heterodimer with Max, which is constitutively expressed. Max also forms homodimers and heterodimers with Mad to inhibit transcription. Thus, this strategy of a ubiquitous bHLH factor interacting with either a positive or negative bHLH factor appears to be a common theme in bHLH-mediated gene regulation.
Possible Evolutionary Conservation of Expression and Function of MyoR and Capsulin.
The finding that MyoR is an inhibitor of skeletal muscle differentiation and is down-regulated early in the myogenic pathway raises the possibility that the related bHLH protein capsulin may perform a similar inhibitory function in lineages in which it is expressed. Indeed, capsulin is expressed in precursors of vascular and visceral smooth muscle and is down-regulated as smooth muscle structural genes are expressed (14). Capsulin also binds the E-box consensus sequence but fails to activate E-box-dependent reporters and may therefore be a transcriptional repressor. This observation might imply the existence of positive-acting bHLH factors in smooth muscle lineages.
The homology between MyoR and capsulin and the Drosophila factor bHLH54F is striking and raises the possibility that these factors may perform similar functions. Consistent with this idea, bHLH54F is expressed in a subset of somatic and visceral muscle cells during Drosophila embryogenesis (15), resembling the combined expression patterns of capsulin and MyoR in the mouse. Whether the functions of bHLH54F in the somatic and visceral muscle lineages of Drosophila were segregated during evolution into MyoR and capsulin, respectively, awaits genetic studies in these organisms.
Acknowledgments
We thank A. Tizenor for assistance with graphics, W. Simpson for editorial assistance, and J. Shelton and J. Stark for histology. We are also grateful to Stan Hollenberg and Barbara Wold for reagents. This work was supported by grants to E.N.O. from the National Institutes of Health, the Muscular Dystrophy Association, and the American Heart Association.
ABBREVIATIONS
- bHLH
basic helix–loop–helix
- MCK
muscle creatine kinase
- En
embryonic day n
- MHC
myosin heavy chain
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF108216).
References
- 1.Yun S, Wold B J. Curr Opin Cell Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin J, Olson E N. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- 3.Rudnicki M A, Schnegelsberg P N J, Stead R H, Braun T, Arnold H H, Jaenisch R. Cell. 1993;71:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 4.Hasty P, Bradley A, Morris J H, Venuti J M, Olson E N, Klein W H. Nature (London) 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- 5.Nabeshima Y K, Hanaoka K, Hayasaka M, Esumi S, Li S, Nonaka I, Nabeshima Y. Nature (London) 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- 6.Rawls A, Valdez M R, Zhang W, Richardson J, Klein W H, Olson E N. Development (Cambridge, UK) 1998;125:2349–2358. doi: 10.1242/dev.125.13.2349. [DOI] [PubMed] [Google Scholar]
- 7.Lassar A B, Davis R L, Wright W E, Kadesh T, Murre C, Voronova A, Baltimore D, Weintraub H. Cell. 1991;66:305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- 8.Hu J S, Olson E N, Kingston R E. Mol Cell Biol. 1992;12:1031–1042. doi: 10.1128/mcb.12.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murre C, McCaw P S, Vaessin H, Caudy M, Jan L Y, Jan Y N, Cabrera C V, Buskin J N, Hauschka S D, Lassar A B, Weintraub H, Baltimore D. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 10.Weintraub H, Dwarki V J, Verma I, Davis R, Hollenberg S, Snider L, Lassar A, Tapscott S J. Genes Dev. 1991;5:1377–1386. doi: 10.1101/gad.5.8.1377. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz J J, Chakraborty T, Martin J, Zhou J, Olson E N. Mol Cell Biol. 1992;12:266–275. doi: 10.1128/mcb.12.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quong M W, Massari M E, Zwart R, Murre C. Mol Cell Biol. 1993;13:792–800. doi: 10.1128/mcb.13.2.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerson C P., Jr Curr Opin Genet Dev. 1993;3:265–274. doi: 10.1016/0959-437x(93)90033-l. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Richardson J A, Olson E N. Mech Dev. 1998;73:23–32. doi: 10.1016/s0925-4773(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 15.Georgias C, Wasser M, Hinz U. Mech Dev. 1997;69:115–124. doi: 10.1016/s0925-4773(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 16.Lin Q, Schwarz J, Buchana C, Olson E N. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan T J, Olson E N. Genes Dev. 1990;4:582–595. doi: 10.1101/gad.4.4.582. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub H, Davis R, Lockshon D, Lassar A. Proc Natl Acad Sci USA. 1990;87:5623–5627. doi: 10.1073/pnas.87.15.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sternberg E A, Spizz G, Perry W M, Vizard D, Weil T, Olson E N. Mol Cell Biol. 1988;8:2896–2909. doi: 10.1128/mcb.8.7.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollenberg S M, Sternglanz R, Cheng P F, Weintraub H. Mol Cell Biol. 1995;15:3813–3822. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edmondson D G, Olson E N. Genes Dev. 1989;3:678–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 22.Hidai H, Bardales R, Goodwin R, Quertermous T, Quertermous E E. Mech Dev. 1998;73:33–43. doi: 10.1016/s0925-4773(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 23.Quaggin S E, Vanden Heuvel G B, Igarashi P. Mech Dev. 1998;71:37–48. doi: 10.1016/s0925-4773(97)00201-3. [DOI] [PubMed] [Google Scholar]
- 24.Massari M E, Rivera R R, Voland J R, Quong M W, Breit T M, Van Donge J J M, De Smit O, Murre C. Mol Cell Biol. 1998;18:3130–3139. doi: 10.1128/mcb.18.6.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons G E, Muhlebach S, Moser A, Masood R, Paterson B M, Buckingham M E, Perriard J C. Development (Cambridge, UK) 1991;113:1017–1029. doi: 10.1242/dev.113.3.1017. [DOI] [PubMed] [Google Scholar]
- 26.Lassar A B, Buskin J N, Lockson D, Davis R L, Apone S, Hauschka S D, Weintraub H. Cell. 1989;58:823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- 27.Neuhold L A, Wold B. Cell. 1993;74:1033–1042. doi: 10.1016/0092-8674(93)90725-6. [DOI] [PubMed] [Google Scholar]
- 28.Molkentin J D, Black B L, Martin J F, Olson E N. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 29.Buckingham M. Trends Genet. 1992;8:144–148. doi: 10.1016/0168-9525(92)90373-C. [DOI] [PubMed] [Google Scholar]
- 30.Kelly A M. In: Handbook of Physiology. Peachey L D, editor. (Baltimore: Williams & Wilkins; 1983. pp. 507–537. [Google Scholar]
- 31.Davis R L, Weintraub H. Science. 1992;256:1027–1030. doi: 10.1126/science.1317057. [DOI] [PubMed] [Google Scholar]
- 32.Brennan T J, Chakraborty T, Olson E N. Proc Natl Acad Sci USA. 1991;88:5675–5679. doi: 10.1073/pnas.88.13.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black B L, Molkentin J D, Olson E N. Mol Cell Biol. 1998;18:69–77. doi: 10.1128/mcb.18.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemercier C, To R Q, Carrasco R A, Konieczny S. EMBO J. 1998;17:1412–1422. doi: 10.1093/emboj/17.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemercier C, To R Q, Swanson B J, Lyons G E, Konieczny S F. Dev Biol. 1997;182:101–113. doi: 10.1006/dbio.1996.8454. [DOI] [PubMed] [Google Scholar]
- 36.Wolf C, Thisse C, Stoetzel B, Gerlinger P, Perrin-Schmitt F. Dev Biol. 1991;142:363–373. doi: 10.1016/0012-1606(91)90086-i. [DOI] [PubMed] [Google Scholar]
- 37.Li, L., Cserjesi, P. & Olson, E. N. (1995) Dev. Biol.280–292. [DOI] [PubMed]
- 38.Spicer D B, Rhee J, Cheung W L, Lassar A B. Science. 1996;272:1476–1480. doi: 10.1126/science.272.5267.1476. [DOI] [PubMed] [Google Scholar]
- 39.Ayer D E, Kretzner L, Eisenman R N. Cell. 1993;72:211–222. doi: 10.1016/0092-8674(93)90661-9. [DOI] [PubMed] [Google Scholar]