Abstract
This study was aimed at examining the spectrum of antimicrobial activity of MUC7 20-mer (N-LAHQKPFIRKSYKCLHKRCR-C; residues 32 to 51 of MUC7, the low-molecular-weight human salivary mucin, comprised of 357 residues) and comparing its antifungal properties to those of salivary histatin 5 (Hsn-5). We also examined the secondary structure of the 20-mer and the possible mechanism of its antifungal action. Our results showed that MUC7 20-mer displays potent killing activity against a variety of fungi and both gram-positive and gram-negative bacteria at micromolar concentrations. Time-dependent killing of Candida albicans and Cryptococcus neoformans by MUC7 20-mer and Hsn-5 indicated differences in killing rates between MUC7 20-mer and Hsn-5. The secondary structure prediction showed that MUC7 20-mer adopts an amphiphilic helix with distinguishable hydrophilic and hydrophobic faces (a characteristic that is associated with antimicrobial activity). In comparison to that of Hsn-5, the fungicidal activity of MUC7 20-mer against C. albicans seems to be independent of fungal cellular metabolic activity, as evidenced by its killing potency at a low temperature (4°C) and in the presence of inhibitors of oxidative phosphorylation in the mitochondrial system. Fluorescence microscopy showed the ability of MUC7 20-mer to cross the fungal cell membrane and to accumulate inside the cells. The internalization of MUC7 20-mer was inhibited by divalent cations. Confocal microscopy of cells doubly labeled with MUC7 20-mer and a mitochondrion-specific dye indicated that mitochondria are not the target of MUC7 20-mer for either C. albicans or C. neoformans.
The need for efficient antimicrobial agents increases with the emergence of pathogens resistant to current therapies. Among the different approaches to finding novel, safe, and effective antimicrobial agents, the discovery and use of naturally occurring antimicrobial peptides are attracting increasing attention. This is so because, unlike many currently used antimicrobial compounds, they show little or no toxicity toward mammalian cells and a low tendency to elicit resistance. The functional and structural properties and therapeutic potential of naturally occurring antimicrobial peptides (ribosomally synthesized) were recently reviewed (17, 35). With respect to structure and function, magainins (39), dermaseptins (22), cecropins (31), and mammalian defensins (18) have been best characterized (3, 35). A small size, amphipathicity, and a net positive charge are the common features of most naturally occurring antimicrobial peptides (6). The majority of these peptides exhibit a random structure in water and a well-defined structure (α helix, β sheet, extended structures, and loops) in a simulated membrane-like environment (17). Their mode of antimicrobial action is not well understood. In most instances, it is mediated not by receptors but rather through peptide-microbial cell membrane lipid interactions resulting in membrane permeation and cell lysis (28).
The human salivary nonimmune defense system includes antimicrobial components such as lactoferrin, lysozyme, and peroxidase (20) and the histidine-rich cationic peptides, histatins (23). Previous studies showed that histatin 5 (24-amino-acid peptide) (Hsn-5) possesses potent fungicidal activity in vitro against Candida albicans (23, 36). Further studies showed that Hsn-5 is targeted to mitochondria and that its cytotoxic activity depends on the metabolic activity of C. albicans (12). The killing of C. albicans by Hsn-5 is accomplished by an increase in membrane potential and permeability and the subsequent release of intracellular ATP (15, 16). It was also shown that Hsn-5 and human neutrophil defensin 1 kill C. albicans via a shared pathway (4).
Our laboratory reported that MUC7 domain 1 (D1), a 51-amino-acid-residue peptide (Table 1) derived from the N terminus of the low-molecular-weight human salivary mucin, MUC7 (comprised of 357 residues), possesses antifungal activity that is comparable to or exceeds the antifungal activity of Hsn-5 (26). It was shown that this peptide is effective against wild-type, azole-resistant, and amphotericin B-resistant C. albicans and Cryptococcus neoformans and against Candida glabrata, Candida krusei, and Saccharomyces cerevisiae (29). It was implicated that the MUC7 D1 (MUC7 51-mer) net positive charge played a key role in its antifungal activity (26, 29). Another, much shorter peptide, MUC7 15-mer (amino acids 3 to 17 of MUC7) (Table 1), which was also derived from the MUC7 N terminus and which showed 53.3% sequence similarity to Hsn-5, was found to be at least sixfold less active against C. albicans than MUC7 51-mer (8). Because seven out of eight positively charged amino acid residues present in the rest of the MUC7 D1 sequence are located within its C-terminal 20 residues, we investigated this MUC7 20-mer peptide (amino acids 32 to 51 of MUC7) (Table 1). Indeed, our initial studies showed that MUC7 20-mer displayed fungicidal activities comparable to or better than those of MUC7 51-mer against C. albicans and C. neoformans, two medically important fungal pathogens. In addition, MUC7 20-mer also showed potent bactericidal activities against Streptococcus mutans (cariogenic bacteria) and Streptococcus gordonii (30).
TABLE 1.
Amino acid sequences and charges of peptides under study
| Peptide | Amino acid sequence | Charge
|
||
|---|---|---|---|---|
| + | − | Net | ||
| MUC7 51-mer | EGRERDHELRHRRHHHQSPKSHFELPHYPGLLAHQKPFIRKSYKCLHKRCR | 13 | 5 | +8 |
| MUC7 15-mer | RERDHELRHRRHHHQ | 5 | 3 | +2 |
| MUC7 20-mer | LAHQKPFIRKSYKCLHKRCR | 7 | 0 | +7 |
| Hsn-5 | DSHAKRHHGYKRKFHEKHHSHRGY | 7 | 2 | +5 |
| Ins-A | GIVEQCCASVCSLYQLENYCN | 0 | 2 | −2 |
In the present study, we examined the spectrum of antimicrobial activity of MUC7 20-mer, its secondary structure, and its possible mechanism of antifungal action. We focused on C. albicans and C. neoformans since candidiasis and cryptococcosis, caused by these organisms, are the most common opportunistic infections in immunocompromised patients, especially patients with human immunodeficiency virus or AIDS. MUC7 20-mer, like Hsn-5, is a basic salivary antimicrobial peptide (pI, 10.58). Thus, it was of interest to determine whether MUC7 20-mer and Hsn-5 kill fungi by similar mechanisms. We also examined and compared the dependence of 20-mer and Hsn-5 fungicidal activities on the metabolic state of the cells; the ability of the 20-mer to cross the plasma membrane and accumulate intracellularly; and the effects of temperature, metabolic inhibitors, and divalent cations on 20-mer internalization. Lastly, we studied the possible intracellular target(s) of the 20-mer.
MATERIALS AND METHODS
Materials. (i) Peptides.
Unlabeled MUC7 20-mer and fluorescein isothiocyanate (FITC)-labeled MUC7 20-mer were purchased from Bio-Synthesis Inc., (Lewisville, Tex.). High-pressure liquid chromatography and mass spectrometry were performed by the company to analyze the purity of the peptides. Recombinant Hsn-5 was produced in Escherichia coli by using vector pET-30b(+). The cloning, expression, and purification of this peptide were done as previously described (34). Bovine insulin chain A (Ins-A) (21 amino acid residues) was purchased from Sigma Chemical Co. (St. Louis, Mo.).
(ii) Other materials.
Carbonyl cyanide m-chlorophenylhydrazone (CCCP) and sodium azide were obtained from Sigma. MitoTracker Red CMXRos and MitoTracker Green FM dyes were purchased from Molecular Probes Inc. (Eugene, Oreg.). Sabouraud dextrose agar (SAB) and tryptic soy agar were obtained from Difco Laboratories (Detroit, Mich.).
Yeast and fungal strains and culture conditions.
For the experiments reported in Table 2, the following strains were used. C. albicans strain DIS, a clinical isolate from a patient with denture-induced stomatitis, was provided by M. Edgerton (Department of Oral Biology, University at Buffalo), and an azole-resistant clinical isolate (no. 12-99) of C. albicans was a gift from Theodore C. White (University of Washington and Seattle Biomedical Research Institute, Seattle, Wash.). Azole-sensitive C. glabrata was purchased from ATCC (ATCC 90030), and its azole-resistant counterpart, clinical isolate 65C, was obtained from John E. Bennett (National Institute of Allergy and Infectious Diseases, Bethesda, Md.). A clinical isolate of C. krusei was obtained from the Erie County Medical Center, Buffalo, N.Y. Amphotericin B-sensitive (CN2) and amphotericin B-resistant (CN2843) C. neoformans strains were obtained from AIDS patients with cryptococcal meningitis and were generously provided by John H. Rex (University of Texas Medical School, Houston). Additionally, S. cerevisiae strain S288C was provided by D. Kosman, Department of Biochemistry, University at Buffalo. All were streaked and grown on SAB plates at 37°C, except for S. cerevisiae, which was grown at 30°C, until large colonies formed. One colony was then picked and resuspended in 10 mM sodium phosphate buffer (pH 7.4), and the concentration was adjusted to 105 cells/ml for the antifungal assay described below.
TABLE 2.
ED50s of MUC7 20-mer and Hsn-5a
| Organism | ED50 (95% confidence limit), μM
|
|
|---|---|---|
| MUC7 20-mer | Hsn-5 | |
| Fungi | ||
| C. albicans (DIS) | 5.85 (4.17-8.67) | 6.68 (6.05-7.37) |
| C. glabrata | 5.02 (3.75-8.22) | 38.7 (30.8-50.7) |
| C. krusei | 5.16 (4.17-6.40) | 6.47 (5.79-7.55) |
| C. neoformans (CN2) | 4.05 (3.16-5.81) | 3.71 (1.92-5.60) |
| S. cerevisiae | 5.23 (4.01-6.76) | 74.0 (58.0-101.) |
| C. albicans (azole resistant) | 2.40 (1.73-3.08) | 6.40 (5.59-7.32) |
| C. glabrata (fluconazole resistant) | 12.3 (9.06-17.1) | 85.3 (78.4-94.0) |
| C. neoformans (amphotericin B resistant) | 4.29 (3.59-4.26) | 3.72 (2.90-4.87) |
| Bacteria | ||
| A. actinomycetemcomitansb | 4.37 (3.74-5.04) | >100 |
| E. coli | 1.61 (1.09-2.14) | Not tested |
| P. aeruginosa | 4.41 (3.65-5.19) | Not tested |
| S. mutansc | 1.39 (1.00-1.90) | 92.0 (85.1-99.3) |
| S. gordonii | 2.43 (0.60-6.58) | Not tested |
| P. gingivalis | <1.00 | Not tested |
For more details on organisms, see Materials and Methods. Ins-A showed no antimicrobial activity. Data for Hsn-5 and fungi are from reference 29.
ED50 of streptomycin, 2.7 μM.
ED50 of streptomycin, 64 μM.
For all subsequent experiments, C. albicans (DIS) and C. neoformans (CN2) were used.
Bacterial strains and culture conditions.
The following bacterial strains were tested: S. mutans GS-5, S. gordonii Challis, E. coli HB101, Pseudomonas aeruginosa ATCC 17648, Actinobacillus actinomycetemcomitans NCTC9710, and Porphyromonas gingivalis 381 and W50. S. mutans, S. gordonii, A. actinomycetemcomitans, and P. aeruginosa were grown anaerobically by candle jar extinction at 37°C on tryptic soy agar plates. P. gingivalis was grown in an anaerobic chamber at 37°C on sheep blood agar plates. E. coli was grown aerobically at 37°C on Luria-Bertani agar plates.
Antifungal and antibacterial activity assays.
For killing assays, twofold serial dilutions of each peptide (100 to 1.56 μM) in 20 μl of 10 mM sodium phosphate buffer (pH 7.4) were incubated with equal volumes of bacterial or fungal cells (105 cells/ml in 10 mM sodium phosphate buffer [pH 7.4]) for 1.5 h at 30°C for S. cerevisiae and 37°C for the rest of the organisms tested. As controls, the cells were incubated without the peptides. At the end of the incubation, the samples were diluted 20-fold with the same buffer; aliquots (∼120 cells) of each sample were plated on appropriate plates, depending on the organism tested, as indicated above. Plates were incubated for 1 to 7 days aerobically or anaerobically, depending on the organism tested. Colonies were then counted, and the loss of cell viability was plotted as a function of protein concentration. Fungicidal activities against C. albicans (DIS) and C. neoformans (CN2) were also tested at 4°C.
For assays of the time-dependent killing activity of peptides, C. albicans (DIS) and C. neoformans (CN2) fungal cells (105 cells/ml in 10 mM sodium phosphate buffer [pH 7.4]) were incubated with 6.5 μM MUC7 20-mer or 6.5 μM Hsn-5 (no peptide was added to control reactions). Aliquots of 40 μl were taken at different times; after 20-fold dilution with phosphate-buffered saline to stop the reaction, the aliquots were plated on SAB plates.
To test the effects of inhibitors of oxidative phosphorylation in the mitochondrial system on peptide-induced killing, C. albicans (DIS) and C. neoformans (CN2) cells (105 cells/ml in 10 mM sodium phosphate buffer [pH 7.4]) were preincubated with either 20 mM sodium azide or 300 μM CCCP for 2 h at 37°C. Killing assays were then carried out as described above. In separate control experiments, both inhibitors were evaluated for their toxicity toward C. albicans and C. neoformans in the absence of peptides. At these concentrations, neither compound had any lethal effect.
In addition, the fungicidal activities of MUC7 20-mer (25 μM) and of Hsn-5 (25 μM) in 10 mM sodium phosphate buffer (pH 7.4) in the presence of various concentrations (1 to 50 mM) of Ca2+ (as CaCl2) or Mg2+ (as MgCl2) were examined.
Statistical analysis.
Molar concentrations of peptides required to kill half the maximal number of cells (ED50s) and 95% confidence limits of ED50s were determined by the PROBIT procedure (SPSS software package 6.1.2 for Macintosh). A time course study of peptide-induced killing was statistically analyzed with the Student t test.
Secondary structure prediction.
For secondary structure prediction, we used the PSI Pred graphical viewer from the Brunel Bioinformatics Group of Brunel University (Uxbridge, United Kingdom). The helical wheel projections of the predicted helical region of each peptide were made by using Genetics Computer Group sequence analysis software.
Fluorescence light microscopy studies.
C. albicans (DIS) and C. neoformans (CN2) cells, 107 in 100 μl of 10 mM sodium phosphate buffer (pH 7.4), were treated with 50 μM FITC-MUC7 20-mer for 45 to 90 min at 37 or 4°C. In separate experiments, the cells were first preincubated with 20 mM sodium azide or 300 μM CCCP for 2 h at 37°C and then treated with 50 μM FITC-MUC7 20-mer. In other experiments, the cells were incubated simultaneously in the presence of 50 mM Ca2+ or Mg2+ and 50 μM FITC-MUC7 20-mer. The cells were then extensively washed with the same phosphate buffer, concentrated by centrifugation, and resuspended in the same buffer. The cell suspension was quickly mounted on slides with sealed coverslips. Fluorescence light micrographs were made on a Nikon Optiphot microscope with a fluorescent light source.
Confocal fluorescence microscopy studies.
The intracellular localization of MUC7 20-mer was investigated in a double-labeling experiment with FITC- MUC7 20-mer and a MitoTracker Red CMXRos mitochondrial probe. C. albicans (DIS) and C. neoformans (CN2) cells (107 in 100 μl of 10 mM sodium phosphate buffer [pH 7.4]) were incubated with 500 nM MitoTracker Red CMXRos for 15 min at room temperature, washed three times with the same buffer, and subsequently incubated with 50 μM FITC-MUC7 20-mer. After 20 min of incubation at 37°C, the suspension was washed extensively with phosphate buffer. For control experiments, the same numbers of cells in the same buffer as that described above were incubated with two mitochondion-specific dyes: first, in 500 nM MitoTracker Red CMXRos, and second, in 500 nM MitoTracker Green FM. The cell suspensions (2 μl) were quickly mounted on slides with sealed coverslips. Confocal fluorescence microscopy was performed by using a Bio-Rad MRC-1024 confocal microscope system with a krypton-argon laser, which outputs 488-, 568-, and 647-nm excitation lines, and a Nikon upright epifluorescence microscope (Zeiss ×60 oil immersion planApp objective with a 1.4 numerical aperture).
RESULTS
Antifungal and antibacterial activities of MUC7 20-mer.
MUC7 20-mer (see Table 1 for the sequence) showed potent activity against a variety of fungi (Table 2), including C. albicans, C. glabrata, C. krusei, C. neoformans, and S. cerevisae. In addition, it was also active against several drug-resistant strains, including azole-resistant C. albicans, fluconazole-resistant C. glabrata, and amphotericin B-resistant C. neoformans. Its potency against all fungi tested was comparable to or exceeded that of full-length MUC7 D1 (51-mer; see Table 1 for the sequence) (29). In order to rule out a nonspecific nature of this activity, Ins-A, an unrelated peptide with a similar chain length (21 residues) (Table 1), was assayed. This peptide had no fungicidal activity (data not shown). In comparison to Hsn-5, MUC7 20-mer showed especially high activity against C. glabrata and S. cerevisiae.
Time-dependent killing of C. albicans (DIS) and of C. neoformans (CN2) indicated differences in killing rates between MUC7 20-mer and Hsn-5 (Table 3). For these experiments, 6.5 μM concentrations of MUC7 20-mer and Hsn-5 were used. For C. albicans, both peptide concentrations fall within the ED50s, which are 5.85 μM (range, 4.17 to 8.67) for the MUC7 20-mer and 6.68 μM (range, 6.06 to 7.37) for Hsn-5 (Table 2). For C. neoformans, 6.5 μM concentrations of the peptides used are somewhat higher than their ED50s, which are 4.05 μM (range, 3.16 to 5.8) for the MUC7 20-mer and 3.71 μM (range, 1.92 to 5.60) for Hsn-5 (Table 2). The results indicated that at equal molar concentrations (6.5 μM), MUC7 20-mer kills both C. albicans and C. neoformans faster than Hsn-5 does (P < 0.05) (Table 3).
TABLE 3.
Time-dependent killing activity of MUC7 20-mer and Hsn-5
| Time (min) | % Loss of viability of the following cells in the presence of the indicated agenta:
|
|||
|---|---|---|---|---|
|
C. albicans (DIS)
|
C. neoformans (CN2)
|
|||
| 20-Mer | Hsn-5 | 20-Mer | Hsn-5 | |
| 5 | 31 ± 8 | 0 ± 0 | 97 ± 1 | 9 ± 2 |
| 15 | 42 ± 5 | 1 ± 3 | 97 ± 2 | 43 ± 3 |
| 30 | 52 ± 1 | 11 ± 4 | 96 ± 2 | 59 ± 5 |
| 45 | 64 ± 3 | 34 ± 2 | 98 ± 1 | 64 ± 1 |
| 90 | 74 ± 6 | 42 ± 3 | 100 ± 0 | 71 ± 7 |
Peptide concentration, 6.25 μM. Data are reported as mean and standard deviation. P values for 20-mer versus Hsn-5 were <0.05.
MUC7 20-mer displayed potent antibacterial activity (Table 2) against S. mutans, S. gordonii, E. coli, and P. gingivalis, with ED50s of less than 3 μM, and A. actinomycetemcomitans and P. aeruginosa, with ED50s of less than 5 μM. On the other hand, Hsn-5 had an ED50 of 92 μM against S. mutans and induced only a 24% loss of cell viability at the highest concentration tested (100 μM) against A. actinomycetemcomitans. Streptomycin and Ins-A were used as positive and negative controls, respectively, in the experiments with S. mutans and A. actinomycetemcomitans. No killing was detected with Ins-A. The ED50s of streptomycin against A. actinomycetemcomitans and S. mutans were 2.7 and 64 μM, respectively.
Effects of temperature on MUC7 20-mer-induced killing.
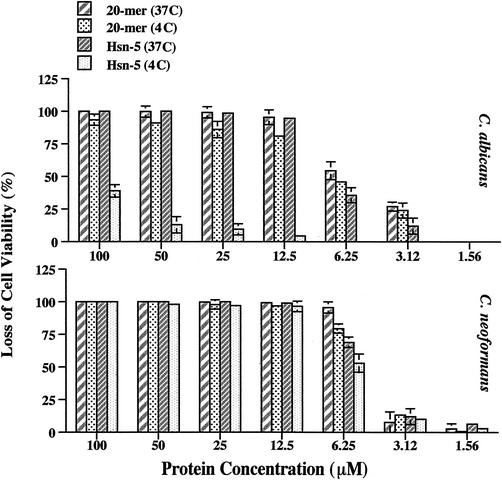
Previous studies reported that no killing of C. albicans by Hsn-5 was detected at 4°C (9) and that C. albicans killing by histatin 3 was highly attenuated at 0°C (37). We performed parallel lethal activity assays at 4°C with Hsn-5 and MUC7 20-mer using C. albicans and C. neoformans. Our results (Fig. 1) confirmed the limited potency of Hsn-5 against C. albicans at 4°C and revealed that MUC7 20-mer sustained almost 90% killing activity against C. albicans. The results also showed that the activities of both MUC7 20-mer and Hsn-5 against C. neoformans at 4°C were barely affected.
FIG. 1.
Effects of temperature on peptide-induced killing of C. albicans (DIS) and C. neoformans (CN2). Fungal cells (2 × 103 in 20 μl of 10 mM sodium phosphate buffer) were incubated with 20 μl of MUC7 20-mer or Hsn-5 peptide (at concentrations ranging from 1.56 to 100 μM in 10 mM sodium phosphate buffer) for 1.5 h at 4 and 37°C. Cell viability was determined by plating on SAB plates. Results represent the mean and standard deviation of duplicate or triplicate experiments.
Effects of CCCP and sodium azide on MUC7 20-mer-induced killing.
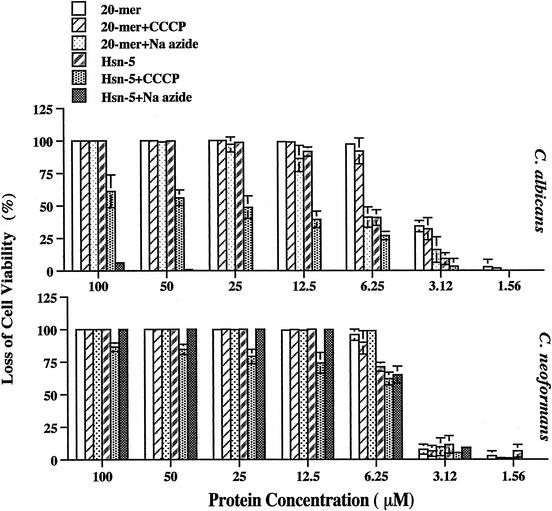
CCCP is an uncoupler of oxidative phosphorylation in the mitochondrial system, and sodium azide inhibits ATP synthesis by inhibiting cytochrome oxidase. It was previously reported that both CCCP and sodium azide were able to protect C. albicans from Hsn-5 killing (10, 16). Our results showed that pretreatment of C. albicans or C. neoformans with CCCP or sodium azide did not protect either organism from killing by MUC7 20-mer (Fig. 2). These results, together with the killing properties at 4°C (Fig. 1), demonstrated that the fungicidal activity of MUC7 20-mer, unlike that of Hsn-5, does not require an active cell metabolic state. Interestingly, though, we found that the killing of C. albicans and C. neoformans by Hsn-5 in the presence of CCCP and sodium azide showed distinct features (Fig. 2)—namely, both CCCP and sodium azide rescued Candida cells from killing by Hsn-5 but were unable to do the same for Cryptococcus cells.
FIG. 2.
Effects of CCCP and sodium azide on peptide-induced killing of C. albicans (DIS) and C. neoformans (CN2). Fungal cells were incubated with 20 mM sodium azide or 300 μM CCCP for 2 h at 37°C. Cells without any pretreatment were used as controls. A 20-μl aliquot (2 × 103 cells) of treated fungal cells was then incubated with 20 μl of MUC7 20-mer or Hsn-5 peptide (1.56 to 100 μM in 10 mM sodium phosphate buffer) for an additional 1.5 h at 37°C. Cell viability was determined by plating on SAB plates. Results represent the mean and standard deviation of duplicate or triplicate experiments.
Effects of divalent cations on MUC7 20-mer-induced killing.
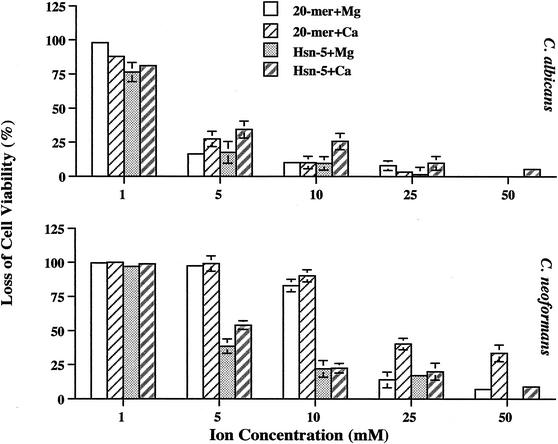
Previous studies showed that divalent cations (Ca2+ and Mg2+) adversely affected the candidacidal activities of human neutrophilic granulocyte defensins (18) and of Hsn-5 (24). Thus, we tested the effects of Ca2+ and Mg2+ on the fungicidal activity of MUC7 20-mer. The addition of 1 mM Ca2+ (as CaCl2) or Mg2+ (as MgCl2) to sodium phosphate buffer (10 mM, pH 7.4) had a limited effect on MUC7 20-mer anticandidal activity (Fig. 3); however, increases in the concentrations of these ions greatly reduced MUC7 20-mer potency. The inhibitory effects of these divalent cations on the antifungal activity of MUC7 20-mer were more pronounced for C. albicans. MUC7 20-mer sustained 90 and 84% killing of C. neoformans in the presence of 10 mM Ca2+ and Mg2+, respectively, while it sustained only 10% anticandidal activity.
FIG. 3.
Effects of cations on peptide-induced killing of C. albicans (DIS) and C. neoformans (CN2). Fungal cells (2 × 103 in 20 μl of 10 mM sodium phosphate buffer) were incubated with 20 μl of MUC7 20-mer or Hsn-5 peptide (final concentrations, 25 μM) in 10 mM sodium phosphate buffer in the presence of either Mg2+ or Ca2+ at concentrations ranging from 1 to 50 mM for 1.5 h at 37°C. Cell viability was determined by plating on SAB plates. Results represent the mean and standard deviation of duplicate or triplicate experiments. At a 25 μM concentration of peptides, without the presence of cations, 100% peptide-induced killing was obtained.
Secondary structure prediction.
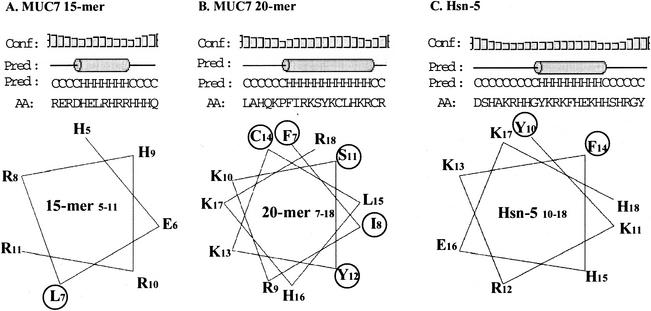
Figure 4 shows the secondary structure prediction for MUC7 20-mer in comparison to those for MUC7 15-mer (histatin-like domain, amino acids 3 to 17 of native MUC7) (Table 1) and Hsn-5 and the helical wheel projections of the predicted helical region of each peptide. As shown at the top of Fig. 4, a certain portion of each peptide sequence was predicted to have a helical structure; the amino acid stretches involved consist of 12 residues out of 20 in MUC7 20-mer, 7 out of 15 in MUC7 15-mer, and 9 out of 24 in Hsn-5. The rest of the sequence exhibits a coil structure. No β-sheet structure was predicted for any of the three peptides. The helical wheel projections of the predicted helical region of each peptide are shown below the secondary structures in Fig. 4. Most antimicrobial peptides exhibit an amphiphilic character. However, neither Hsn-5 nor its active domain (residues 11 to 24) adopts amphiphilic structures (8, 35), and this finding was confirmed by our study. On the other hand, the secondary structure prediction results obtained for MUC7 20-mer indicated that this peptide adopts an amphipathic helix with distinguishable hydrophilic and hydrophobic faces. The hydrophobic face is made up of C14, F7, R18, S11, L15, I8, and Y12, with five hydrophobic residues (C14, F7, S11, I8, and Y12). The hydrophilic face is formed by K10, K17, K13, R9, and H16. We also acquired peptide conformations from far-UV circular dichroism spectra in an aqueous solution and a nonpolar medium (organic solvent trifluoroethanol [TFE] which simulates a membrane environment) (data not shown). The analysis of these spectra indicated that in the aqueous medium, all peptides exist in a random conformation, and that in TFE, they adopt a helical structure; these results are consistent with the secondary structure prediction.
FIG. 4.
Secondary structure predictions and helical wheel projections for MUC7 15-mer, MUC7 20-mer, and Hsn-5. For the secondary structure prediction (shown at the top), a PSI Pred graphical viewer was used. Conf, confidence of prediction; Pred, predicted secondary structure; AA, target sequence. The cylinder denotes helix structure (H), and the line denotes coil structure (C). The helical wheel projections of the predicted helical region of each peptide were made by using Genetics Computer Group sequence analysis software. Hydrophobic residues are displayed in circles.
Internalization and localization of MUC7 20-mer.
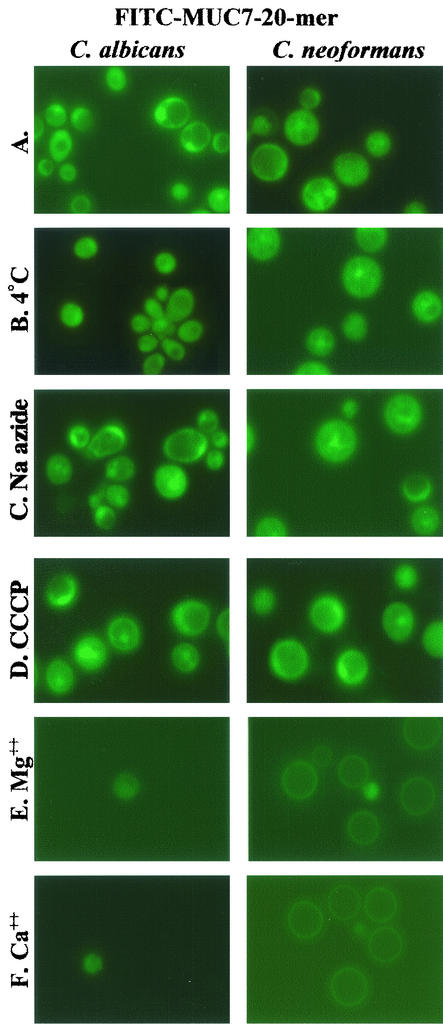
In order to visualize the association of MUC7 20-mer with fungi or bacteria and determine its fungal cellular localization, FITC-labeled MUC7 20-mer was used. The fungicidal activity of FITC-MUC7 20-mer is about 10% lower than that of unlabeled MUC7 20-mer (data not shown). Figure 5 shows the fluorescence light micrographs of fungi (C. albicans and C. neoformans) incubated with FITC-MUC7 20-mer. The fluorescent active peptide was visible on the cell membrane and intracellularly in both Candida and Cryptococcus, indicating that MUC7 20-mer penetrated the cell wall and cell membrane and accumulated inside the cells. A low incubation temperature (4°C) (Fig. 5B) and pretreatment of cells with sodium azide (Fig. 5C) and CCCP (Fig. 5D) did not affect the uptake of MUC7 20-mer. FITC-MUC7 20-mer was seen within all of the cells with a fluorescence intensity similar to that seen in untreated cells. However, the uptake of the peptide was significantly limited in the presence of the divalent cations Mg2+ and Ca2+ (Fig. 5E and F, respectively). FITC-MUC7 20-mer was seen within only a very small number of cells. The fluorescence intensity was also low. An interesting observation was that FITC-MUC7 20-mer was visualized on the circumference of C. neoformans in the presence of cations; this characteristic was not seen with C. albicans, indicating that the binding of the peptide to C. neoformans was affected much less than that to C. albicans. A higher fluorescence signal was detected within C. neoformans cells treated with cations than within C. albicans cells so treated. FITC-labeled peptide was also seen on the cell membrane of and inside A. actinomycetemcomitans cells (data not shown), indicating internalization of MUC7 20-mer in bacterial cells as well.
FIG. 5.
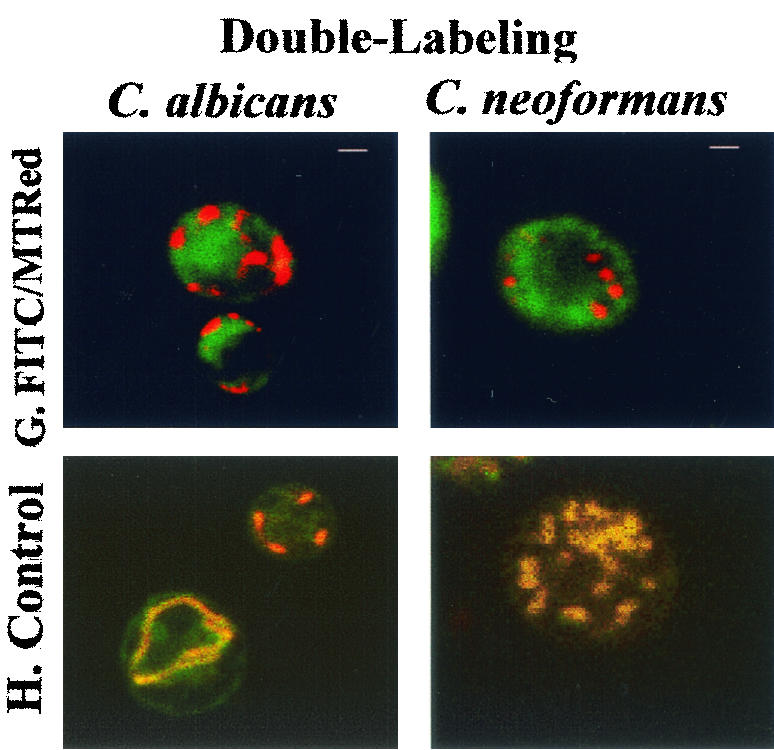
Fluorescence light microscopy and confocal laser microscopy of internalization of FITC-MUC7 20-mer by C. albicans (DIS) and C. neoformans (CN2). (A to F) Fluorescence light microscopy. (G and H) Confocal laser microscopy. (A) 37°C. (B) 4°C. (C) Cells pretreated with 20 mM sodium azide. (D) Cells pretreated with 300 μM CCCP. (E) Cells in the presence of 50 mM Mg2+. (F) Cells in the presence of 50 mM Ca2+. Cells (107) were treated with 50 μM FITC- MUC7 20-mer in 100 μl of sodium phosphate buffer (10 mM, pH 7.4) for 45 to 90 min at 37°C (except for cells in panel B, which were treated at 4°C). Fluorescence light micrographs were made on a Nikon Optiphot microscope with a fluorescent light source. (G and H) Confocal fluorescence microscopy of cells labeled with FITC-MUC7 20-mer and a mitochondrion-specific marker, MitoTracker Red CMXRos (G), or (in a control experiment) with MitoTracker Red CMXRos and MitoTracker Green FM (H). Cells were incubated for 15 min at 37°C with 500 nM MitoTracker Red CMXRos in 10 mM phosphate buffer (pH 7.4), washed twice, and subsequently incubated for 20 min at 37°C with either 50 μM FITC-MUC7 20-mer in 10 mM phosphate buffer (pH 7.4) (G) or 500 nM MitoTracker Green FM (H). Confocal fluorescence microscopy was performed with a Bio-Rad MRC-1024 confocal microscope system. Bars, 1 μM.
In addition, confocal fluorescence microscopy was used to study the intracellular localization of MUC7 20-mer. Double labeling with FITC-MUC7 20-mer and MitoTracker Red CMSRos, a mitochondrion-specific dye, did not show overlapping patterns, suggesting that mitochondria are not the intracellular target of MUC7 20-mer in C. albicans or C. neoformans (Fig. 5G). In the control experiment with two mitochondrion-specific dyes (MitoTracker Red CMSRos and MitoTracker Green FM), both dyes localized to the mitochondria of both fungi, as shown by identical (overlapping) staining patterns (Fig. 5H).
DISCUSSION
Human saliva is fundamentally important in the host innate nonimmune defense system against oral pathogens. Salivary mucins, together with other groups of salivary proteins (proline-rich proteins, cystatins, statherins, and amylase), protect the oral cavity from microbial infections through more general protective mechanisms rather than the direct killing of microorganisms. The antimicrobial effect of mucins has been attributed largely to agglutination activity—trapping and clearing the microorganisms from the system. The selective binding of salivary mucins to microbial adhesins prevents the subsequent attachment of microorganisms to host surfaces (32, 33). Conversely, mucins bound to tissues may serve as docking ports facilitating the colonization of microorganisms such as Streptococcus (7). Thus, salivary mucins play an intriguing, paradoxical role in the dynamics of the oral flora. This study, together with two previous studies (26, 29), further complicates the unique relationship of MUC7 and microbial pathogens because it was shown that MUC7-derived peptides (possibly generated in vivo by proteolytic enzymes) can directly kill bacteria (this study) and fungi (26, 29; this study), making MUC7 a multifaceted, critical component of oral defense mechanisms. Another study (19) showed that the N-terminal region of MUC7 (amino acids 1 to 144) contains a structural determinant for the binding of oral streptococci and exhibits candidacidal activity.
It is important to note that the broad-spectrum antimicrobial activity of MUC7 20-mer reported here is specific, since the control peptide, Ins-A (composed of 21 amino acid residues), showed no lethal activity toward fungi or bacteria. MUC7 20-mer displayed both antifungal and antibacterial activities at micromolar concentrations, and the activities were comparable. Hsn-5 required more than a 10-fold-higher concentration for killing bacteria than for killing fungi (Table 2).
Antimicrobial peptides are classified according to their mode of action as inhibitors of protein biosynthesis, inhibitors of DNA or RNA synthesis, lytic peptides that disrupt the membrane, and inhibitors of microbial cellular metabolism (1, 27, 38). Earlier studies showed that Hsn-5 is taken up into the intracellular space possibly by means of a receptor-mediated mechanism, after which it interacts with an intracellular target, mitochondria (5, 12). A recent study demonstrated that it subsequently inhibits respiration and induces the formation of reactive oxygen species, leading to cell death (13). We have demonstrated, by using lethal assays, metabolic inhibitors, and confocal laser microscopy, that MUC7 20-mer and Hsn-5 may have different targets. Our preliminary data also showed that both peptides cause disturbances of C. albicans and C. neoformans plasma membrane potentials, indicating membranolytic activity (data not shown). FITC-MUC7 20-mer not only was associated with the plasma membrane but also was internalized, suggesting that its mechanism of killing does not involve a direct effect on the membrane alone. Other studies showed that cell membrane permeabilization (depolarization) by itself is not necessarily the crucial event in the killing of microorganisms. To explain this finding, the “aggregate channel model” (of pore formation) has been postulated (11). In this model, the peptides are allowed to cross the membrane through pores formed by the peptides but also have an intracellular target(s) on which to exert their killing activities. MUC7 20-mer may work via such a mechanism.
In contrast to the characteristics of Hsn-5, 20-mer peptide internalization and killing potency were not affected (blocked) by a low temperature or the presence of inhibitors of ATP synthesis (CCCP and sodium azide). These results indicate that its action is not dependent on cellular metabolic activity (respiration). The 20-mer does not target mitochondria, as shown by double labeling and confocal microscopy. In agreement with the characteristics of Hsn-5, internalization and killing were affected by the addition of the divalent cations Ca2+ and Mg2+. We speculate that, as with Hsn-5, the fungicidal activity of the 20-mer may depend on the initial interactions between the positively charged 20-mer molecules and the negatively charged head groups of the yeast cell membrane bilayer. The presence of divalent cations reduces the 20-mer interaction with fungal cells and its internalization. However, binding of the 20-mer to C. neoformans was affected much less than was that to C. albicans in the presence of the cations. These results were evidenced by the detection of a higher fluorescence signal associated with the membrane of C. neoformans than with that of C. albicans. These observations, coupled with the lethal assay results, further suggest that the interaction of the peptide with the cell membrane and the further internalization of the 20-mer are crucial for the 20-mer to exert its fungicidal activity. The dependence of Hsn-5 on Candida cellular metabolic activity to exert killing is quite unique, as evidenced by our results demonstrating that the Hsn-5-induced killing of Cryptococcus and the 20-mer-induced killing of both Candida and Cryptococcus are not dependent on cellular metabolic activity. In addition, a recent study (25) showed that after pretreatment of C. albicans cells with sodium azide (blocking cellular respiration), the uptake of Hsn-5 variant dhvar4 and consequently the killing by dhvar4 were barely affected, and those of variant dhvar5 were affected only moderately.
Differences in the activities of MUC7 20-mer and Hsn-5 are also correlated with their structural differences. We obtained this finding by secondary structure prediction (Fig. 4). The amphiphatic helix is a membrane-binding motif in many antimicrobial proteins and peptides. Both Hsn-5 and MUC7-derived peptides (15-mer and 20-mer) have some degree of helical structure, as indicated by secondary structure predictions (and confirmed by circular dichroism analysis; data not shown). However, the helices that Hsn-5 and MUC7 15-mer adopt are weakly amphipathic, in contrast to that of MUC7 20-mer, which is strongly amphipathic. While many antimicrobial peptides are characterized as having helices, this characteristic is not a prerequisite for activity, as demonstrated by the relative lack of activity of MUC7 15-mer, a peptide with a significant α-helical structure (8). The stronger amphipathicity of MUC7 20-mer may be the key to understanding its potency. Having the polar side chain aligned along one side and the hydrophobic residues aligned along the opposite side of the helical wheel allows an optimal interaction of the peptide with the cell membrane and facilitates the entrance of the peptide into the cytoplasm.
Most natural antimicrobial peptides are also positively charged. The specific role of a positive peptide charge for the interaction with the negatively charged membrane has long been recognized and has been documented by a variety of attempts to induce or to improve antimicrobial activity by charge modification (2, 21). It would also seem that this charge could account for the greater susceptibility of C. neoformans to small cationic peptides, since its unique polysaccharide capsule contributes to its increased negative charge. At a physiologic pH (7.4), MUC7 15-mer contains five positively and three negatively charged residues, so that it carries a net positive charge of 2. MUC7 20-mer contains seven positively and no negatively charged residues, for a net positive charge of 7. Hsn-5 also carries seven positively charged residues but carries two negatively charged residues, which decrease its net positive charge to 5. Here we showed that MUC7 20-mer, which is much more amphipathic than Hsn-5 and which has a higher net positive charge, is also more potent against many of the fungi and bacteria tested (lower ED50s) (Table 2). Other investigators showed that a histatin variant with increased amphipathicity (and net positive charge) had enhanced candidacidal activity (14). It is inferred from these data that amphipathicity and higher charge are characteristics associated with potency. Recent data on the antifungal activity of MUC7 12-mer (12 C-terminal residues of the 20-mer; RKSYKCLHRKCR) also support this inference (L. A. Bobek, S. Mashhoon, and H. Situ, J. Dent. Res., abstr. 1798, 2002). MUC7 12-mer has a net positive charge of 6 (versus 7 in the 20-mer). It also exhibits an amphipathic helix. Its ED50 against C. albicans is 2.2 μM (versus 5.8 μM for the 20-mer). When the first and second positive amino acid residues (R and K) were substituted with A, the ED50 increased to 14.6 μM. This substitution decreases the net positive charge to 4, and even though it adds two more hydrophobic residues, these disrupted the hydrophilic phase of the helix. These results indicate that the amphipathic nature of the peptide and the higher net positive charge are important for activity.
In summary, MUC7 20-mer is potent against a broad range of microorganisms, including fungi, that are resistant to conventional clinically used drugs, and it appears to work via a mechanism distinct from that of Hsn-5. These characteristics are those which would make 20-mer an ideal drug for patients who have developed resistance to conventional antimicrobial agents. It is also possible that 20-mer could act synergistically with other antimicrobial agents in saliva (e.g., Hsn-5), making it an important ingredient of artificial saliva formulations. Finally, at this stage, it is not known whether 20-mer exerts similar antimicrobial activities in vivo. Although some important questions and concerns remain regarding the expression of its activity in physiological and pathological situations, 20-mer represents a prototypic molecule useful for the development of new antimicrobial therapeutic agents.
Acknowledgments
We thank B. Kritzman and C. Smith for help with antimicrobial assays (Table 2), G. Intini for help with secondary structure predictions, and W. Sigurdson and G. Martins for performing confocal microscopy. We also thank G. Wei for preparation of control samples for confocal microscopy. Lastly, we thank C. Smith for reading of the manuscript and valuable additions to the discussion section.
This study was supported by NIH/NIDCR grant DE09820.
REFERENCES
- 1.Bartizal, K., G. Abruzzo, C. Trainor, D. Krupa, K. Nollstadt, D. Schmatz, R. Schwartz, M. Hammond, J. Balkovec, and F. Vanmiddlesworth. 1992. In vitro antifungal activities and in vivo efficacies of 1,3-beta-d-glucan synthesis inhibitors L-671,329, L-646,991, tetrahydroechinocandin B, and L-687,781, a papulacandin. Antimicrob. Agents Chemother. 36:1648-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessalle, R., H. Haas, A. Goria, I. Shalit, and M. Fridkin. 1992. Augmentation of the antibacterial activity of magainin by positive-charge chain extension. Antimicrob. Agents Chemother. 36:313-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Lucca, A. J. 2000. Antifungal peptides: potential candidates for the treatment of fungal infections. Expert Opin. Investig. Drugs 9:273-299. [DOI] [PubMed] [Google Scholar]
- 4.Edgerton, M., S. E. Koshlukova, M. W. Araujo, R. C. Patel, J. Dong, and J. A. Bruenn. 2000. Salivary histatin 5 and human neutrophil defensin 1 kill Candida albicans via shared pathways. Antimicrob. Agents Chemother. 44:3310-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edgerton, M., S. E. Koshlukova, T. E. Lo, B. G. Chrzan, R. M. Straubinger, and P. A. Raj. 1998. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J. Biol. Chem. 273:20438-20447. [DOI] [PubMed] [Google Scholar]
- 6.Feder, R., A. Dagan, and A. Mor. 2000. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 275:4230-4238. [DOI] [PubMed] [Google Scholar]
- 7.Gibbons, R. J., and J. V. Qureshi. 1978. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect. Immun. 22:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gururaja, T. L., J. H. Levine, D. T. Tran, G. A. Naganagowda, K. Ramalingam, N. Ramasubbu, and M. J. Levine. 1999. Candidacidal activity prompted by N-terminus histatin-like domain of human salivary mucin (MUC7). Biochim. Biophys. Acta 1431:107-119. [DOI] [PubMed] [Google Scholar]
- 9.Gyurko, C., U. Lendenmann, M. S. Lamkin, C. Champagne, R. F. Troxler, and F. G. Oppenheim. 1998. Uptake of FITC-labeled human histatin 5 by Candida albicans. J. Dent. Res. 77:286. [Google Scholar]
- 10.Gyurko, C., U. Lendenmann, R. F. Troxler, and F. G. Oppenheim. 2000. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob. Agents Chemother. 44:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmerhorst, E. J., P. Breeuwer, W. van't Hof, E. Walgreen-Weterings, L. C. Oomen, E. C. Veerman, A. V. Amerongen, and T. Abee. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274:7286-7291. [DOI] [PubMed] [Google Scholar]
- 13.Helmerhorst, E. J., R. F. Troxler, and F. G. Oppenheim. 2001. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 98:14637-14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmerhorst, E. J., W. van't Hof, P. Breeuwer, E. C. Veerman, T. Abee, R. F. Troxler, A. V. Amerongen, and F. G. Oppenheim. 2001. Characterization of histatin 5 with respect to amphipathicity, hydrophobicity, and effects on cell and mitochondrial membrane integrity excludes a candidacidal mechanism of pore formation. J. Biol. Chem. 276:5643-5649. [DOI] [PubMed] [Google Scholar]
- 15.Koshlukova, S. E., M. W. Araujo, D. Baev, and M. Edgerton. 2000. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect. Immun. 68:6848-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshlukova, S. E., T. L. Lloyd, M. W. Araujo, and M. Edgerton. 1999. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J. Biol. Chem. 274:18872-18879. [DOI] [PubMed] [Google Scholar]
- 17.Lehrer, R. I., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 18.Lehrer, R. I., T. Ganz, D. Szklarek, and M. E. Selsted. 1988. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J. Clin. Investig. 81:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, B., S. Rayment, C. Gyurko, F. G. Oppenheim, G. D. Offner, and R. F. Troxler. 2000. The recombinant N-terminal region of human salivary mucin MG2 (MUC7) contains a binding domain for oral streptococci and exhibits candidacidal activity. Biochem. J. 345:557-564. [PMC free article] [PubMed] [Google Scholar]
- 20.Mandel, I. D. 1987. The functions of saliva. J. Dent. Res. 66:623-627. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzaki, K., K. Sugishita, M. Harada, N. Fujii, and K. Miyajima. 1997. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1327:119-130. [DOI] [PubMed] [Google Scholar]
- 22.Mor, A., V. H. Nguyen, A. Delfour, D. Migliore-Samour, and P. Nicolas. 1991. Isolation, amino acid sequence, and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry 30:8824-8830. [DOI] [PubMed] [Google Scholar]
- 23.Oppenheim, F. G., T. Xu, F. M. McMillian, S. M. Levitz, R. D. Diamond, G. D. Offner, and R. F. Troxler. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 263:7472-7477. [PubMed] [Google Scholar]
- 24.Patel, R., S. E. Koshlukova, T. L. Lloyd, M. W. Araujo, and M. Edgerton. 1999. Candidacidal activity of salivary Hsts in physiologic inorganic ion buffer. J. Dent. Res. 78:342. [Google Scholar]
- 25.Ruissen, A. L., J. Groenink, E. J. Helmerhorst, E. Walgreen-Weterings, W. van't Hof, E. C. Veerman, and A. V. Nieuw Amerongen. 2001. Effects of histatin 5 and derived peptides on Candida albicans. Biochem. J. 356:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satyanarayana, J., H. Situ, S. Narasimhamurthy, N. Bhayani, L. A. Bobek, and M. J. Levine. 2000. Divergent solid-phase synthesis and candidacidal activity of MUC7 D1, a 51-residue histidine-rich N-terminal domain of human salivary mucin MUC7. J. Pept. Res. 56:275-282. [DOI] [PubMed] [Google Scholar]
- 27.Shai, Y. 1995. Molecular recognition between membrane-spanning polypeptides. Trends Biochem. Sci. 20:460-464. [DOI] [PubMed] [Google Scholar]
- 28.Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1462:55-70. [DOI] [PubMed] [Google Scholar]
- 29.Situ, H., and L. A. Bobek. 2000. In vitro assessment of antifungal therapeutic potential of salivary histatin-5, two variants of histatin-5, and salivary mucin (MUC7) domain 1. Antimicrob. Agents Chemother. 44:1485-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, C. J., and L. A. Bobek. 2001. Bactericidal and fungicidal activity of salivary mucin (MUC7) peptide fragments. J. Dent. Res. 80:601. [Google Scholar]
- 31.Steiner, H., D. Hultmark, A. Engstrom, H. Bennich, and H. G. Boman. 1981. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292:246-248.7019715 [Google Scholar]
- 32.Tabak, L. A. 1995. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu. Rev. Physiol. 57:547-564. [DOI] [PubMed] [Google Scholar]
- 33.Tabak, L. A. 1998. Protein structure and function relationships: mucins, p. 189-197. In B. Guggenheim and S. Shapiro (ed.), Oral biology at the turn of the century (misconceptions, truths, challenges and prospects). S. Karger, Basel, Switzerland.
- 34.Tsai, H., and L. A. Bobek. 1997. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and -resistant Candida species. Antimicrob. Agents Chemother. 41:2224-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van’t Hof, W., E. C. Veerman, E. J. Helmerhorst, and A. V. Amerongen. 2001. Antimicrobial peptides: properties and applicability. Biol. Chem. 382:597-619. [DOI] [PubMed] [Google Scholar]
- 36.Xu, T., S. M. Levitz, R. D. Diamond, and F. G. Oppenheim. 1991. Anticandidal activity of major human salivary histatins. Infect. Immun. 59:2549-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu, Y., I. Ambudkar, H. Yamagishi, W. Swaim, T. J. Walsh, and B. C. O'Connell. 1999. Histatin 3-mediated killing of Candida albicans: effect of extracellular salt concentration on binding and internalization. Antimicrob. Agents Chemother. 43:2256-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota, T. 1997. Kinds of antimicrobial agents and their mode of actions. Nippon Rinsho 55:1155-1160. [PubMed] [Google Scholar]
- 39.Zasloff, M. 1987. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 84:5449-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]