Abstract
Trypanosoma cruzi, the protozoan pathogen that causes Chagas' disease, can be found in the blood of infected individuals for their entire life span. This presents a serious challenge in safeguarding blood products. Transmission of T. cruzi from blood products is a frequent occurrence in Latin America, where Chagas' disease is endemic. This study was designed to determine whether T. cruzi could be inactivated in human platelet concentrates and plasma by a photochemical treatment process with long-wavelength UV A light (UVA, 320 to 400 nm) plus the psoralen amotosalen HCl (Cerus Corporation). Units of platelet concentrates (300 ml) and plasma (300 ml) were intentionally contaminated with approximately 106 T. cruzi trypomastigotes, the T. cruzi form found in the bloodstream, per ml. The viability of T. cruzi after photochemical inactivation was determined by their ability to replicate in 3T3 fibroblasts. Controls, including treatment with 150 μM amotosalen or 3 J/cm2 UVA alone, did not lead to reduction of the viability of T. cruzi in plasma or platelet concentrates. However, treatment with 150 μM amotosalen plus 3 J/cm2 UVA inactivated T. cruzi to undetectable levels in plasma and platelet concentrates. This represented a >5.4-log reduction of T. cruzi in platelet concentrates and >5.0-log reduction of T. cruzi in plasma. We conclude that the amotosalen plus UVA photochemical inactivation technology is effective in inactivating high levels of protozoan pathogens, such as T. cruzi, in platelet concentrates and plasma, as has been previously shown for numerous viruses and bacteria.
Trypanosoma cruzi is a vector-borne protozoan pathogen that causes Chagas' disease, which is responsible for serious morbidity and mortality. Once infection is established, therapy for T. cruzi is difficult; available drugs are toxic, and a minimum of 90 days of treatment is required to achieve cure. Even under optimal treatment conditions, a cure is achieved in less than 50% of cases (8). The World Health Organization estimates that 18 to 20 million people in Latin America are infected with T. cruzi (27). T. cruzi establishes a life-long blood and tissue infection (3). Because of this, T. cruzi remains a serious threat to the public blood supply (26a).
T. cruzi infection by blood transfusion frequently occurs in areas of Latin America where blood products are not screened for T. cruzi antibodies (21; Wendel et al., abstr. 271, 1992). The risk of acquiring T. cruzi infection from a blood product that is seropositive is estimated to be between 12 and 25% (1). The World Health Organization and Pan American Health Organization have aggressively promoted screening all blood products for T. cruzi by serology; however, logistical difficulties have interfered with universal implementation in Latin America (21). To date, United States blood banks have not implemented screening for T. cruzi serologies. The immigration of Latin Americans into the United States ensures that there is a risk of transfusion infection with T. cruzi (6, 10-12, 22). Indeed, at least six documented transfusion-associated T. cruzi infections have occurred in the United States and Canada (5, 7, 24), and probably many more undocumented transfusion-associated infections have occurred.
Long-wavelength UV A light (UVA, 320 to 400 nm) plus psoralen (amotosalen HCl) photochemical treatment (Cerus Corporation) has been shown to be effective in inactivation of high levels of a broad spectrum of bacteria and viruses in therapeutic units of human plasma and platelet concentrates (9, 14). Amotosalen is a nucleic acid-targeting reagent that intercalates and forms cross-links into the double helix or single-strand hairpin loops of nucleic acids (DNA and RNA). When illuminated with UVA, it forms covalent monoadducts with pyrimidine bases on a single strand of nucleic acid. Additional illumination causes the monoadduct to link with a second pyrimidine base, producing an inter- or intrastrand crosslink and thus disabling the transcription and replication processes. The technology is simple to implement and could eventually substitute for extensive testing of blood products for viral and bacterial contamination.
Amotosalen plus UVA treatment of platelets does not alter platelet function in in vitro tests, including: platelet count, morphology, ATP secretion, hypotonic shock response, platelet shape change, platelet aggregation, and platelet activation by p-selectin expression (13, 14). The amotosalen plus UVA photoinactivation technology has received a CE mark (a symbol that shows that the goods meet the European Union’s product safety standards) in Europe, and the regulatory approval process in the United States has been initiated. This technology has not previously been tested for inactivation of blood-borne trypanosomatid infections, such as T. cruzi, Trypanosoma brucei spp. (agents of African sleeping sickness), or Leishmania spp.
We report here that UVA plus amotosalen treatment of human platelet concentrates and plasma is extremely effective in inactivating T. cruzi and thus should protect patients from T. cruzi infection transmitted by blood products.
MATERIALS AND METHODS
T. cruzi Tulahuen strain was used for these experiments (19). T. cruzi trypomastigotes used for these experiments were grown on BALB/c mouse NIH clone A31 3T3 fibroblast cells (CCL 163, American Type Culture Collection) in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, l-glutamine, penicillin, and streptomycin (culture medium). Trypomastigotes were collected from tissue culture supernatant by centrifugation, counted with a hemacytometer, and resuspended in normal saline.
The process of photochemical inactivation with amotosalen plus UVA (Helinx Technology) has been developed by Cerus Corporation, Concord, Calif. Approximately 3 × 109 (range, 2.1 × 108 to 1.2 × 1010) T. cruzi trypomastigotes in 6 ml of normal saline were added to either two pooled, ABO-matched single-donor units of platelet concentrates (approximately 570 ml) or approximately 570 ml of pooled random donor plasma. Platelet concentrates were collected on an Amicus Cell Separator at the Puget Sound Blood Center (Seattle, Wash.) and suspended in approximately 65% normal saline and 35% autologous plasma. Plasma was obtained as fresh frozen plasma, collected in acid citrate dextrose solution, from the Sacramento Blood Center (Sacramento, Calif.). An aliquot of the saline suspension was diluted at the same ratio in culture medium to serve as a control for the effects of platelet concentrates or plasma alone on the viability of the organisms.
After mixing, the platelet concentrates or plasma was divided into two 285-ml aliquots in PL 2410 illumination containers (Baxter Healthcare Corporation). One container was used as a control without amotosalen, and 15 ml of sterile saline, in lieu of amotosalen, was added to this container. The second (test) container was sterilely connected to one line of a pouch containing 15 ml of 3 mM amotosalen in saline. The other line of the amotosalen pouch was sterilely connected to an empty 1-liter PL 2410 illumination container, forming a chain. The infected platelet concentrate or plasma mixture was passed back and forth through the amotosalen container three times to ensure adequate mixing, ending in a PL 2410 illumination container (final amotosalen concentration was approximately 150 μM).
Samples were taken from both the control container and the amotosalen-treated container immediately after mixing and after each UVA illumination (1, 2, and 3 J/cm2). The plasma or platelet concentrates spiked with T. cruzi were illuminated in serial 1-J/cm2 increments for a total UVA treatment of 1, 2, or 3 J/cm2. Treatment was performed on a UVA light apparatus that provided 320- to 400-nm long-wavelength UVA light above and below the containers while shaking them continuously (Fenwal model FX-1019; Baxter Healthcare Corporation). The control units were not actually illuminated but were sampled in parallel with the test units to demonstrate stability of the organism in the blood component. After the 3-J/cm2 treatment sample was taken, each control unit received a single 3-J/cm2 UVA treatment, which served as a control for the effect of UVA without amotosalen. As an additional control, plasma samples containing T. cruzi were removed from the control unit and exposed to a 137cesium source delivering 440 cGy/min of gamma irradiation, for a total of 2,500 cGy.
To assay the viability of trypomastigotes, samples were diluted in culture medium, either 1:3 or serially 1:10, through 10−7. One tenth milliliter of each dilution was transferred to each of five replicate wells of a 96-well tissue culture plate seeded 18 h previously with 1,000 3T3 cells in 0.1 ml of culture medium. An inverted microscope was used to examine the microtiter plates for the presence of live trypomastigotes every 3 to 4 days. Wells were scored as positive for growth if T. cruzi trypomastigotes were present any time during the 7 to 28 days following plating.
This study consisted of four replicate independent inactivation experiments in platelet concentrates and an additional four replicates in plasma. For each replicate, the method developed by Reed and Muench was used to estimate the 50% tissue culture infectious dose (TCID50, the dilution at which 50% of the wells are infected) from the numbers of positive and negative wells at each dilution (18). The level of T. cruzi inactivation was determined by subtracting the TCID50 after illumination from the TCID50 of the control unit sampled concurrently. Results for the study of each blood component were calculated as the mean and standard deviation of the four replicate experiments.
RESULTS
T. cruzi trypomastigotes were spiked into human platelet concentrates or plasma to titers of approximately 106/ml. Half of the material was treated with UVA plus the psoralen amotosalen, and half was reserved for controls.
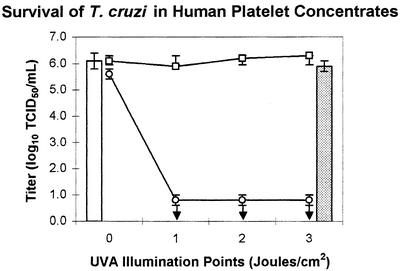
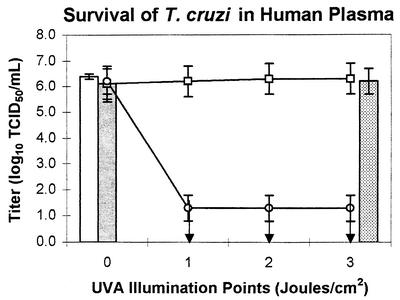
Tables 1 and 2 contain the results, expressed as log10 TCID50 of trypomastigotes in the various samples, and Fig. 1 and 2 graphically depict the log reduction of viable organisms. Exposure of T. cruzi to human platelet concentrates or plasma alone (control samples) had little effect on viability of T. cruzi over the course of the experiment, as evidenced by the mean titer of the control samples at the various illumination points, which varied by less than 0.5 log. The viability of T. cruzi was not significantly affected by a 3-J/cm2 UVA treatment in the absence of amotosalen (control samples with 3-J/cm2UVA illumination) or by amotosalen in the absence of UVA (0-J/cm2 test samples). In both cases, the mean T. cruzi titer varied by less than 0.5 log from the titer of organisms in culture medium alone.
TABLE 1.
Inactivation of T. cruzi in human platelet concentrates by amotosalen and UVA
| Condition | Mean titer (log10 TCID50/ml) ± SD (n = 4)
|
|||
|---|---|---|---|---|
| Preillumination | UVA (J/cm2)
|
|||
| 1 | 2 | 3 | ||
| T. cruzi in medium only | 6.1 ± 0.3 | |||
| Untreated controla | 6.1 ± 0.4 | 5.9 ± 0.6 | 6.2 ± 0.5 | 6.2 ± 0.1 |
| UVA-only control | 5.9 ± 0.1 | |||
| Amotosalen-only control | 5.6 ± 0.2 | |||
| Treated test titer (with amotosalen) | <0.8 ± 0.2 | <0.8 ± 0.2 | ≤0.8 ± 0.2b | |
| Log inactivation | >5.1 ± 0.6 | >5.4 ± 0.5 | ≥5.4 ± 0.1 | |
Without amotosalen or UVA. Controls were not actually exposed to the UVA indicated but were sampled at these points in parallel with the illuminated test samples.
In one of the four replicates, one sample yielded a positive well in an unexpected location. This was likely the product of cross-contamination from an adjacent control well with a high titer of T. cruzi.
TABLE 2.
Inactivation of T. cruzi in human plasma by amotosalen and UVA or by gamma irradiation
| Condition | Mean titer (log10 TCID50/ml) ± SD (n = 4)
|
|||
|---|---|---|---|---|
| Preillumination | UVA (J/cm2)
|
|||
| 1 | 2 | 3 | ||
| T. cruzi in medium only | 6.1 ± 0.5a | |||
| Untreated controlb | 6.1 ± 0.5 | 6.2 ± 0.8 | 6.3 ± 0.8 | 6.3 ± 0.6 |
| UVA-only control | 6.1 ± 0.2 | |||
| Amotosalen-only control | 6.2 ± 0.5 | |||
| Treated test titer (with amotosalen) | <1.3 ± 0.5 | <1.3 ± 0.5 | <1.3 ± 0.5 | |
| Log inactivation | >4.9 ± 0.8 | >5.0 ± 0.8 | >5.0 ± 0.6 | |
| Gamma irradiatedc | 6.1 ± 0.7 | |||
Three replicates. The fourth replicate had a titer of >7.
Without amotosalen or UVA. Controls were not actually exposed to the UVA indicated but were sampled at these points in parallel with the illuminated test samples.
Dose, 2,500 cGy, two replicates.
FIG. 1.
T. cruzi in medium only (open bar) and after 3-J/cm2 UVA only (shaded bar). After the untreated control unit had been sampled at all illumination points, it was exposed to a single 3-J/cm2 UVA illumination (open squares). These samples were not exposed to UVA, but the unit was sampled at the indicated time points in parallel with the treated test unit (open circles). Arrows indicate that the T. cruzi titer was below the level of detection. Platelet units were pooled, inoculated with T. cruzi, and then split to generate the test and control units.
FIG. 2.
T. cruzi in medium only (open bar), after gamma irradiation (stippled bar), and after 3-J/cm2 UVA only (shaded bar). After the untreated control unit had been sampled at all illumination points, it was exposed to a single 3-J/cm2 UVA illumination (open squares). These samples were not exposed to UVA, but the unit was sampled at the indicated time points in parallel with the treated test unit (open circles). Arrows indicate that the T. cruzi titer was below the level of detection. Platelet units were pooled, inoculated with T. cruzi, and then split to generate the test and control units.
Treatment of T. cruzi with 150 μM amotosalen and as little as 1 J/cm2 of UVA led to complete abrogation of the ability of T. cruzi to infect 3T3 fibroblasts. The limit of detection of <1.3 log10 TCID50/ml (plasma) or <0.8 log10 TCID50/ml (platelets) was governed by the amount of plasma or platelet concentrates that could be plated without interfering with the viability of the 3T3 fibroblasts. No T. cruzi were detected in any of the samples treated with 150 μM amotosalen and 1, 2, or 3 J/cm2 UVA illumination. The initial titer of T. cruzi was 6.1 ± 0.5 log10 TCID50/ml in plasma and 6.1 ± 0.4 log10 TCID50/ml in platelet concentrates (Table 1 and Fig. 1).
Photochemical treatment with amotosalen and UVA resulted in inactivation of >5.0 ± 0.6 logs of T. cruzi in plasma and inactivation of ≥5.4 ± 0.1 logs of T. cruzi in platelet concentrates (Table 2 and Fig. 2). In one replicate of the inactivation studies in platelet concentrates, a single infected well was found in an unexpected location in the dilution series for one sample. Based on the location of the well, it is most likely that the positive result was due to cross-contamination from an adjacent control well with a high titer of T. cruzi. However, because this cannot be demonstrated to be the case, the data were analyzed as if there truly were a single surviving organism in that one sample. This is why the titer in platelets after the 3-J/cm2 UVA treatment is expressed as “greater than or equal to” instead of simply “greater than.”
To compare photochemical treatment with amotosalen and UVA to an inactivation process currently used on blood products, two experiments were performed in which plasma spiked with T. cruzi was exposed to 2,500 cGy of γ-irradiation, the dose of γ-irradiation commonly used to inactivate leukocytes prior to transfusion. In sharp contrast to the UVA plus amotosalen treatment results, γ-irradiation did not lead to a significant reduction of T. cruzi titers: 6.1 log10 TCID50/ml after γ-irradiation, compared to 6.2 ± 0.5 log10 TCID50/ml in the 0-J/cm2 control (Table 2 and Fig. 2).
DISCUSSION
The UVA and amotosalen photochemical treatment method was assessed for its ability to inactivate blood-borne protozoan pathogens. This technology has been shown to inactivate many viral and bacterial pathogens (9, 14), but inactivation of blood-borne parasites has not previously been evaluated. This UVA and amotosalen photochemical treatment is currently under review for approval in Europe, and the regulatory approval process in the United States has been initiated.
To assess the efficacy of the photochemical treatment process for inactivation of trypanosomatid parasites, we chose T. cruzi because it poses a serious problem for transfusion medicine. Most seropositive individuals have lifelong circulating T. cruzi, usually without overt symptoms (3, 15, 25, 26a). The risk of transmission from a seropositive blood product is estimated to be between 12 and 25% (26a). We tested the extracellular trypomastigote form of T. cruzi because they have the potential to contaminate plasma or platelet concentrates due to their small size and motility. The photochemical treatment process was able to inactivate T. cruzi to below the limit of detection in platelet concentrates and plasma, an average of >5.3 log10 TCID50/ml and >4.9 log10 TCID50/ml, respectively. Since the titer of T. cruzi in the blood of chronically infected individuals has been estimated to be only about 5 parasites/ml (3), this technique appears to have sufficient capacity to inactivate all of the T. cruzi that would be found in a plasma or platelet unit from a chronically infected donor.
T cruzi inoculation of immunocompromised mice is another sensitive method to detect T. cruzi that could have been used in these studies. The 3T3 fibroblast tissue culture system used in this report can detect as few as 1 to 2 T. cruzi trypomastigotes per tissue culture well (unpublished data); this method is as sensitive as inoculation of immunocompromised mice. The failure of the 2,500-cGy dose of γ-irradiation, which is commonly used to inactivate leukocytes in blood products, to significantly reduce T. cruzi titers indicates that current U.S. blood-processing procedures do not address potential contamination with this pathogen, underscoring the potential of the photochemical treatment process.
Though the exact numbers are not known, it is estimated that thousands of persons in Latin America are infected annually with T. cruzi as a result of transfusion with contaminated blood products (20). Testing for anti-T. cruzi antibodies with at least two different serological tests and discarding positive units, can generally prevent T. cruzi infection from blood products (20). Although serologic testing of blood products is performed in many areas of Latin America, there are still areas of Latin America where testing has not been implemented and transmission occurs unabated (20, 21). The probability of receiving an infected blood unit in Latin America has been estimated to be as high as 1 in 10 in Bolivia and as low as 1 in 50,000 in the seven countries with universal serological testing (20). In Bolivia, where the T. cruzi seropositive rate in blood donors is 25%, screening has not been implemented because there simply would not be enough blood available if all units seropositive for T. cruzi were discarded (20). The amotosalen and UVA photochemical treatment process could inactivate T. cruzi in these blood products, rendering them safer for transfusion.
Testing of blood products for antibodies to T. cruzi has not been implemented in the United States and Canada. Estimates of the risk to the U.S. and Canadian blood supply vary widely. Over seven million people who originated from countries where Chagas' disease is endemic reside in the United States, and it is estimated that over 100,000 people are asymptomatically infected with T. cruzi in the United States and Canada (8, 20). Screening of blood donors in Los Angeles County (California) has demonstrated T. cruzi seropositivity rates of 1 in 500 (22). However, screening of 100,019 blood donors in the southwestern United States identified only three donors who were repeatedly seropositive (10). In addition, look-back studies of 11,430 cardiac surgery patients who received massive transfusions in the United States and Canada failed to demonstrate transmission of T. cruzi (12), yet transmission of T. cruzi via infected blood products does occur in the United States and Canada; at least six cases of transmission of T. cruzi infection by blood product transfusion have been documented (5, 7, 24). Clearly, treating blood products with the photochemical treatment process could reduce, if not eliminate, this risk.
Gentian violet treatment of blood products to inactivate T. cruzi has been used in endemic areas since the 1950s (16). This process is poorly accepted by patients because of discoloration of the skin and because of its uncertain safety. In addition, gentian violet may not inactivate other agents that contaminate transfused blood. Other pathogen inactivation systems under evaluation for platelet concentrates and plasma include dimethylene blue and light (23, 26), riboflavin and light (24), and an ethylene amine derivative (V.I. Technologies, Inc., Watertown, Mass.) (4). In addition, methods for inactivating pathogens in packed red blood cells are under development. These include S-303 (Cerus Corporation) and dimethylene blue and Inactine (24). A recent presentation suggested that T. cruzi was inactivated by an ethylene amine derivative treatment of contaminated red blood cells (17).
In this publication, we have demonstrated that amotosalen and UVA photochemical treatment effectively inactivates high levels of T. cruzi in contaminated plasma and platelet concentrates to below the level of detection, >5.0 log10 TCID50/ml for plasma and >5.4 log10 TCID50/ml for platelets. This suggests that amotosalen and UVA photochemical treatment will prevent transfusion-associated T. cruzi infection in addition to providing protection from other viral and bacterial transfusion-associated infections.
Acknowledgments
We acknowledge Frederick S. Buckner, University of Washington, for advice and T. cruzi parasites, Lynette Sawyer, Cerus Corporation, for editorial assistance, and the assistance of Baxter Healthcare Corporation, the Puget Sound Blood Center, and the Sacramento Blood Center.
Financial support for these studies was provided by Cerus Corporation and Baxter Healthcare Corporation.
REFERENCES
- 1.Avila, H. L., J. B. Pereira, O. Thiemann, W. Degrave, C. M. Morel, and L. Simpson. 1993. Detection of Trypanosoma cruzi in blood specimens of chronic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J. Clin. Microbiol. 31:2421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brener, Z. 1992. Trypanosoma cruzi: taxonomy, morphology, and life cycle, p. 13-29. In S. Wendel, Z. Brener, M. E. Carmago, and A. Rassi (ed.), Chagas’ disease (American trypanosomiasis): its impact on transfusion and clinical medicine. ISBT, Sao Paulo, Brazil.
- 3.Centurion-Lara, A., L. K. Barrett, and W. C. Van Voorhis. 1994. Quantitation of parasitemia by competitive polymerase chain reaction amplification of parasite kDNA minicircles during chronic infection with Trypanosoma cruzi. J. Infect. Dis. 170:1334-1339. [DOI] [PubMed] [Google Scholar]
- 4.Chapman, J. 2000. Progress in improving the pathogen safety of red cell concentrates. Vox Sang. 78(Suppl. 2):203-204. [PubMed] [Google Scholar]
- 5.Cimo, P. L., W. E. Luper, and M. A. Scouros. 1993. Transfusion-associated Chagas' disease in Texas: report of a case. Tex. Med. 89:48-50. [PubMed] [Google Scholar]
- 6.Galel, S. A., and L. V. Kirchhoff. 1996. Risk factors for Trypanosoma cruzi infection in California blood donors. Transfusion 36:227-231. [DOI] [PubMed] [Google Scholar]
- 7.Grant, I. H., J. W. Gold, M. Wittner, H. B. Tanowitz, C. Nathan, K. Mayer, L. Reich, N. Wollner, L. Steinherz, and F. Ghavimi. 1989. Transfusion-associated acute Chagas’ disease acquired in the United States. Ann. Intern. Med. 111:849-851. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhoff, L. V. 1993. American trypanosomiasis (Chagas' disease) — a tropical disease now in the United States. N. Engl. J. Med. 329:639-644. [DOI] [PubMed] [Google Scholar]
- 9.Knutson, F., R. Alfonso, K. Dupuis, V. Mayaudon, L. Lin, L. Corash, and C. F. Hogman. 2000. Photochemical inactivation of bacteria and HIV in buffy-coat-derived platelet concentrates under conditions that preserve in vitro platelet function. Vox Sang. 78:209-216. [DOI] [PubMed] [Google Scholar]
- 10.Leiby, D. A., M. H. Fucci, and R. J. Stumpf. 1999. Trypanosoma cruzi in a low- to moderate-risk blood donor population: seroprevalence and possible congenital transmission. Transfusion 39:310-315. [DOI] [PubMed] [Google Scholar]
- 11.Leiby, D. A., E. J. Read, B. A. Lenes, A. J. Yund, R. J. Stumpf, L. V. Kirchhoff, and R. Y. Dodd. 1997. Seroepidemiology of Trypanosoma cruzi, etiologic agent of Chagas' disease, in US blood donors. J. Infect. Dis. 176:1047-1052. [DOI] [PubMed] [Google Scholar]
- 12.Leiby, D. A., F. J. Rentas, K. E. Nelson, V. A. Stambolis, P. M. Ness, C. Parnis, H. A. McAllister, Jr., D. H. Yawn, R. J. Stumpf, and L. V. Kirchhoff. 2000. Evidence of Trypanosoma cruzi infection (Chagas' disease) among patients undergoing cardiac surgery. Circulation 102:2978-2982. [DOI] [PubMed] [Google Scholar]
- 13.Lin, L., R. Alfonso, B. Behrman, L. Corten, P. B. Damonte, R. Dikeman, K. Dupuis, D. Hei, C. Y. Lin, H. F. Londe, K. Metchette, T. Phan, A. A. Reames, M. Rheinschmidt, A. Savoor, J. Tessman, and L. Corash. 1998. Photochemical treatment of platelet concentrates with a novel psoralen and UVA to enhance the safety of platelet transfusions. Infusther. Tranfsusmed. 25:39-48. [Google Scholar]
- 14.Lin, L., D. N. Cook, G. P. Wiesehahn, R. Alfonso, B. Behrman, G. D. Cimino, L. Corten, P. B. Damonte, R. Dikeman, K. Dupuis, Y. M. Fang, C. V. Hanson, J. E. Hearst, C. Y. Lin, H. F. Londe, K. Metchette, A. T. Nerio, J. T. Pu, A. A. Reames, M. Rheinschmidt, J. Tessman, S. T. Isaacs, S. Wollowitz, and L. Corash. 1997. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion 37:423-435. [DOI] [PubMed] [Google Scholar]
- 15.Moser, D. R., L. V. Kirchhoff, and J. E. Donelson. 1989. Detection of Trypanosoma cruzi by DNA amplification with the polymerase chain reaction. J. Clin. Microbiol. 27:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nussenzweig, V., R. Sonntag, A. Biancalana, J. L. Frietas, V. Amato-Neto, and J. Kloetzel. 1953. Action of certain dyes on T. cruzi in vitro. The use of gentian violet to prevent the transmission of Chagas' disease by blood transfusion. Hospital (Rio de Janeiro) 44:731-744. [PubMed] [Google Scholar]
- 17.Pereira, M. C., D. Serbryanik, A. Purmal, M. Jorge, and V. Zavizion. 2002. Inactivation of Virulent Trypanosoma cruzi trypomastigotes by the Inactine Process. Transfusion 41(S):87S-88S.
- 18.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 19.Reed, S. G. 1988. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infection. J. Immunol. 140:4342-4347. [PubMed] [Google Scholar]
- 20.Schmunis, G. A. 1999. Prevention of transfusional Trypanosoma cruzi infection in Latin America. Mem. Inst. Oswaldo Cruz 94(Suppl. 1):93-101. [DOI] [PubMed] [Google Scholar]
- 21.Schmunis, G. A., F. Zicker, J. R. Cruz, and P. Cuchi. 2001. Safety of blood supply for infectious diseases in Latin American countries, 1994-1997. Am. J. Trop. Med. Hyg. 65:924-930. [DOI] [PubMed] [Google Scholar]
- 22.Shulman, I. A., M. D. Appleman, S. Saxena, A. L. Hiti, and L. V. Kirchhoff. 1997. Specific antibodies to Trypanosoma cruzi among blood donors in Los Angeles, California. Transfusion 37:727-731. [DOI] [PubMed] [Google Scholar]
- 23.Skripchenko, A. A., and S. J. Wagner. 2000. Inactivation of WBCs in RBC suspensions by photoactive phenothiazine dyes: comparison of dimethylmethylene blue and MB. Transfusion 40:968-975. [DOI] [PubMed] [Google Scholar]
- 24.Snyder, E. L., and R. Y. Dodd. 2001. Reducing the risk of blood transfusion. Hematology. Am. Soc. Hematol. Educ. Program 433-442. [DOI] [PubMed]
- 25.Sturm, N., W. Degrave, C. Morel, and L. Simpson. 1989. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplastid minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol. Biochem. Parasitol. 33:205-214. [DOI] [PubMed] [Google Scholar]
- 26.Wagner, S. J., A. Skripchenko, D. Robinette, D. A. Mallory, and L. Cincotta. 1998. Preservation of red cell properties after virucidal phototreatment with dimethylmethylene blue. Transfusion 38:729-737. [DOI] [PubMed] [Google Scholar]
- 26a.Wendel, S., Z. Brener, M. E. Camargo, and A. Rassi (ed.). 1992. Chagas’ disease (American trypanosomiasis): its impact on transfusion and clinical medicine. International Society for Blood Transfusion, Sociedade Brasilera de Hematologia e Hemoterapia, Sao Paulo, Brazil.
- 27.World Health Organization. 1991. Control of Chagas' disease. World Health Organization Tech. Rep. Ser. 811:1-93. [PubMed] [Google Scholar]