Abstract
Rabbit neutrophil peptide-1 (NP-1), a prototypic α-defensin, protects cells in vitro from infection by clinical and laboratory isolates of herpes simplex virus type 2 (HSV-2). Incubation of concentrated virus stocks for 1 h with noncytotoxic concentrations of NP-1 reduces subsequent infection by >98%. Pretreating cells with NP-1 for 1 h prior to inoculation with untreated virus also prevents infection. NP-1, a cationic peptide, does not compete with viral envelope glycoproteins for binding to cellular heparan sulfate receptors, but it prevents viral entry. No VP16, a major viral tegument protein, is transported to the cell nucleus in the presence of NP-1. Infectious center assays demonstrate that NP-1 also inhibits cell-to-cell viral spread. Thus, NP-1 prevents virally mediated fusion events, entry, and cell-to-cell spread. This unique mechanism of anti-HSV activity, coupled with established antibacterial and possible anti-human immunodeficiency virus type 1 activities of defensins, render this family of compounds excellent candidates for further development as topical microbicides.
Novel strategies to prevent sexually transmitted infections (STIs) are urgently needed. While barrier methods remain the most effective protection against transmission of STI-causing pathogens, this modality generally requires male partner initiation and/or consent. In contrast, topical microbicides for vaginal or rectal application that are active at the mucosal surface could provide at-risk individuals with methods that can be self-initiated.
Earlier approaches to topical microbicide development focused on spermicidal detergents, especially nonoxynol-9 (N-9)—the most commonly used contraceptive worldwide. Although N-9 was initially considered safe, it is highly cytotoxic to primary human cervical or vaginal epithelial cells in vitro (13) and in vivo (5). In a recently completed clinical trial, women who used N-9 gel became infected with human immunodeficiency virus (HIV) at a 50% higher rate than women who used a placebo gel, and they had a significantly higher incidence of inflammatory lesions than the placebo group (5). This experience highlights the need for novel candidate topical microbicides and for their rigorous preclinical evaluation.
Defensins are endogenous, cysteine-rich, 3- to 4-kDa cationic peptides that exert antimicrobial activity through membrane permeabilization (11, 42, 43). Three subfamilies of defensins exist among mammals. In humans, α-defensins are found in neutrophils (19), T and B lymphocytes (1), Paneth cells of the small intestine (24), and portions of the female genital tract (29). In rabbits, but not in humans, α-defensins are also prominent in pulmonary alveolar macrophages (10).
β-Defensins are produced by many, if not all, human epithelial cells, and their expression can be constitutive, induced, or both. At least four different β-defensins are expressed by humans (18), and numerous additional β-defensin genes were recently identified in the human genome (32). Circular minidefensins constitute the third mammalian defensin subfamily and were first delineated in the leukocytes and bone marrow of rhesus monkeys (20, 38, 40). These minidefensins are cyclic octadecapeptides with three intramolecular cysteine disulfide bonds, and they can protect cells in vitro from infection by HIV type 1 (HIV-1) (7).
Although certain human and rabbit α-defensins (8, 17) have been shown to inactivate herpes simplex virus (HSV) and other enveloped viruses, their mechanism of antiviral activity is unknown. Possibly, the insertion of defensins into the viral lipid envelope interferes with its function in ways analogous to their effects on bacterial cell membranes. Defensins may also play a role in the degradation and inactivation of phagocytosed virions (41). Recent studies suggest that human α-defensins contribute to the anti-HIV-1 activity found in CD8 cytotoxic T-lymphocyte supernatants (44).
Genital herpes is the most common cause of genital ulceration in the developed world. Seroepidemiologic studies suggest that one of five persons aged 12 or above nationwide has already been infected with HSV type 2 (HSV-2) (9), and an estimated 500,000 new cases of herpes occur in the United States each year (9, 35). Although both HSV-1 and HSV-2 can cause genital herpes, HSV-2 predominates and causes intermittent lifelong recurrences.
NP-1 is a prototypic rabbit α-defensin that is abundant in rabbit granulocytes (polymorphonuclear [PMN] cells) and rabbit alveolar macrophages. NP-1 was initially purified from rabbit alveolar macrophages (25, 34), and it was first called MCP-1 (macrophage cationic peptide-1). Later sequencing proved that NP-1 from rabbit PMNs (33) and MCP-1 were identical. These studies were undertaken to explore the mechanism of NP-1's activity against HSV-2.
MATERIALS AND METHODS
Peptide and other reagents.
Lyophilized NP-1 was prepared from sterile peritoneal exudates of rabbits as previously described (33), lyophilized, and stored at −80°C until used. The sequence of NP-1 is VVCACRRALCLPRERRAGFCRIRGRIHPLCCRR (33, 34). Stock solution was prepared by dissolving the lyophilized peptide in sterile 0.1% acetic acid in distilled water at a final concentration of 1 mg/ml and it was stored at −20°C. Prior to each experiment, the stock solution was dissolved in phosphate-buffered saline (PBS) to a final concentration ranging from 0.25 to 250 μg/ml. Control buffer consisted of a solution of 0.1% acetic acid in distilled water similarly diluted in PBS. Heparin and N-9 were purchased from Sigma (St. Louis, Mo.). Acyclovir was obtained from Glaxo-Wellcome (Research Triangle Park, N.C.). HSV-2 recombinant glycoprotein B (gB-2) was generated using the Bac-to-Bac system (Gibco) and purified by heparin-affinity chromatography. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining confirmed purity (14).
Vero (monkey kidney epithelial) and CaSki (human cervical epithelial) cells were obtained from the American Type Culture Collection and grown as previously described (14, 16). Three viral strains were laboratory-adapted isolates: HSV-1(17), HSV-2(333), and HSV-2(G). The MMA and MTWTA strains were clinical isolates from STD clinic patients and have been partially sequenced (39). The DT-1 (acyclovir-susceptible) and DT-2 (acyclovir-resistant) strains were isolated from a neonate (23).
Antiviral activity of NP-1.
NP-1 (10 to 100 μg/ml) or its peptide-free vehicle was incubated with concentrated stocks of HSV-2 (∼108 PFU/ml) for 1 h at 37°C. The mixtures were then diluted ∼10,000-fold to yield noninhibitory peptide levels (≤0.01 μg/ml), and duplicate aliquots containing 100 to 500 PFU/well were inoculated onto Vero cell monolayers. Alternatively, cells that had been preincubated with 25 μg of NP-1/ml or vehicle for 1 h at 37°C were either washed extensively or left alone prior to inoculating them with virus. Plaques were quantified 48 h postinfection.
Time course assays.
Confluent monolayers of cells were cooled to 4°C and inoculated with ∼200 to 1,000 PFU of virus/well at 4°C for 3 h. After unbound virus was removed by washing the cells three times, the cultures were temperature shifted to 37°C for 1 h to permit penetration. After inactivating any nonpenetrant viruses by washing the monolayer with a citrate buffer, pH 3.0 (12, 31), fresh medium was added and plaques were counted 48 h postinfection. As further described below, NP-1 or its vehicle was added (i) during the 4°C binding period (t = 0 h); (ii) when cells were shifted to 37°C (t = 3 h); or (iii) at select times post-citrate treatment to determine whether the compound inhibited infection if present during these time windows. Additionally, to determine if the drug could remove bound virus after the 4°C adsorption period, in some experiments the cells were washed thrice with 25 μg of NP-1/ml (5 min, 5 min, and 1 h) before being temperature shifted.
Binding studies.
To determine if NP-1 inhibited binding of the viral envelope or the envelope glycoprotein B (gB-2) to cells, binding studies with purified HSV-2 or recombinant gB-2 were conducted as previously described (6). CaSki cells were exposed to serial dilutions of purified HSV-2 for 5 h at 4°C or to recombinant gB-2 for 1 h at 37°C in the absence or presence of NP-1 or heparin as a control. After the binding period, unbound virus or gB-2 and the peptide were removed by washing the cells extensively. Cell-bound virus or glycoprotein was analyzed by Western blotting of cell lysates with anti-gD or anti-gB monoclonal antibodies (Abs), respectively (antibody 1103 for virus and antibody 1123 for recombinant gB; Goodwin Institute Plantation). Blots were subsequently incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) and developed using the ECL kit (DuPont, Boston, Mass.) as previously described (28). Western blots were scanned and analyzed using the GELDOC 2000 Bio-Rad system linked to an IBM PC, and the relative number of viral particles or quantity of gB-2 was determined.
VP16 indirect immunofluorescence.
The ability of VP16, a viral tegument protein, to translocate to the host cell nucleus was examined as described previously (27). Concentrated stocks of virus (∼109 PFU/ml) were preincubated with 25 μg of NP-1/ml, 0.1% acetic acid, or 100 μg of acyclovir/ml for 1 h at 37°C. The mixture was diluted and used to infect Vero cells on glass coverslips at a multiplicity of infection (MOI) of 100 PFU/cell. Four hours postinfection, cells were washed, fixed in 0.5% paraformaldehyde, and permeabilized with 100% acetone. The cells were blocked overnight with 10% goat serum and 1% bovine serum albumin in PBS, then incubated with a 1:50 dilution of anti-HSV monoclonal Ab (mouse anti-VP16; Santa Cruz Biotechnology), washed extensively, and subsequently incubated with a 1:500 dilution of a goat anti-mouse Ab conjugated to fluorescein isothiocyanate (FITC; Calbiochem). Cells were preserved overnight in a 0.1% solution of Mowoil (Sigma) with 2.5% 1,4-diazabicyclo-octane (Sigma) as an antibleaching agent and sealed under a coverslip with nail polish. Cells were visualized under a Zeiss Axioskop fluorescence microscope.
Infectious center assay.
Infectious center assays were performed as previously described (12, 30). Briefly, Vero cells (donor cells) were exposed to virus at 37°C to allow entry (MOI of 3 to 5 PFU/cell). Cells were washed with citrate buffer (pH 3.0) to inactivate residual extracellular virus 1 to 2 h after infection. Then, 4 to 5 h after infection, the infected cells were detached with trypsin-EDTA and counted, and ∼100 cells were plated onto duplicate monolayers of uninfected cells in medium containing pooled human IgG (Sigma) in the presence of NP-1 or control buffer. The pooled human immunoglobulin neutralizes infection by virus released into the medium. Plaques were quantified after 48 h by using a black plaque immunoassay (15).
Cytotoxicity assays.
The cytotoxic effects of NP-1 compared with that of N-9 on Vero or CaSki cells were determined by quantifying cell viability using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay (Cell Titer 96; Promega). Cytotoxicity was examined following 1, 4, 8.5, or 20 h of exposure (acute) or a 2-h daily exposure for 7 consecutive days (chronic) to serial dilutions of NP-1, N-9, or 0.1% acetic acid buffer.
Statistical analysis.
The data presented are means with standard deviations. Differences between groups were compared by using the Mann-Whitney test, as indicated below in the figure legends.
RESULTS
NP-1 inhibits HSV-1 and HSV-2 infection.
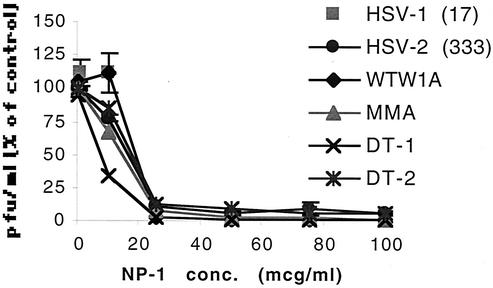
Preincubating concentrated stocks of laboratory or clinical isolates of HSV-1 and HSV-2 with NP-1 followed by dilution by 10,000-fold significantly inhibited viral infection (Fig. 1). The antiviral activity appeared similar for all strains evaluated, including an acyclovir-resistant clinical isolate, DT-2. These results suggest that NP-1 irreversibly inactivates HSV. In a clinical setting, microbicides will be applied to mucosal surfaces prior to viral challenge. To simulate this scenario, cells were treated with NP-1 for 1 h and then challenged with virus. At a concentration of 25 μg/ml, NP-1 reduced HSV-2 infection by 98.7% ± 0.7%. However, if the cells were extensively washed prior to viral inoculation, the inhibitory effects were reduced and infection was inhibited by 45.5% ± 1.6%. The observation that pretreatment of cells with NP-1 followed by extensive washing still partially inhibits infection suggests that the defensin may interact with or persist locally on cell surfaces. This is consistent with in vivo immunohistochemical findings in a rabbit syphilitic orchitis model (3, 4).
FIG. 1.
Direct inactivation of virus by NP-1. Laboratory and clinical isolates of HSV (at a concentration of ∼108 PFU/ml) were mixed with increasing concentrations of NP-1 (or 0.1% acetic acid diluted in PBS for control) and incubated at 37°C for 1 h. The virus-drug mixture was then diluted to yield 100 to 500 PFU/ml and noninhibitory concentrations of drug prior to infecting cells. Plaques were quantified 48 h postinfection. Results are presented as PFU formed in the presence of NP-1 as a percentage of PFU formed in the presence of control buffer. Each point is the mean of two independent experiments performed in duplicate; error bars indicate standard deviations. Isolates include the laboratory strains HSV-1(17) and HSV-2(333) and the clinical isolates WTW1A, MMA, DT-1 (acyclovir susceptible), and DT-2 (acyclovir resistant).
Antiviral activity of NP-1 is greatest if added prior to viral penetration.
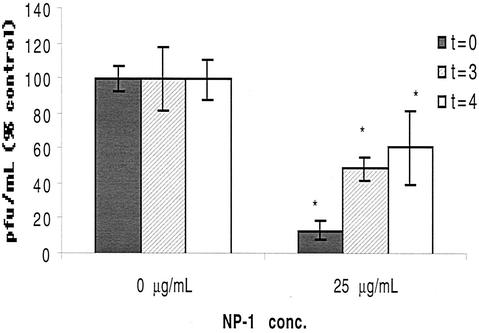
We further explored how NP-1 inhibits HSV infection with time course studies. Viral attachment can be experimentally separated from entry by temperature. Cells were precooled to 4°C and exposed to virus for 3 h to allow viral attachment. Unbound virus and drug were removed by washing, and cells were shifted to 37°C to allow penetration. After a 1-h penetration period, cells were washed with citrate buffer to inactivate extracellular virus and fresh medium was added. NP-1 or control acetic acid buffer was added initially (t = 0 h), at the time of temperature shift (t = 3 h), or 1 h post-citrate treatment (i.e., t = 4 h). Results are depicted in Fig. 2. NP-1 inhibited ∼90% of viral infection when added at t = 0 h. An antiviral effect persisted, albeit reduced, if the peptide was added at the time of temperature shift or even 1 h postentry. At later time points (3 and 7 h post-citrate treatment), there was no reduction in viral plaque numbers but there was a marked diminution in plaque size, suggesting that the peptide might be preventing cell-to-cell spread (data not shown).
FIG. 2.
Antiviral activity of NP-1 added at different times during viral infection. Cells were cooled to 4°C and inoculated with HSV-2(G) in the presence of control buffer or NP-1 added at different times as described in Materials and Methods. Shown are the means of two experiments, each performed in triplicate; error bars indicate standard deviations. Results are presented as PFU formed in the presence of NP-1 as a percentage of PFU formed in control wells. At each time point, the effects of NP-1 were compared to those of control medium by using the Mann-Whitney test; asterisks indicate a significant reduction in viral infection (P < 0.004).
One possibility to explain a postbinding effect of NP-1 could be that the peptide removes bound virus. We assessed this by washing the cells thrice (5 min, 5 min, and 1 h) at 4°C with the peptide after the binding period but prior to shifting the plates to 37°C. Such washing (with 25 μg/ml) reduced viral infection by only 15% ± 8.3%, suggesting that removal of bound virus did not account for the postbinding antiviral activity. These results suggested that NP-1 acted primarily on extracellular virus, but they did not indicate how.
Effect of NP-1 on binding.
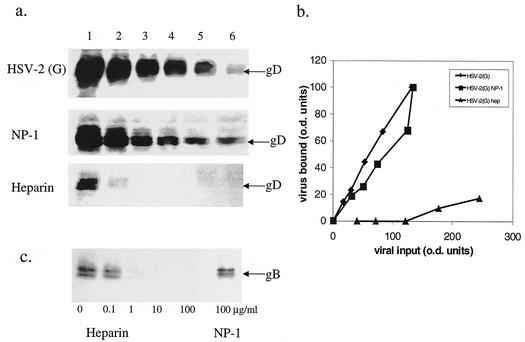
We speculated that NP-1 might prevent HSV-2 infection by competing with gB-2 for its heparan sulfate receptor sites on the cell plasma membrane. gB-2 plays the key role in mediating binding of HSV-2 to cells and is also required for viral entry (6). To assess this possibility, we examined the effects of NP-1 on viral and recombinant gB-2 binding to cells. NP-1 failed to inhibit the binding of virus or gB-2 to CaSki cells, whereas heparin completely abolished viral and glycoprotein binding (Fig. 3). Thus, NP-1 does not competitively inhibit viral binding.
FIG. 3.
Effects of NP-1 and heparin on binding of HSV-2 or recombinant gB-2 to cells. (a) Cells were exposed to twofold dilutions of purified HSV-2(G) (equivalent to an MOI of ∼1, 0.5, 0.25, 0.12, 0.06, and 0.03 PFU/cell; lanes 1 to 6, respectively) for 5 h at 4°C in the absence (upper gel) or presence of 25 μg of NP-1/ml (middle gel) or 100 μg of heparin/ml (lower gel). After washing away unbound virus, cell lysates were prepared and separated by SDS-PAGE, and cell-bound virus was visualized by Western blotting with anti-gD monoclonal Ab. The gels are representative of at least two independent experiments. (b) The relative particle numbers of viral input and bound virus were quantified by densitometry scanning of the Western blots of equal portions of input and bound virus. (c) Alternatively, cells were exposed to recombinant gB-2 (10−5 μg/cell) for 1 h at 37°C in the absence or presence of the indicated concentrations of heparin or 100 μg of NP-1/ml, and the bound gB-2 was compared by Western blotting of cell lysates probed with anti-gB monoclonal Ab.
NP-1 prevents translocation of VP16 to the cell nucleus.
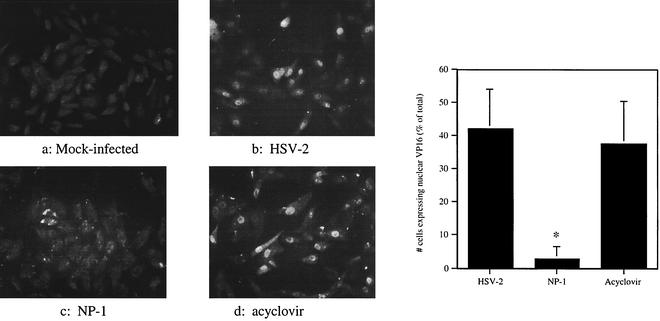
The ability of VP16, a viral tegument protein, to translocate to the nucleus is one of the earliest detectable measures of viral entry (27). To assess the effects of NP-1 on VP16 translocation, cells were mock infected or infected with HSV-2 that had been preincubated for 1 h with NP-1, acetic acid buffer control, or acyclovir, a known inhibitor of DNA polymerase. After 4 h, cells were fixed and the number of cells with immuno-detectable VP-16 in the nucleus was quantified by fluorescence microscopy. As shown in Fig. 4, NP-1, but not acyclovir, prevents VP16 from being translocated to the nucleus, suggesting that NP-1 blocks viral entry. Thus, NP-1 evidently acts to prevent an early postbinding step, perhaps fusion of the viral envelope and cell membrane.
FIG. 4.
Effect of NP-1 or acyclovir on VP-16 translocation to the host cell nucleus. Cells were mock infected (a) or inoculated with HSV-2(G) that had been preincubated for 1 h at 37°C with 0.1% acetic acid (b), 25 μg of NP-1/ml (c), or 100 μg of acyclovir/ml (d). After 4 h at 37°C, cells were fixed, permeabilized, and incubated with mouse monoclonal anti-VP16 Ab, followed by incubation with goat anti-mouse IgG conjugated to FITC. Cells were visualized using a Zeiss Axioskop fluorescence microscope. The number of cells expressing VP16 in the nucleus as a percentage of the total cells visualized per high-power field were counted in four to eight fields per experiment. The graph depicts the mean ± standard deviation obtained from three to four independent experiments; the asterisk indicates a significant reduction in VP16 translocation, by the Mann-Whitney test (P < 0.0001).
Inhibition of cell-cell spread.
The observation that the addition of NP-1 postinfection resulted in a reduction in viral plaque size suggested that NP-1 might also prevent another fusion event, the spread of virus from infected cells to neighboring uninfected cells. To explore this possibility, infectious center assays were conducted. Uninfected cells were cocultured with ∼100 infected cells in the presence of pooled immunoglobulin. Under these experimental conditions, HSV spreads presumably via intercellular junctions created by local fusion events between the plasma membrane of infected and neighboring uninfected cells, but released virus is neutralized by the IgG. The number and size of plaques formed in the presence of NP-1 or control buffer were compared. NP-1 reduced the number of plaques by 59.0% ± 16.6% compared to control acetic acid buffer. Moreover, infectious centers formed in the presence of NP-1 were significantly smaller than control infectious centers (Fig. 5). These results suggest that NP-1 prevents cell-to-cell spread of virus and would be effective at inhibiting transmission of cell-associated HSV.
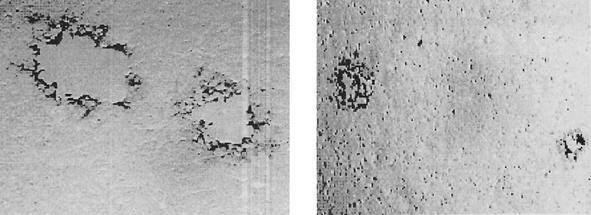
FIG. 5.
Effect of NP-1 on cell-to-cell spread. Vero cells were infected with HSV-2(333) at 37°C to allow viral entry and treated with citrate buffer 2 h after infection to inactivate residual extracellular virus. Five hours after infection, cells were trypsinized, counted, and plated onto monolayers of uninfected cells in the presence of pooled human immunoglobulin to neutralize released virus and 0.1% acetic acid (left) or 25 μg of NP-1/ml (right). After 48 h plaques were visualized by immunostaining.
NP-1 exhibits little cytotoxicity.
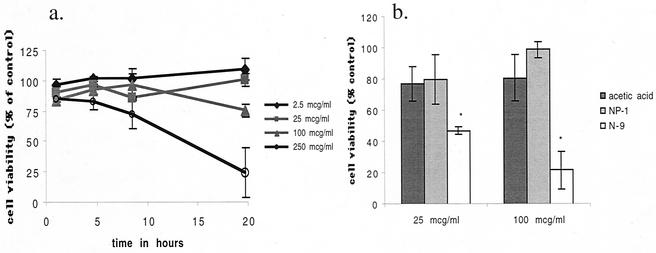
To further explore the potential use of NP-1 as a topical agent, we compared cell viability following a single exposure to NP-1 at different concentrations and for increasing time periods. The results are summarized in Fig. 6a. No cytotoxic effect was observed even after 20 h of exposure to NP-1 at the concentration of 25 or 100 μg/ml, concentrations that largely abolish HSV infection. Cytotoxicity was observed following exposure of cells to 250 μg of NP-1/ml, but only after a 10-h exposure. Moreover, no toxicity was observed in the setting of repeated exposure of CaSki cells to NP-1 (2 h/day for 7 days) (Fig. 6b). In contrast, repeated exposure of CaSki cells to the same concentrations of N-9 was significantly cytotoxic (Fig. 6b). In addition, it has been previously reported that a single exposure to N-9 (100 μg/ml or 0.01% [vol/vol]) for 1 h is cytotoxic (13).
FIG. 6.
Cells were incubated for increasing lengths of time in the presence of the indicated concentrations of NP-1. Results are presented as cell viability in the presence of drug as a percentage of cell viability in the absence of compound and are means ± standard deviations of two independent experiments performed in triplicate. To assess cytotoxicity following chronic exposure to NP-1 or N-9, cells were exposed to 25- or 100-μg/ml concentrations of each (or the equivalent amount of acetic acid buffer) for 2 h each day for 7 consecutive days. Results are presented as cell viability in the presence of drug as a percentage of cell viability in the presence of medium and are means ± standard deviations of quadruplicates. Asterisks indicate a P value of <0.05 (Mann-Whitney).
DISCUSSION
Harnessing defensins, natural antimicrobial peptides, as topical microbicides to prevent the transmission of STIs offers distinct advantages. An ideal topical microbicide would be effective against multiple pathogens, including HIV, HSVs, Chlamydia trachomatis, Neisseria gonorrhoeae, and Treponema pallidum. Although each of these pathogens has been found to be susceptible to one or more defensin-like peptides, at present no one host defense peptide is known to be active against all of these agents. The α-defensin used in these studies, rabbit NP-1, is active against T. pallidum, but not against N. gonorrhoeae (4). Although the activity of rabbit NP-1 against HIV-1 has not been described, a recent study demonstrated that human α-defensins 1, 2, and 3 have anti-HIV activity and may be a component of CD8 antiviral factor, the anti-HIV activity secreted by CD8 T cells from HIV-infected long-term nonprogressors (44). In addition, certain circular (θ) minidefensins afford substantial protection to human cells challenged by M- and T-tropic HIV-1 strains in vitro (7).
Results obtained in these studies show that NP-1 inactivates HSV and prevents viral entry and cell-to-cell spread. NP-1 does not prevent viral binding but, rather, blocks fusion events important in HSV acquisition and transmission. NP-1 prevents entry of cell-free virus, as evidenced by inhibition of VP16 translocation to the cell nucleus, and prevents cell-to-cell spread, as evidenced by a reduction in plaque size when NP-1 was present in the culture medium postentry and in infectious center assays. Inhibition of cell-to-cell spread is important for topical microbicide development because a source of sexual acquisition also may be cell-associated virus. Following infection of a cell, HSV spreads to neighboring cells via intercellular junctions created by local fusion events between the plasma membranes of infected and uninfected cells. Presumably, NP-1 prevents these fusion events by targeting the membrane of the infected and/or uninfected cell.
The observation that NP-1 inhibits viral entry independent of any effects on viral attachment makes this peptide distinct from most other compounds in development as topical microbicides. Most candidate microbicides are detergents, surfactants, or polyanionic compounds (2, 12, 15, 26). Among the candidate agents presently being evaluated are the detergents SDS and C31G and the sulfated polymers dextran sulfate, carageenan, polystyrene sulfonate, cellulose sulfate, and PRO 2000 (a naphthalene sulfonate polymer). Preliminary studies suggest that these sulfated (or sulfonated) polymers competitively inhibit binding of HSV to cell surface heparan sulfate (12).
The observation that a peptide produced by neutrophils might exhibit antiviral activity is consistent with observations suggesting that neutrophils play an important role in the initial response to genital herpes. Studies in mice have shown that large numbers of PMN cells infiltrate the vaginal mucosa within 24 h following vaginal HSV-2 infection (21, 22). Neutrophil depletion of the mice prior to vaginal inoculation resulted in significantly enhanced local viral replication, suggesting that PMNs play a key role in HSV clearance. The exact mechanism by which neutrophils help clear HSV is unknown; however, murine neutrophils completely lack α-defensins. Consequently, the beneficial effects of neutrophils observed in this murine model are more likely attributable to phagocytosis of free virus or virus-infected cells, release of antiviral cytokines, antibody-dependent cell-mediated cytotoxicity, and/or as-yet-unidentified factors.
A potential role of defensins in the host response to vaginal viral infection is suggested by the recent finding that human β-defensin-1 (HBD-1) is especially prominent within the female reproductive tract. In situ hybridization localized the HBD-1 mRNA to the epithelial layers of the vagina, ectocervix, endocervix, uterus, and fallopian tubes (36, 37). Moreover, the α-defensin HD-5 has also been identified in the upper genital tract of women.
The results obtained warrant further exploration of endogenous host defense peptides as candidate microbicides. Because these peptides generally have a rapid onset of action and exhibit little or no cytotoxicity at therapeutic concentrations, they have potential for topical vaginal and rectal application. The unique mechanism of anti-HSV activity of NP-1 also suggests that it may be useful alone, or in combination with other candidate agents, in the development of multitargeted combination therapies to prevent STIs.
Acknowledgments
This work was supported by Public Health Service grants AI 37940, AI 22839, and AI 37945.
REFERENCES
- 1.Agerberth, B., J. Charo, J. Werr, B. Olsson, F. Idali, L. Lindbom, R. Kiessling, H. Jornvall, H. Wigzell, and G. H. Gudmundsson. 2000. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96:3086-3093. [PubMed] [Google Scholar]
- 2.Baba, M., M. Nakajima, D. Schols, R. Pauwels, J. Balzarini, and E. De Clercq. 1988. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antivir. Res. 9:335-343. [DOI] [PubMed] [Google Scholar]
- 3.Borenstein, L. A., T. Ganz, S. Sell, R. I. Lehrer, and J. N. Miller. 1991. Contribution of rabbit leukocyte defensins to the host response in experimental syphilis. Infect. Immun. 59:1368-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borenstein, L. A., M. E. Selsted, R. I. Lehrer, and J. N. Miller. 1991. Antimicrobial activity of rabbit leukocyte defensins against Treponema pallidum subsp. pallidum. Infect. Immun. 59:1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2000. CDC statement on study results of product containing nonoxynol-9. JAMA 284:1376.. [DOI] [PubMed] [Google Scholar]
- 6.Cheshenko, N., and B. C. Herold. 2002. Glycoprotein B plays predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-cell spread. J. Gen. Virol. 83:2247-2252. [DOI] [PubMed] [Google Scholar]
- 7.Cole, A. M., T. Hong, L. M. Boo, T. Nguyen, C. Zhao, G. Bristol, J. A. Zack, A. J. Waring, O. O. Yang, and R. I. Lehrer. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daher, K. A., M. E. Selsted, and R. I. Lehrer. 1986. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 10.Ganz, T., M. P. Sherman, M. E. Selsted, and R. I. Lehrer. 1985. Newborn rabbit alveolar macrophages are deficient in two microbicidal cationic peptides, MCP-1 and MCP-2. Am. Rev. Respir. Dis. 132:901-904. [DOI] [PubMed] [Google Scholar]
- 11.Gundmundsson, G. H., and B. Agerberth. 1999. Neutrophil antibacterial peptides, multifunctional effector molecules in the mammalian immune system. J. Immunol. Methods 232:45-54. [DOI] [PubMed] [Google Scholar]
- 12.Herold, B. C., N. Bourne, D. Marcellino, R. Kirkpatrick, D. M. Strauss, L. J. Zaneveld, D. P. Waller, R. A. Anderson, C. J. Chany, B. J. Barham, L. R. Stanberry, and M. D. Cooper. 2000. Poly(sodium 4-styrene sulfonate): an effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. J. Infect. Dis. 181:770-773. [DOI] [PubMed] [Google Scholar]
- 13.Herold, B. C., R. Kirkpatrick, D. Marcellino, A. Travelstead, V. Pilipenko, H. Krasa, J. Bremer, L. J. Dong, and M. D. Cooper. 1999. Bile salts: natural detergents for the prevention of sexually transmitted diseases. Antimicrob. Agents Chemother. 43:745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold, B. C., I. Scordi-Bello, N. Cheshenko, D. Marcellino, et al. 2002. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J. Virol. 76:11236-11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 41:2776-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold, B. C., D. WuDunn, N. Soltys, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrer, R. I., K. Daher, T. Ganz, and M. E. Selsted. 1985. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J. Virol. 54:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehrer, R. I., and T. Ganz. 2002. Defensins of vertebrate animals. Curr. Opin. Immunol. 14:96-102. [DOI] [PubMed] [Google Scholar]
- 19.Lehrer, R. I., A. K. Lichtenstein, and T. Ganz. 1993. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 11:105-128. [DOI] [PubMed] [Google Scholar]
- 20.Leonova, L., V. N. Kokryakov, G. Aleshina, T. Hong, T. Nguyen, C. Zhao, A. J. Waring, and R. I. Lehrer. 2001. Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 70:461-464. [PubMed] [Google Scholar]
- 21.Milligan, G. N. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73:6380-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milligan, G. N., N. Bourne, and K. L. Dudley. 2001. Role of polymorphonuclear leukocytes in resolution of HSV-2 infection of the mouse vagina. J. Reprod. Immunol. 49:49-65. [DOI] [PubMed] [Google Scholar]
- 23.Oram, R. J., D. Marcellino, D. Strauss, E. Gustafson, C. L. Talarico, A. K. Root, P. L. Sharma, K. Thompson, J. D. Fingeroth, C. Crumpacker, and B. C. Herold. 2000. Characterization of an acyclovir-resistant herpes simplex virus type 2 strain isolated from a premature neonate. J. Infect. Dis. 181:1458-1461. [DOI] [PubMed] [Google Scholar]
- 24.Oullette, A. J., and C. L. Bevins. 2001. Paneth cell defensins and innate immunity of the small bowel. Inflamm. Bowel Dis. 7:43-50. [DOI] [PubMed] [Google Scholar]
- 25.Patterson-Delafield, J., D. Szklarek, R. J. Martinez, and R. I. Lehrer. 1981. Microbicidal cationic proteins of rabbit alveolar macrophages: amino acid composition and functional attributes. Infect. Immun. 31:723-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce-Pratt, R., and D. M. Phillips. 1996. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of human immunodeficiency virus-1. Biol. Reprod. 54:173-182. [DOI] [PubMed] [Google Scholar]
- 27.Pomeranz, L. E., and J. A. Blaho. 1999. Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection. J. Virol. 73:6769-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qie, L., D. Marcellino, and B. C. Herold. 1999. Herpes simplex virus entry is associated with tyrosine phosphorylation of cellular proteins. Virology 256:220-227. [DOI] [PubMed] [Google Scholar]
- 29.Quayle, A. J., E. M. Porter, A. A. Nussbaum, Y. M. Wang, C. Brabec, K. P. Yip, and S. C. Mok. 1998. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am. J. Pathol. 152:1247-1258. [PMC free article] [PubMed] [Google Scholar]
- 30.Roller, R. J., and B. C. Herold. 1997. Characterization of a BHK(TK−) cell clone resistant to postattachment entry by herpes simplex virus types 1 and 2. J. Virol. 71:5805-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roller, R. J., and D. Rauch. 1998. Herpesvirus entry mediator HVEM mediates cell-cell spread in BHK(TK−) cell clones. J. Virol. 72:1411-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schutte, B. C., J. P. Mitros, J. A. Bartlett, J. D. Walters, H. P. Jia, M. J. Welsh, T. L. Casavant, and P. B. McCray, Jr. 2002. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. USA 99:2129-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selsted, M. E., D. M. Brown, R. J. DeLange, S. S. Harwig, and R. I. Lehrer. 1985. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J. Biol. Chem. 260:4579-4584. [PubMed] [Google Scholar]
- 34.Selsted, M. E., D. M. Brown, R. J. DeLange, and R. I. Lehrer. 1983. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J. Biol. Chem. 258:14485-14489. [PubMed] [Google Scholar]
- 35.Service, G. S. 1996. Sexually transmitted diseases, England 1995: new cases seen at NHS genito-urinary medicine clinics. HM Stationery Office, London, England.
- 36.Svinarich, D. M., R. Gomez, and R. Romero. 1997. Detection of human defensins in the placenta. Am. J. Reprod. Immunol. 38:252-255. [DOI] [PubMed] [Google Scholar]
- 37.Svinarich, D. M., N. A. Wolf, R. Gomez, B. Gonik, and R. Romero. 1997. Detection of human defensin 5 in reproductive tissues. Am. J. Obstet. Gynecol. 176:470-475. [DOI] [PubMed] [Google Scholar]
- 38.Tang, Y. Q., J. Yuan, G. Osapay, K. Osapay, D. Tran, C. J. Miller, A. J. Ouellette, and M. E. Selsted. 1999. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498-502. [DOI] [PubMed] [Google Scholar]
- 39.Terhune, S. S., K. T. Coleman, R. Sekulovich, R. L. Burke, and P. G. Spear. 1998. Limited variability of glycoprotein gene sequences and neutralizing targets in herpes simplex virus type 2 isolates and stability on passage in cell culture. J. Infect. Dis. 178:8-15. [DOI] [PubMed] [Google Scholar]
- 40.Tran, D., P. A. Tran, Y. Q. Tang, J. Yuan, T. Cole, and M. E. Selsted. 2002. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277:3079-3084. [DOI] [PubMed] [Google Scholar]
- 41.Van Strijp, J. A., L. A. Miltenburg, M. E. van der Tol, K. P. Van Kessel, A. C. Fluit, and J. Verhoef. 1990. Degradation of herpes simplex virions by human polymorphonuclear leukocytes and monocytes. J. Gen. Virol. 71:1205-1209. [DOI] [PubMed] [Google Scholar]
- 42.White, S. H., W. C. Wimley, and M. E. Selsted. 1995. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 5:521-527. [DOI] [PubMed] [Google Scholar]
- 43.Wimley, W. C., M. E. Selsted, and S. H. White. 1994. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 3:1362-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, L., W. Yu, T. He, J. Yu, R. E. Caffrey, E. A. Dalmasso, S. Fu, T. Pham, J. Mei, J. J. Ho, W. Zhang, P. Lopez, and D. D. Ho. 2002. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science 298:995-1000. [DOI] [PubMed] [Google Scholar]