Abstract
The M184V mutation in human immunodeficiency virus (HIV) reverse transcriptase is associated with high-level resistance to both (−)2′,3′-dideoxy-3′-thiacytidine (3TC) and (−)2′,3′-dideoxy-5-fluoro-3′-thiacytidine as well as low-level resistance to 2′,3′-dideoxyinosine, 2′,3′-dideoxycytidine, and abacavir. This mutation is also associated with diminished HIV replicative fitness as well as several functional changes in enzyme activity, including diminutions in polymerase processivity, pyrophosphorylysis, and nucleotide primer unblocking. Despite the fact that M184V encodes up to 1,000-fold resistance to 3TC, we asked whether this drug might still display some antiviral effect in regard to viruses containing this mutation. Cell-free assays revealed that high concentrations of 3TC triphosphate (i.e., >100 μM) could affect chain termination and/or inhibit purified reverse transcriptase containing the M184V substitution. This effect became more pronounced with elongation of reverse transcriptase products. In newly infected cells (i.e., peripheral blood mononuclear cells), we found that the amount of full-length reverse transcriptase product was diminished in the presence of 2 to 10 μM 3TC, although no decrease in the first product of the reverse transcriptase reaction, i.e., minus strong-stop DNA, was observed. In the presence of two other HIV inhibitors, e.g., nevirapine and indinavir, 3TC exerted additive effects in tissue culture at concentrations only marginally higher than the 50% inhibitory concentration (IC50). Reverse transcriptases cloned from clinical isolates harboring M184V in the context of multidrug resistance had similar IC50 values for 3TC triphosphate compared to reverse transcriptase containing only the M184V mutation. These results suggest that viruses containing M184V can retain a higher degree of sensitivity to 3TC than previously assumed.
Human immunodeficiency virus (HIV) drug resistance is due to the error-prone nature of reverse transcriptase, which is responsible for the transformation of the HIV-1 RNA genome into its viral DNA form (6, 8, 37). Resistance can occur against each of the categories of anti-HIV drugs now in use (4, 7, 12-13, 16-17, 19, 21-23, 40, 42), including nucleoside reverse transcriptase inhibitors, which cause DNA chain termination, nonnucleoside reverse transcriptase inhibitors, which act as noncompetitive inhibitors of reverse transcriptase, and protease inhibitors, which block the action of the HIV protease enzyme.
Among the nucleoside reverse transcriptase inhibitors, lamivudine (3TC) has been widely used in combination therapy due to its antiviral efficacy and relatively low toxicity. However, resistance to 3TC can develop quickly and at high levels, due to an M184V mutation in the reverse transcriptase (12, 44). The same substitution in the active site of the enzyme (24) can also confer lower-level resistance to 2′,3′-dideoxyinosine, 2′, 3′-dideoxycytidine, and abacavir (16, 45). In addition, however, M184V apparently alters a number of other characteristics of reverse transcriptase, including diminished rates of nucleotide primer unblocking and/or pyrophosphorylysis (15), diminished processivity of DNA polymerization (5), and increased fidelity, as measured in biochemical assays (47).
The diminished rates of nucleotide primer unblocking and pyrophosphorylysis help to explain that viruses containing both M184V and multiple mutations associated with resistance to zidovudine, e.g., at positions 41, 70, 215, and 219, can be resensitized to zidovudine (44). In addition, M184V is associated with a lower incidence of thymine analog mutations in patients who are multiple-drug experienced as well as lower phenotypic resistance to both zidovudine and stavudine (1). The biochemical changes described above are probably responsible for the fact that viruses containing M184V displayed reduced replication kinetics or fitness in tissue culture (5).
For these reasons, it may be beneficial to maintain the M184V mutation in treatment once it has developed rather than allow viruses containing this mutation to be overgrown by fitter wild-type viruses (11, 46). Indeed, patients who received 3TC monotherapy and developed M184V maintained significantly lower viral loads over 1 year than patients who either received no drug or received zidovudine as monotherapy (18, 32). However, it is not known whether 3TC can still maintain a degree of antiviral activity against viruses that contain M184V, in spite of the fact that this mutation confers high-level resistance against the compound.
We have now studied purified reverse transcriptases from viruses containing the M184V mutation in this context. We now report that the active form of 3TC, i.e., 3TC triphosphate, maintained a degree of antiviral activity against reverse transcriptases containing M184V even though relatively high concentrations of 3TC triphosphate were required to achieve this effect. Consistently, the PCR products of these reactions showed a decline in levels of full-length viral DNA in newly infected activated peripheral blood mononuclear cells (PBMCs) in the presence of micromolar concentrations of 3TC in the absence of a concomitant decrease in levels of minus-strong stop DNA. Additive inhibitory effects were observed at micromolar concentrations of 3TC when M184V-containing viruses were studied in tissue culture in the presence of 3TC and two other active drugs. In addition, reverse transcriptases containing multiple resistance-associated mutations, with or without M184V, as well as drug resistance-unrelated mutations were cloned from clinical isolates and had similar or lower 50% inhibitory concentration (IC50) values for 3TC triphosphate compared to a singly mutated M184V reverse transcriptase. These findings support the concept that 3TC might continue to have some degree of antiviral activity even after the M184V mutation has emerged.
MATERIALS AND METHODS
Molecular cloning.
The proviral recombinant clone HxB2D was used as a wild-type HIV-1 plasmid. The M184V mutation in reverse transcriptase was introduced into HxB2D by site-directed mutagenesis as previously described (16). Mutated and wild-type reverse transcriptases were expressed with an Escherichia coli expression system (25-26, 33). Briefly, the M184V mutation was introduced into the His-tagged reverse transcriptase expression plasmid pRT6H-PROT. The reverse transcriptase coding sequence of a BamHI fragment (between nucleotides 121 and 1968) of pRT6H-PROT was subcloned into the corresponding site in pGEM-3Z (Promega, Madison, Wis.). The reverse transcriptase mutation at amino acid position 184 (M184V) was introduced by replacing the BstXI fragment (nucleotides 580 to 808) with that excised from the M184V mutated proviral plasmid. The BamHI fragments were then reintroduced into plasmid pRT6H-PROT, and the presence of mutations was confirmed by sequencing.
Similarly, M184V-containing reverse transcriptases from clinical isolates were cloned by replacing the reverse transcriptase region of XhoI-BglII in plasmid pRT6H-PROT with the entire region of the reverse transcriptase fragment amplified by PCR from DNA extracted from HIV-1-infected peripheral blood mononuclear cells (PBMCs) through an intermediate plasmid, psp72, with the reverse transcriptase-containing XhoI-BglII fragment. The presence of mutations was ascertained by HIV reverse transcriptase genotyping, by PCR with two pairs of doubly fluorescent-labeled primers in bidirectional sequencing, with sequences elucidated with an automated system and the manufacturer's software (Visible Genetics Inc., Toronto, Ontario, Canada). In these cases, samples were obtained with informed consent from patients treated at the Infectious Diseases service of our hospital.
Recombinant reverse transcriptase purification.
Recombinant wild-type and mutant reverse transcriptases were expressed in E. coli and purified as described previously (33). Briefly, reverse transcriptase expression in bacteria was induced by isopropyl-β-d-thiogalactopyranoside. The reverse transcriptase molecules were processed into heterodimers by an HIV-1 viral protease coexpressed in the bacteria. The bacteria were lysed, sonicated, and centrifuged, and supernatants were applied to a nickel-nitrilotriacetate-Sepharose column (Qiagen, Mississauga, Canada). The column was washed, and histidine-tagged reverse transcriptase was eluted with an imidazole gradient. Reverse transcriptase-containing fractions were pooled, passed through DEAE-Sepharose (Pharmacia, Montreal, Canada), and loaded onto SP-Sepharose (Pharmacia). Fractions containing purified reverse transcriptase were pooled, concentrated on cartridges (Millipore, Ontario, Canada), and dialyzed overnight against storage buffer (50 mM Tris [pH 7.0], 25 mM NaCl, 1 mM EDTA, and 50% glycerol). The final product, the p66/p51 reverse transcriptase heterodimer, was aliquoted and stored at −20°C.
Reverse transcriptase assays.
For quantification of reverse transcriptase DNA polymerase activity, reverse transcriptase reaction buffer containing 50 mM Tris (pH 7.8), 5 mM MgC12, 60 mM KCl, 10 mM dithiothreitol, and 5 μM dTTP with 2.5 μCi of [3H]dTTP (70 to 90 mCi/mM), 5 U of template or primer, i.e., poly(rA)/(oligo(dT)12-18 (Pharmacia), and various amounts of wild-type or mutant reverse transcriptase (i.e., 0, 10, 20, or 40 ng) were included in 50-μl reaction mixes that were incubated at 37°C for 5 min, and the reactions were quenched with 0.2 ml of 10% cold trichloroacetic acid and 20 mM sodium pyrophosphate. After 30 min on ice, the precipitated products were filtered on 96-well plates with glass fiber filters (Millipore) and sequentially washed with 10% trichloroacetic acid and 95% ethanol.
The radioactivity of incorporated products was analyzed by liquid scintillation spectrometry. An active unit of reverse transcriptase was defined as the amount of enzyme that incorporated 1 pmol of dTTP in 10 min at 37°C. For determinations of resistance, 10 μM each of the four deoxynucleoside triphosphates with 2.5 μCi of [3H]dTTP and 30 nM RNA template or primer, prepared as described below, were used in each reaction with the buffers as described above. Various concentrations of the reverse transcriptase inhibitor 3TC triphosphate (i.e., 0, 0.1, 0.5, 2.5, 10, 40, 160, and 640 μM) and 10 U of reverse transcriptase were employed in 50-μl reaction volumes at 37°C for 30 min.
Templates and primers.
HIV RNA template was prepared in vitro with the MEGAscript transcription kit (Ambion, Austin, Tex.) from linearized plasmid pHIV-PBS. The template consists of a 497-bp HIV-1 sequence spanning the R region of the HIV-1 long terminal repeat, and a portion of gag (3).
For primer extension assays, we used the 18-nucleotide DNA primer dPR and the 20-nucleotide DNA primers pAR and PA (purchased from Bio S&T, Montreal, Canada), which are complementary to the HIV-1 primer binding site (PBS), the R region of the long terminal repeat, and the 5′ end of gag, respectively. The primers were [γ-32P]ATP labeled by using T4 kinase (Life Technology, Burlington, Ontario, Canada) and filtered with a Sephadex G-25 column. The template-primer complex was prepared as follows. The template and primer were mixed at a ratio of 1:2, denatured at 85°C for 5 min, and then sequentially cooled to 55°C for 8 min and 37°C for 10 min to allow specific annealing of primer to the template. For reverse transcriptase assays, HIV RNA template and dPR primer were mixed at a ratio of 1:3 and annealed as above.
Primer extension assay.
The reaction mixture contained 50 mM Tris (pH 7.8), 5 mM MgC12, 60 mM KCl, and 10 mM dithiothreitol, and 50 nM HIV RNA template and 100 nM primer were included in the reaction. We used 100 μM each of dATP, dGTP, and dTTP with 2.5 μM dCTP. Twenty units of reverse transcriptase were included in a total volume of 20 μl, and reaction mixtures were incubated for 30 min at 37°C. Products were extracted with phenol-chloroform, denatured by boiling for 5 min, and electrophoresed in a 5% denaturing polyacrylamide gel. Full-length DNA (runoff band) was analyzed by molecular imaging (Bio-Rad Instruments, Mississauga, Ontario, Canada), and the relative radioactive intensities of product bands were calculated.
Generation of infectious virus.
MT-2 and H9 cells were routinely maintained in RPMI 1640 medium (Life Technologies, Mississauga, Canada), supplemented with 10% fetal bovine serum (Flow Laboratories, Toronto, Canada), 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. HIV-1 virus stocks were generated by transfection of 2 μg of proviral DNA (HxB2D and HxB2-M184V) into 5 × 105 MT-2 cells with Lipofectin according to the manufacturer's recommendations (Life Technologies, Montreal, Canada). Recombinant wild-type and M184V viruses were harvested from these MT-2 cell transfections and used to achieve chronic infection of H9 cells or PBMCs.
Seed viruses (M184V and wild type) that were used in subsequent infection experiments in PBMCs for quantitative PCR analysis were harvested from chronically infected H9 cells to diminish the presence of cellular debris that could have arisen had these viruses been harvested from acute MT-2 infections. Sequencing confirmed that those viruses into which M184V had been introduced retained this mutation after passage and did not contain other mutations in the reverse transcriptase. Viral particles in culture fluids were harvested and ultracentrifuged (80,000 × g for 1 h at 4°C). Pelleted viruses were resuspended in fresh medium, evaluated for p24 (CA) content (Abbott Laboratories, North Chicago, Ill.) and reverse transcriptase activity, and kept frozen at −70°C until further study.
Detection of reverse transcriptase products in infected PBMCs.
PBMCs (2 × 106 cells) derived from healthy donors were activated with phytohemagglutinin and grown in the presence of interleukin-2 as previously described (39); they were then infected with HxB2D or HxB2D-M184V viruses (equivalent to 10 ng of p24). Following a 2-h adsorption at 37°C, the cells were pelleted at 4°C and resuspended in fresh medium containing either no drug or various concentrations of 3TC. Cells were either untreated or treated with various concentrations of 3TC for 3 h to ensure that the drugs would act against HIV-1 reverse transcription from the time of viral adsorption. The viruses used for infection were diluted into medium supplemented with various concentrations of 3TC.
PCR detection of HIV cDNA in newly infected cells has been described previously (27, 36). Briefly, PBMCs that had been exposed to virus for 36 h were pelleted, washed twice with medium at 4°C, and resuspended in lysis buffer containing 0.5% sodium dodecyl sulfate and 1 mg of proteinase K per ml for 6 h; extracted DNA was then analyzed by quantitative PCR with the primer pairs PS/PA and PS/A55 (PS was [γ-32P]ATP labeled by T4 kinase) to detect both full-length and minus strong-stop DNA products. The resulting amplified DNA products were electrophoresed in a 4% nondenaturing polyacrylamide gel and visualized by autoradiography.
Infections.
Infections were carried out as reported previously (39). Briefly, phytohemagglutinin-activated PBMCs were infected with 0.1 50% tissue culture infectious dose of wild-type or HxB2D-M184V viruses. Following a 2-h adsorption at 37°C, cells were pelleted at 4°C, resuspended in fresh medium, and adjusted to the proper concentration, and 106 cells per well were distributed into 96-well plates containing constant concentrations of two unrelated HIV inhibitors, i.e., the protease inhibitor indinavir and the nonnucleoside reverse transcriptase inhibitor nevirapine and either no additional drug or 3TC.
It should be noted that the purpose of this study was not to demonstrate synergy among the three drugs in the combination but merely to show that 3TC could exert a modest level of antiviral activity under the conditions employed. For this reason, a series of preliminary experiments was first performed, leading to the use of drug concentrations that were 75% below the usual IC50 for both indinavir and nevirapine, while a variety of concentrations of 3TC were included in the wells under conditions in which 3TC was present. Cells were refed at 3-day intervals with fresh medium containing drugs at the same concentrations as those initially employed. Culture fields were tested for reverse transcriptase activity at 7 or 10 days after infection.
Drugs.
Both 3TC and 3TC triphosphate were gifts of Shire Biochem Inc., Laval, Quebec, Canada. Nevirapine was kindly provided by Boehringer-Ingelheim, Inc., Laval, Quebec, Canada, and indinavir was a gift of Merck-Frosst Pharmaceuticals, Montreal, Canada.
RESULTS
Inhibitory effects of 3TC triphosphate on M184V reverse transcriptase in cell-free assays.
DNA polymerization catalyzed by reverse transcriptase is a processive reaction that uses the products generated in one step as the substrate for the next. Thus, the inhibitory effects of a given inhibitor may not be observed if the DNA chain that is polymerized is insufficiently long, while these effects will be pronounced as chain elongation proceeds beyond a critical point. This is true for HIV wild-type reverse transcriptase in both cell-free assays and within cells at early stages of infection (33, 36).
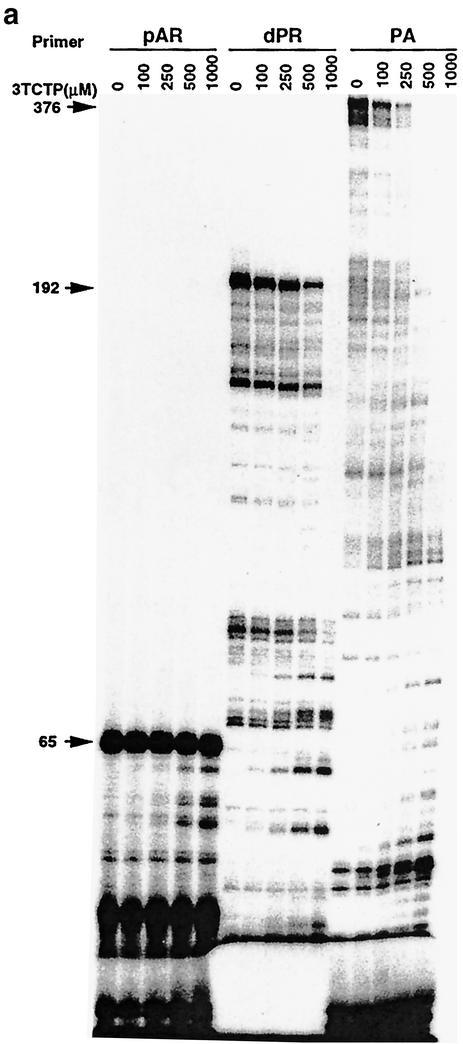
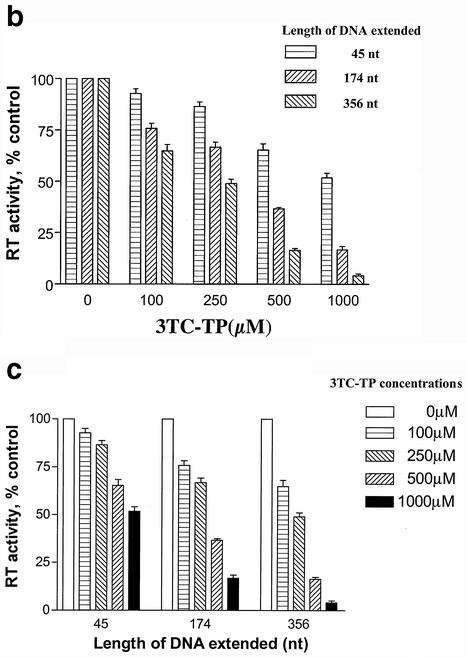
We now show that 3TC-resistant M184V reverse transcriptase can also be inhibited by 3TC triphosphate in primer extension assays, although these inhibitory effects were not obvious until the concentration of 3TC triphosphate reached 500 μM in reactions in which reverse transcriptase products had been elongated to 65 nucleotides (Fig. 1a). In contrast, inhibitory effects were detected at 100 μM 3TC triphosphate in reactions in which reverse transcriptase products had elongated to 376 nucleotides (Fig. 1a). Dose-dependent inhibition of 3TC triphosphate for M184V reverse transcriptase is shown in Fig. 1b, while the relationship between inhibitory effect and product length is demonstrated in Fig. 1c. At 500 μM drug, the extent of inhibition was less than 40% when the reverse transcriptase product was elongated to 65 nucleotides but was greater than 80% when the DNA chain length reached 376 nucleotides.
FIG. 1.
Inhibition of recombinant mutated M184V in reactions performed with different primers, i.e., pAR, dPR, and PA, in the presence of 3TC triphosphate. (a) Radioautographic results of reaction mixtures that contained 50 mM Tris (pH 7.8), 5 mM MgCl2, 60 mM KCl, 20 mM dithiothreitol, 50 nM HIV RNA template, 100 nM labeled primer, 10 U of reverse transcriptase, 100 μM each of the four deoxynucleoside triphosphates with the exception of dCTP, for which 2.5 μM was employed, and 3TC triphosphate (3TC-TP) concentrations of 0, 100, 250, 500, and 1,000 μM (from left to right in each panel). After 60 min at 37°C, reaction products were boiled, electrophoresed in a 5% denaturing polyacrylamide gel, and visualized by autoradiography. Numbers on the left identify the positions of runoff (full-length) products (in nucleotides [nt]). (b and c) Analysis of the reverse transcriptase (RT) activity results in panel a by molecular imaging.
To compare differences in sensitivity between wild-type and M184V reverse transcriptases for 3TC triphosphate, we also show IC50 values calculated on the basis of differential reverse transcriptase products obtained with both wild-type reverse transcriptase and M184V reverse transcriptase in the presence of 3TC triphosphate (Table 1). As expected, the increase in degree of resistance to 3TC triphosphate manifested by M184V reverse transcriptase relative to wild-type reverse transcriptase increased in proportion to the length of the DNA product that was extended, i.e., increases of 56-, 62-, and 65-fold for DNA products of 45, 174, and 356 nucleotides, respectively. These results indicate that the activity of M184V reverse transcriptase can be inhibited by 3TC triphosphate but only at high concentrations.
TABLE 1.
IC50 values of 3TC triphosphate for wild-type and M184V reverse transcriptases calculated from primer extension assaysa
| DNA length, extended (nt) | Mean IC50 of 3TC (μM) ± SD
|
||
|---|---|---|---|
| Wild type | M184V | Increase (fold) | |
| 45 | 15.6 ± 3.3 | 891.0 ± 75.0 | 56 |
| 174 | 6.4 ± 0.8 | 391.0 ± 49.0 | 62 |
| 356 | 3.8 ± 0.2 | 248.0 ± 18.0 | 65 |
The values are means of two independent experiments based on Molecular Analyst scanning.
Inhibitory effects of 3TC on M184V reverse transcriptase mutant in PBMCs.
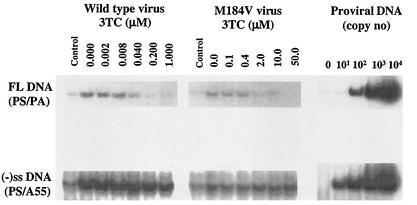
The above data, obtained in cell-free assays, prompted us to assess the relationship between M184V reverse transcriptase and 3TC triphosphate in newly infected cells, in which as little as 10 kb of extended reverse transcriptase product can be detected. Theoretically, lower concentrations of 3TC should be required in tissue culture to inhibit the synthesis of full-length reverse transcriptase products compared to the concentrations of 3TC triphosphate in cell-free assays, which use relatively short templates. The results in Fig. 2 show that little or no decrease in synthesis of early DNA product, i.e., minus-strong stop DNA, was seen regardless of the concentration of 3TC used, consistent with earlier reports performed with wild-type virus (36, 49). In contrast, dose-dependent inhibitory effects were observed for full-length products. As little as 2 μM 3TC sufficed to partially inhibit the generation of full-length reverse transcriptase products in the case of mutated M184V virus grown in PBMCs, although, of course, far lower concentrations of drug were able to block synthesis of the same products in studies performed with wild-type virus (Fig. 2, left panel).
FIG. 2.
PCR analysis of inhibition of synthesis of minus-strand strong-stop DNA [(−)ss DNA] and full-length viral DNA (FL DNA) by 3TC triphosphate in experiments in which PBMCs had been acutely infected for 36 h with either wild-type or M184V HIV-1 viruses that had been harvested from chronically infected H9 cells as described in Materials and Methods. PCR products were electrophoresed on 4% acrylamide gels under nondenaturing conditions and visualized by autoradiography. Controls in each panel designate results with PBMCs that had been coincubated with virus for 2 h at 4°C. Copy number of proviral DNA is indicated on the right side of the gel.
The results in Fig. 2 and other duplicate experiments were used to calculate IC50 values for 3TC in regard to synthesis of full-length viral DNA product in infected PBMCs. The values obtained were 0.039 ± 0.018 and 9.2 ± 2.7 μM for wild-type and M184V-containing viruses, respectively, i.e., a difference of 236-fold. In regard to synthesis of minus strong-stop DNA, it was not possible to perform such calculations because the levels of DNA product were not significantly reduced in the presence of the inhibitor. This is not an unexpected finding, as previous results have shown that minus strong-stop DNA is found inside virus particles prior to infection and that the levels of such product produced inside infected cells are not impacted to a significant extent by antiviral drugs (36, 49). These results suggest that 3TC might be able to suppress replication of M184V viruses at a concentration of 1 to 10 μM in primary cells, such as PBMCs, that possess relatively low concentrations of deoxynucleoside triphosphates.
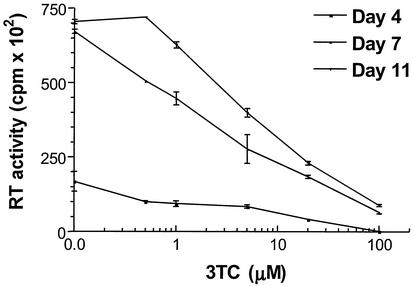
Studies involving infection of PBMCs confirmed the results obtained by PCR that less than 10 μM 3TC could inhibit replication of M184V virus in tissue culture. The results in Fig. 3 show that the presence of reverse transcriptase activity in culture fluids increased significantly at 7 days after infection. Consistent with the PCR results, viral replication was observed to decrease gradually in the presence of increasing concentrations of 3TC. IC50 values for 3TC at days 7 and 11 were both approximately 7 μM, although the amounts of reverse transcriptase in culture fluids at day 11 were higher than those detected at day 7.
FIG. 3.
Inhibition by 3TC of M184V virus in de novo infection of PBMCs. Phytohemagglutinin-activated PBMCs were infected with 0.1 50% tissue culture infectious dose of M184V virus. Following a 2-h adsorption at 37°C, the cells were pelleted at 4°C, resuspended in fresh medium, and adjusted to the proper concentration, and 106 cells per well were distributed into 96-well plates containing various concentrations of 3TC or no drug. Half of the supernatants were replaced at intervals of 3 days. The supernatants were removed and tested for reverse transcriptase (RT) activities at 4, 7, or 11 days after infection. Results shown are means of three independent measurements ± standard deviation.
Combinatorial effects of 3TC with other reverse transcriptase inhibitors in tissue culture.
Previous studies have shown that the M184V mutation in reverse transcriptase confers high-level resistance to 3TC and that concentrations of this drug higher than 200 μM are usually required to document an antiviral effect. We assessed whether lower doses of 3TC could be used to inhibit HIV replication in an assay that was specifically designed for this purpose by simultaneously employing suboptimal concentrations of two other unrelated drugs. Accordingly, one protease inhibitor, i.e., indinavir, and one nonnucleoside reverse transcriptase inhibitor, i.e., nevirapine, were used at 25% of the usual IC50 values together with various concentrations of 3TC to study the replication of the M184V virus. It should be emphasized that the purpose of this study was not to assess synergy among the three drugs in the mixture; this would have necessitated three-way variations in drug concentrations. Rather, the system that we employed was designed solely to assess the ability of 3TC to exert an antiviral effect against viruses containing M184V.
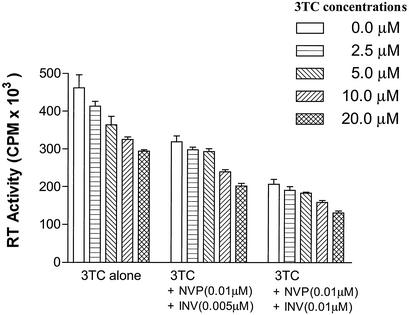
As shown in Fig. 4 and Table 2, the inhibitory effects of 3TC increased as 3TC concentrations increased in the additional presence of indinavir (0.005 μM) and nevirapine (0.01 μM). With combinations of indinavir (0.005 μM) and nevirapine (0.01 μM) and various levels of 3TC, 7.3% additive effects were observed for 3TC even at concentrations as low as 2.5 μM in PBMCs, and this rose to 19.5% and 28.5% at 10.0 and 20.0 μM 3TC, as shown in Table 2. These concentrations of 3TC are significantly below those required for demonstration of antiviral activity against M184V-containing viruses in more conventional assays. Slightly lower additive effects for 3TC were observed when a higher concentration of indinavir (i.e., 0.01 μM) was used with the same concentration of nevirapine (0.01 μM) and various concentrations of 3TC in PBMCs, i.e., 3.7%, 10.4%, and 16.5% at 2.5 μM, 10 μM, and 20 μM 3TC, respectively.
FIG. 4.
Combinatorial effects of 3TC and other HIV inhibitors in PBMCs. The same procedures were used as in Fig. 3, but two other HIV inhibitors were also included. Briefly, after adsorption at 37°C, activated PBMCs were resuspended in fresh medium and adjusted to the proper concentrations, and 106 cells per well were distributed into 96-well plates containing constant concentrations of two HIV inhibitors, indinavir (INV) and nevirapine (NVP), as well as either no additional drug or various concentrations of 3TC. Half of the culture fluids were replaced at 3-day intervals. Culture fluids were tested for reverse transcriptase (RT) activity at 7 and 10 days after infection. Results shown are means of three independent measurements ± standard deviation.
TABLE 2.
Incremental effect of 3TC triphosphate in PBMCs in combination with other antiviral drugs against recombinant HIV-1 containing M184Va
| Drug combination (μM) | Concn of 3TC (μM) | Additional antiviral effect (%) |
|---|---|---|
| Indinavir (0.005) plus nevirapine (0.01) | 2.5 | 7.3 |
| 5.0 | 8.3 | |
| 10.0 | 19.5 | |
| 20.0 | 28.1 | |
| Indinavir (0.01) plus nevirapine (0.01) | 2.5 | 3.7 |
| 5.0 | 5.0 | |
| 10.0 | 10.4 | |
| 20.0 | 16.5 |
The viral inoculum was 0.1 50% tissue culture infections dose per cell. Values were calculated from the mean values of the experiment shown in Fig. 4.
These differences reflect the fact that the higher concentrations of indinavir exerted a more profound antiviral effect in this system, making it more difficult to detect an additional incremental benefit of 3TC. Indeed, our ability to detect an incremental benefit of 3TC in this system was lost when three-way variations in drug concentrations that involved significant increases in the doses of either indinavir or nevirapine were performed (not shown). It is worth noting that variations in inhibitory effects for 3TC in PBMCs were observed among experiments; this could reflect the use of different batches of PBMCs, e.g., in Fig. 3 and Fig. 4. However, consistent results were observed within each individual experiment.
Additive results similar to those described above were also obtained when other drugs were employed in these protocols in regard to M184V-containing viruses, e.g., 3TC plus nelfinavir plus efaverenz (data not shown). Other nucleoside reverse transcriptase inhibitors besides 3TC were not employed in these experiments because the potential effects of M184V re these compounds might have complicated interpretation of the results.
Sensitivities of reverse transcriptases containing both M184V and other mutations.
It is possible that other mutations in reverse transcriptase combined with M184V might affect the enzymatic sensitivity to 3TC triphosphate. Accordingly, the reverse transcriptase regions of several multiply nucleoside reverse transcriptase inhibitor-resistant viruses were cloned from clinical samples, and mutations were determined by genotyping. The viruses employed were from recent cases of HIV infection and may reflect the current situation better than lab strains generated a decade ago or longer.
A number of recombinant reverse transcriptases containing multiple mutations including M184V were generated and tested for sensitivity to 3TC triphosphate in cell-free assays. The results in Table 3 show that the multiply mutated reverse transcriptases that were tested displayed various levels of resistance to 3TC triphosphate. Those reverse transcriptases harboring multiple nucleoside reverse transcriptase inhibitor mutations besides M184V, i.e., RT-1 and RT-3, showed similar levels of resistance to 3TC triphosphate, i.e., ≈50- to 100-fold, as did the reverse transcriptase that contained only M184V. Reverse transcriptases cloned from clinical isolates lacking M184V were approximately as sensitive to 3TC triphosphate as the wild-type enzyme.
TABLE 3.
IC50 values for 3TC triphosphate of wild-type reverse transcriptase and those derived from clinical and cloned samplesa
| Origin of enzyme | Mean IC50 for 3TC (μM) ± SD | Drug resistance-associated mutations | Polymorphisms (other changes) |
|---|---|---|---|
| Wild type-HxB2D | 1.59 ± 0.23 | ||
| M184V-HxB2D | 220.00 ± 14.0 | M184V | |
| RT-1 | 152.00 ± 9.60 | M41L, T69N, K70R, L100I, K103N, M184V, K219Q | K43Q, V60I, V118I, I135V, Q207E, K223Q, L228H |
| RT-2 | 4.48 ± 0.34 | E122K, D123E, I178M, G196E, Q207L, R211K, L214F | |
| RT-3 | 167.00 ± 8.70 | M41L, D67N, K70R, M184V, H208Y, T215F, K219Q | K43M, D121H, I135T, K154X, P157R, G196E, Q207H, R211K, L214F, D218E |
| RT-4 | 5.82 ± 0.36 | I94S, E122K, S134G, V245E |
RT-1 to RT-4 were cloned from clinical samples. The IC50 values shown are means ± standard deviation calculated from two independently performed experiments.
DISCUSSION
Reverse transcriptase is a flexible molecule that can retain polymerase activity despite multiple amino acid substitutions. However, certain of these mutations may change the character of the enzyme, causing alterations in viral fitness. As an example, drug resistance-associated mutations are commonly found at lower frequency in plasma once the drug that selected theses mutations is withdrawn from therapy (11, 46). Some scientists have suggested that it might be beneficial to maintain selective pressure so as to retain mutations that impact viral fitness to the greatest extent. One such mutation may be M184V, associated with high-level resistance to 3TC, because it has multiple pleiotropic effects on reverse transcriptase function (5, 15, 30, 44, 47).
Mutant viruses might replicate more slowly than wild types, especially in cells that possess low concentrations of deoxynucleoside triphosphates, e.g., primary CD4 lymphocytes (5). 3TC may be able to retain some antiviral activity in such cells if intracellular concentrations of dCTP are low. For this reason, the IC50 values of 3TC for wild-type virus are about 10-fold lower in PBMCs than in cell lines (data not shown). Variations in IC50 values for 3TC in experiments performed with PBMCs may be higher than in cell lines because of variable concentrations of deoxynucleoside triphosphate pools in the former populations.
The M184V substitution can suppress the effects of mutations at positions 41, 210, 215, and 219 that confer resistance to zidovudine (44) and increase the sensitivity of the enzyme for adefovir and tenofovir (29, 48). M184V results in diminished pyrophosphorylysis relative to wild-type enzyme (15) or reverse transcriptases containing various mutations associated with resistance to zidovudine, although zidovudine-resistant reverse transcriptase is associated with enhanced pyrophosphorylysis (2, 28). For this reason, we explored the effect of M184V on sensitivity to 3TC triphosphate in the context of other mutations in reverse transcriptase.
We surmised that combinations of such mutations with M184V might have one of three different consequences: additive or synergistic effects, antagonistic effects, or no effect with regard to sensitivity to 3TC triphosphate. Previously, we reported that a K65R M184V double mutant displayed higher resistance to 3TC triphosphate than did the M184V mutant alone, while mutations at positions 41 and 215 had no effect in this regard when combined with M184V (34-35). Other mutations may have no effect with M184V, since IC50 values obtained with reverse transcriptases containing multiple mutations (RT-1 and RT-3) were similar to those of reverse transcriptases containing only the M184V single mutation (Table 3).
Our data show that 3TC may still have moderate inhibitory effects against HIV-1, even if the M184V mutation is present. When combined with other drugs, 3TC had additive effects at concentrations of between 2.5 and 20 μM (Table 3). It is difficult to compare these tissue culture concentrations with levels of 3TC that patients might maintain in their plasma. For example, average peak plasma levels of 3TC are in the range of approximately 2 to 10 μM, and troughs are about 0.3 μM to 1 μM (9, 43). Of course, the in vivo situation is complex due to a multitude of factors, including drug absorption, transportation, entry into cells, and rates of phosphorylation. As stipulated, variations in the concentration of drugs in plasma can be broad among individuals (9, 43). The rate of phosphorylation of 3TC in the PBMCs of different patients can also be variable (31, 38, 41), although this may, in fact, be more efficient than what occurs with some other drugs, e.g., zidovudine (10).
The IC50 value of 3TC for M184V virus in PBMCs can also vary from ≈4 μM to 100 μM, depending on the batch of cells employed, and in this context, it is important that direct comparisons between wild-type and mutated viruses always be made at the same time and on the same cells. In this study, the IC50 of 3TC for the M184V virus varied between about 7 μM (Fig. 3) and 20 μM (Fig. 4). It should also be remembered that resting lymphocytes in vivo maintain relatively low deoxynucleoside triphosphate pools; this can potentiate the effect of drugs such as 3TC because the ratio of inhibitor: substrate may be higher in this circumstance than might be the case in dividing cells (14). Thus, the in vivo benefit of 3TC in the aftermath of the M184V mutation might be greater in nondividing than activated lymphocytes. Thus, even if intracellular levels of 3TC triphosphate in PBMCs of treated patients are lower than levels of either the di- or monophosphates (31), this may not rule out an effect of 3TC triphosphate as a chain terminator in this circumstance. Our tissue culture system may not be adequately sensitive to detect antiviral activity by 3TC at lower concentrations than those employed in this study.
Although M184V results in discrimination by reverse transcriptase against 3TC triphosphate, this mutated enzyme also possesses diminished pyrophosphorylysis activity and a lesser ability to rescue terminator-blocked DNA chain synthesis (15). In this context, a molecule of 3TC triphosphate, once incorporated, might only be displaced with relative difficulty, hence allowing 3TC to continue to function as a chain terminator, albeit at greatly reduced efficiency. This is also suggested by our chain termination experiments with 3TC both in tissue culture and in cell-free systems (see above). Of course, we recognize that these relationships are complex, both in cells and in vivo, e.g., the ability of 3TC to be phosphorylated might be affected by the presence of other drugs, such as dideoxycytidine (20).
Considerable evidence exists that M184V reverse transcriptase is less processive than the wild-type enzyme (5) and that M184V-containing viruses are less fit than their wild-type counterparts (5). The current findings add to evidence that drug resistance is not only complex but always a relative rather than an absolute phenomenon, even for a mutation such as M184V that can confer high-level resistance to a compound such as 3TC.
Acknowledgments
This research was supported by grants from the Canadian Institutes of Health Research. Y. Quan was supported in part by a fellowship from the Canadian HIV Trials Network.
REFERENCES
- 1.Ait-Khaled, M., C. Stone, G. Amphlett, B. Clotet, S. Staszewski, C. Katlama, and M. Tisdale. 2002. M184V is associated with a low incidence of thymidine analogue mutations and low phenotypic resistance to zidovudine and stavudine. AIDS 16:1686-1689. [DOI] [PubMed] [Google Scholar]
- 2.Arion, D., N. Kaushik, S. McCormick, G. Borkow, and M. A. Parniak. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 37:15908-15917. [DOI] [PubMed] [Google Scholar]
- 3.Arts, E., X. Li, Z. Gu, L. Kleiman, M. A. Parniak, and M. A. Wainberg. 1994. Comparison of deoxyoligonucleotide and tRNAlys3 as primers in an endogenous HIV-1 in vitro reverse transcription/template switching reaction. J. Biol. Chem. 269:14672-14680. [PubMed] [Google Scholar]
- 4.Atkinson, B., J. Isaacson, M. Knowles, E. Mazabel, and A. K. Patick. 2000. Correlation between human immunodeficiency virus genotypic resistance and virologic response in patients receiving nelfinavir monotherapy or nelfinavir with lamivudine and zidovudine. J. Infect. Dis. 182:420-427. [DOI] [PubMed] [Google Scholar]
- 5.Back, N. K. T., M. Nijhuis, W. Keulen, et al. 1996. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 15:4040-4049. [PMC free article] [PubMed] [Google Scholar]
- 6.Bebenek, K., J. Abbotts, J. D. Roberts, S. H. Wilson, and T. A. Kunkel. 1989. Specificity and mechanism of error-prone replication by human immunodeficiency virus-1 reverse transcriptase. J. Biol. Chem. 264:16948-16956. [PubMed] [Google Scholar]
- 7.Boucher, C. A. B., N. Cammack, P. Schipper, R. Schuurman, P. Rouse, M. A. Wainberg, and J. A. Cameron. 1993. High-level resistance to (−) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 37:2231-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer, J. C., K. Bebenek, and T. A. Kunkel. 1992. Unequal human immunodeficiency virus type 1 reverse transcriptase error rates with RNA and DNA templates. Proc. Natl. Acad. Sci. USA 89:6919-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno, R., M. B. Regazzi, V. Ciappina, P. Villani, P. Sacchi, M. Montagna, R. Panebianco, and G. C. Filice. 2001. Comparison of the plasma pharmacokinetics of lamivudine during twice and once daily administration in patients with HIV. Clin. Pharmacokinet. 40:695-700. [DOI] [PubMed] [Google Scholar]
- 10.Cammack, N., P. Rouse, C. Marr, P. Reid, R. Boehme, J. Coates, C. Penn, and J. Cameron. 1992. Cellular metabolism of (−) enantiomeric 2′-deoxy-3′-thiacytidine. Biochem. Pharmacol. 43:2059-2064. [DOI] [PubMed] [Google Scholar]
- 11.Devereux, H. L., M. Youle, M. A. Johnson, and C. Loveday. 1999. Rapid decline in detectability of HIV-1 drug resistance mutations after stopping therapy. AIDS 13:F123-F127 [PubMed] [Google Scholar]
- 12.Gao, Q., Z. Gu, M. A. Parniak, J. Cameron, N. Cammack, C. Boucher, and M. A. Wainberg. 1993. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 37:1390-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, Q., Z. Gu, M. A. Parniak, X. Li, and M. Wainberg. 1992. In vitro selection of variants of human immunodeficiency virus type 1 resistant to 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine. J. Virol. 66:12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, W. Y., R. Agbaria, J. S. Driscoll, and H. Mitsuya. 1994. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′,3′-dideoxynucleoside analogs in resting and activated human cells. J. Biol. Chem. 269:12633-12638. [PubMed] [Google Scholar]
- 15.Gotte, M., D. Arion, M. A. Parniak, and M. Wainberg. 2000. The M184V mutation in the reverse transcriptase of human immunodeficiency virus type 1 impairs rescue of chain-terminated DNA synthesis. J. Virol 74:3579-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, Z., Q. Gao, X. Li, M. A. Parniak, and M. Wainberg. 1992. Novel mutation in the human immunodeficiency virus type 1 reverse transcriptase gene that encodes cross-resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine. J. Virol. 66:7128-7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu, Z., Q. Gao, H. Fang, H. Salomon, M. A. Parniak, E. Goldberg, J. Cameron, and M. Wainberg. 1994. Identification of a codon 65 in the IKKK motif of reverse transcriptase that encodes M.A. human immunodeficiency virus resistance to 2′,3′-dideoxycytidine and 2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 38:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingrand, D., J. Weber, C. A. Boucher, C. Loveday, C. Robert, A. Hill, and N. Cammack. 1995. Phase I/II study of 3TC (lamivudine) in HIV-positive, asymptomatic or mild AIDS-related complex patients: sustained reduction in viral markers. AIDS 9:1323-1329. [DOI] [PubMed] [Google Scholar]
- 19.Kellam, P., C. A. B. Boucher, and B. A. Larder. 1992. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contribute to the development of high-level resistance to zidovudine. Proc. Natl. Acad. Sci. USA 89:1934-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kewn, S., G. J. Veal, P. G. Hoggard, M. G. Barry, and D. J. Back. 1997. Lamivudine (3TC) phosphorylation and drug interactions in vitro. Biochem. Pharmacol. 54:589-595. [DOI] [PubMed] [Google Scholar]
- 21.Larder, B. A., G. Darby, and D. D. Richman. 1989. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science 243:1731-1734. [DOI] [PubMed] [Google Scholar]
- 22.Larder, B. A., and S. D. Kemp. 1989. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science 246:1155-1158. [DOI] [PubMed] [Google Scholar]
- 23.Larder, B. A., K. E. Coates, and D. Kemp. 1991. Zidovudine-resistant human immunodeficiency virus selected by passage in cell culture. J. Virol. 65:5232-5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larder, B. A., D. J. M. Purifoy, K. L. Powell, and G. Darby. 1987. Site specific mutagenesis of AIDS virus reverse transcriptase. Nature 327:716-717. [DOI] [PubMed] [Google Scholar]
- 25.Le Grice, S. F. J., C. E. Cameron, and S. J. Benkovic. 1995. Purification and characterization of human immunodeficiency virus type 1 reverse transcriptase. Methods Enzymol. 262:130-144. [DOI] [PubMed] [Google Scholar]
- 26.Le Grice, S. F. J., and F. Gruninger-Leitch. 1990. Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur. J. Biochem. 187:307-314. [DOI] [PubMed] [Google Scholar]
- 27.Li, X., Y. Quan, E. J. Arts, et al. 1996. Human immunodeficiency virus type 1 nucleocapsid protein (NCp7) directs specific initiation of minus-strand DNA synthesis primed by human tRNALys3 in vitro: studies of viral RNA molecules mutated in regions that flank the primer binding site. J. Virol. 70:4996-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer, P. R., S. E. Matsuura, A. G. So, and W. A. Scott. 1998. Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc. Natl. Acad. Sci. USA 95:13471-13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, M. D., K. E. Anton, A. S. Mulato, P. D. Lamy, and J. M. Cherrington. 1999. Human immunodeficiency virus type 1 expressing the lamivudine-associated M184V mutation in reverse transcriptase shows increased susceptibility to adefovir and decreased replication capability in vitro. J. Infect. Dis. 179:92-100. [DOI] [PubMed] [Google Scholar]
- 30.Miller, V., T. Stark, A. E. Loeliger, and J. M. Lange. 2002. The impact of the M184V substitution in HIV-1 reverse transcriptase on treatment response. HIV Med. 3:135-145. [DOI] [PubMed] [Google Scholar]
- 31.Moore, K. H., J. E. Barrett, S. Shaw, G. E. Pakes, R. Churchus, A. Kapoor, J. Lloyd, M. G. Barry, and D. Back. 1999. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS. 13:2239-2250. [DOI] [PubMed] [Google Scholar]
- 32.Pluda, J. M., T. P. Cooley, J. S. Montaner, et al. 1995. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in patients with advanced human immunodeficiency virus infection. J. Infect. Dis. 171:1438-1447. [DOI] [PubMed] [Google Scholar]
- 33.Quan, Y., C. Liang, P. Inouye, and M. A. Wainberg. 1998. Enhanced impairment of chain elongation by inhibitors of HIV reverse transcriptase in cell-free reactions yielding longer DNA products. Nucleic Acids Res. 26:5692-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan, Y., Z. Gu, X. Li, C. Liang, M. A. Parniak, and M. A. Wainberg. 1998. Endogenous reverse transcriptase assays reveal synergy between combinations of the M184V and other drug resistance-conferring mutations in interactions with nucleoside analog triphosphates. J. Mol. Biol. 277:237-247. [DOI] [PubMed] [Google Scholar]
- 35.Quan, Y., Z. Gu, X. Li, Z. Li, C. D. Morrow, and M. A. Wainberg. 1996. Endogenous reverse transcription assays reveal high-level resistance to the triphosphate of (−)2′-dideoxy-3′-thiacytidine by mutated M184V human immunodeficiency virus type 1. J. Virol. 70:5642-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quan, Y., L. W. Rong, C. Liang, and M. A. Wainberg. 1999. Reverse transcriptase inhibitors can selectively block the synthesis of differently sized viral DNA transcripts in cells acutely infected with human immunodeficiency virus type 1. J. Virol. 73:6700-6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts, J. D., K. Bebenek, and T. A. Kunkel. 1988. The accuracy of reverse transcriptase from HIV-1. Science 242:1171-1173. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez, J. F., J. L. Rodriguez, J. Santana, H. Garcia, and O. Rosario. 2000. Simultaneous quantitation of intracellular zidovudine and lamivudine triphosphates in human immunodeficiency virus-infected individuals. Antimicrob. Agents Chemother. 44:3097-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salomon, H., A. Belmonte, K. Nguyen, Z. Gu, M. Gelfand, and M. A. Wainberg. 1994. Comparison of cord blood and peripheral blood mononuclear cells as targets for viral isolation and drug sensitivity studies involving human immunodeficiency virus type 1. J. Clin. Microbiol. 32:2000-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schinazi, R., F. Lloyd, Jr., M.-H. Nguyen, et al. 1993. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob. Agents Chemother. 37:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solas, C., Y. F. Li, M. Y. Xie, J. P. Sommadossi, and X. Zhou. 1998. Intracellular nucleotides of (−)-2′,3′-deoxy-3′-thiacytidine in peripheral blood mononuclear cells of a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 42:2989-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.St Clair, M. H., J. L. Martin, G. Tudor-Williams, M. Bach, C. L. Vavro, D. M. King, P. Kellam, S. D. Kemp, and B. A. Larder. 1991. Resistance to dideoxyinosine and sensitivity to AZT induced by a mutation in HIV-1 reverse transcriptase. Science 253:1557-1559. [DOI] [PubMed] [Google Scholar]
- 43.Tatsunami, S., A. Ito, K. Kawata, R. Kuwabara, K. Fukutake, and K. Yam. 2000. Pharmacokinetic consideration on administration regimen of lamivudine in Japanese patients infected with HIV-1. Int. J. Clin. Pharmacol. Ther. 38:333-338. [DOI] [PubMed] [Google Scholar]
- 44.Tisdale, M., S. D. Kemp, N. R. Parry, and B. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. USA 90:5653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tisdale, M., T. Alnadaf, and D. Cousens. 1997. Combination of mutations in human immunodeficiency virus type 1 reverse transcriptase required for resistance to the carbocyclic nucleoside 1592U89. Antimicrob. Agents Chemother. 41:1094-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verhofstede, C., F. Van Wanzeele, B. Van der Gucht, N. De Cabooter, and J. Plum. 1999. Interruption of reverse transcriptase inhibitors or a switch from reverse transcriptase to protease inhibitors resulted in a fast reappearance of virus strains with a reverse transcriptase inhibitor-sensitive genotype. AIDS 13:2541-2546. [DOI] [PubMed] [Google Scholar]
- 47.Wainberg, M. A., W. C. Drosopoulos, H. Salomon, et al. 1996. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science 271:1282-1285. [DOI] [PubMed] [Google Scholar]
- 48.Wainberg, M. A., M. D. Miller, Y. Quan, et al. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antiviral Ther. 4:87-94. [DOI] [PubMed] [Google Scholar]
- 49.Zack, J. A., S. J. Arrigo, S. R. Weitman, A. S. Go, A. Haislip, and I. S. Y. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]