Abstract
The bactericidal activities of ciprofloxacin, ofloxacin, levofloxacin, moxifloxacin, and gatifloxacin were tested in three models of rifampin-tolerant Mycobacterium tuberculosis persisters. Model 1 was a 100-day-old, unshaken, anaerobically adapted culture in which serial dilutions of the quinolones were incubated for 5 days and CFU counts were then done In models 2 and 3, 100 mg of rifampin/liter was added to the 100-day culture for 5 or 7 days to produce tolerant organisms that did not grow on plates; the rifampin was then washed off, fresh medium was added to allow recovery of growth on plates, and the culture was incubated for 7 days before CFU counts. In model 2, the quinolones were added after rifampin had been washed off, whereas in model 3 the quinolones were added to the cultures containing rifampin. In models 1 and 2, ciprofloxacin had the least bactericidal activity, ofloxacin and levofloxacin had greater activities, and moxifloxacin and gatifloxacin had the greatest activities. In model 3, ofloxacin had no detectable activity whereas moxifloxacin killed about log10 0.279 CFU of the persisters per ml at concentrations attainable in lesions; isoniazid had virtually no activity. These findings predict that ofloxacin will not be found to have effective sterilizing activity in clinical studies now planned whereas moxifloxacin will be able to shorten treatment.
Chemotherapy is the main weapon for control of tuberculosis, since it interrupts the transmission of the disease. The most effective way of improving the results of chemotherapy would be the use of new drugs to shorten the present treatment period of 6 months or more (2, 3, 12). The drugs would do this by their sterilizing activity against bacilli that persist despite chemotherapy (10). There is current interest in whether this might be achieved by the use of quinolones either in addition to the four main drugs or substituted for ethambutol. In the chemotherapy of tuberculosis, isoniazid is the main bactericidal drug during the first 2 days of treatment with the standard regimen being 2 months of ethambutol-isoniazid-rifampin-pyrazinamide followed by 4 months of isoniazid-rifampin (7). After the second day, rifampin and to a lesser extent pyrazinamide are the sterilizing drugs mainly responsible for killing persistent organisms, some of which survive through many months of treatment (11). These persistent organisms, present in the lesions even at the start of treatment, are likely to be old, stationary phase, and microaerophilically adapted (16). Thus, it is pertinent to examine the bactericidal activity of the quinolones against persistent organisms in an in vitro model (10). Since they survive in the presence of rifampin during treatment, it is even more relevant to test them in models consisting of rifampin-tolerant persisters that survive in liquid medium cultures to which is added a high concentration of rifampin, as described previously (4, 5). After exposure to rifampin, the persisters rapidly lose the ability to grow on plates of solid medium, but they are alive and able to grow in liquid medium subcultures free of rifampin. In such subcultures, they regain the ability to grow on plates and are then fully susceptible to rifampin. Among the several populations of bacilli that are dormant or semidormant, rifampin-tolerant persisters are likely to survive the longest during chemotherapy and are therefore the most difficult to kill with any new antibacterial drug. Model 2 has been used to screen many thousands of molecules in a search for new drugs with high sterilizing activity (A. R. M. Coates and Y. Hu, unpublished data).
MATERIALS AND METHODS
Fluoroquinolones.
Small quantities of ciprofloxacin, levofloxacin, moxifloxacin, and gatifloxacin were obtained by recrystallization of the pure compound from treatment tablets. Their purity was established by mass, UV, and proton nuclear magnetic resonance spectrometry and by elemental analysis. Pure ofloxacin was added as a fifth quinolone. These samples were coded and were initially examined blindly for MIC determination and in models 1 and 2.
Bacterial culture models.
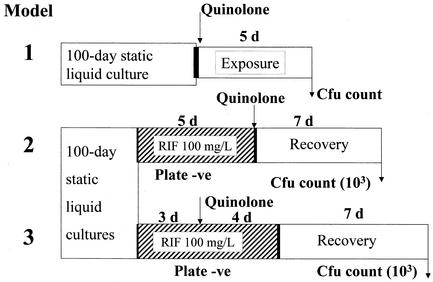
Mycobacterium tuberculosis strain H37Rv was used in all experiments. The Difco media (Becton Dickinson Laboratories, Sparks, Md.) used were plates of 7H11 medium, supplemented with oleic acid-albumin-dextrose-catalase complex, and 7H9 liquid medium containing 0.05% Tween 80, supplemented with 10% (wt/vol) albumin-oleic acid-dextrose-catalase complex. MICs of the five quinolones against strain H37Rv were measured as the lowest concentration to yield no colonies when segments of plates of 7H11 medium, containing quinolone concentrations in 1.414-fold steps (half a twofold step), were inoculated with about 106 log-phase bacilli and incubated for 4 weeks. For the models (all of which are patented), cultures were grown in 10-ml volumes of 7H9 medium in screw-cap 28-ml plastic Universal containers without shaking for 100 to 115 days (“100-day cultures”). To obtain evenly dispersed bacilli prior to experimental treatment, the sedimented clumps of bacilli in each of a set of 10-ml cultures were broken up by vortexing with 2-mm-diameter glass beads for 5 min, followed by sonication in a water bath sonicator (Branson Ultrasonic) for 5 min. After sonication, the 10-ml volumes from each culture of the set were pooled (without the glass beads) and then distributed again in 10-ml volumes in Universal containers. All tests were carried out in duplicate. Figure 1 shows the procedures used in the models.
FIG. 1.
Diagrammatic representation of models of rifampin-tolerant M. tuberculosis. Abbreviations: RIF, rifampin; d, day; −ve, negative.
Model 1.
Each of the five quinolones was added, in final concentrations increasing by twofold steps from 1.56 to 100 mg/liter, to the sonicated 100-day cultures, each concentration being added to a separate 10-ml culture. After incubation for 5 days, counts of viable CFU were set up by inoculating a pair of 7H11 plates with 100 μl of 10-fold serial dilutions of the culture.
Model 2.
Rifampin at 100 mg/liter was added to each of a set of sonicated 100-day cultures, which were then incubated for 5 days. After the first day of incubation, no colonies could be obtained on plates inoculated from the culture. After washing cultures twice with phosphate-buffered saline by centrifugation, fresh rifampin-free 7H9 medium was added to make up the volume to 10 ml and the quinolones were added in the same concentrations as in model 1. After further incubation for 7 days, CFU counts were set up by inoculating 1 ml from each container onto a 7H11 plate. These plates were incubated for 2 weeks, and the colonies, which were very small, were counted and marked. After a further 2 weeks of incubation, any additional unmarked colonies, which had grown slowly, were added to the count. Control studies have shown that plate counts begin to yield colonies on subculture after about 4 days of incubation of the rifampin-free cultures.
Model 3.
The procedure was similar to that for model 2, but only ofloxacin and moxifloxacin, in a range that included more low concentrations, and isoniazid were added to the 100-day culture at 3 days after addition of rifampin. At the end of the 7-day incubation period (4 days with quinolones plus rifampin), all cultures were washed, replaced with drug-free medium, and incubated for a further 7 days before CFU counts were set up.
The bactericidal activities of the quinolones in the three models could be described in summary form by the following abbreviations: MSC50, minimal bactericidal concentration killing half the stationary-phase bacteria, appropriate to model 1, and MDC50, minimal bactericidal concentration killing half the dormant bacteria, appropriate to models 2 and 3 (2).
Statistics.
The accuracy of the counts in the three models was estimated by analysis of variance of the log10 CFU counts in the duplicate experiments with the Stata package (Stata Corp., College Station, Tex.). Falls in counts at time T were defined as log10 CFU count before exposure − log10 CFU count at time T.
RESULTS
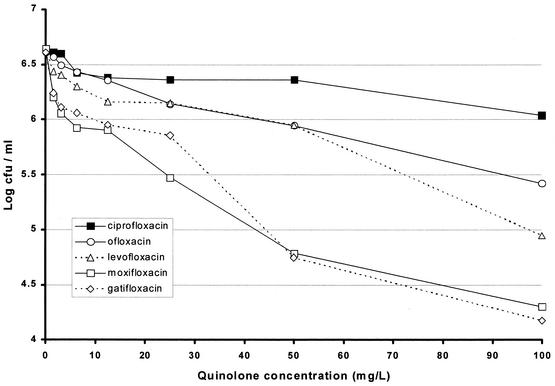
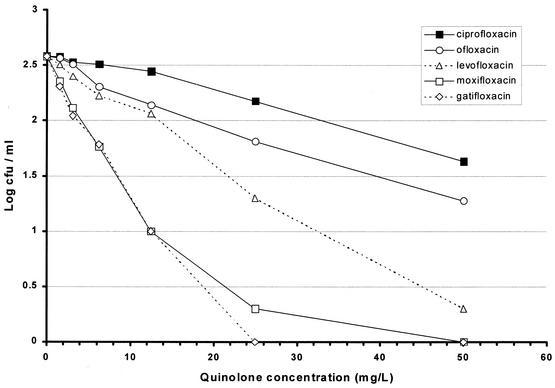
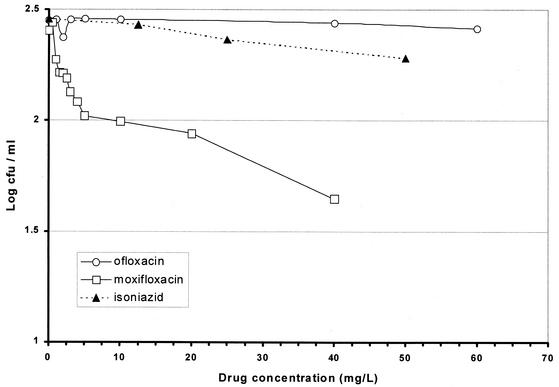
The MIC of ofloxacin was the highest at 0.707 mg/liter with a MIC of levofloxacin of half the value for ofloxacin and MICs of moxifloxacin and gatifloxacin at least fourfold lower (Table 1). The results with model 1 showed the least bactericidal activity with ciprofloxacin, slightly greater activity with ofloxacin and levofloxacin, and the greatest activities with moxifloxacin and gatifloxacin (Fig. 2). Details of the falls in counts as a result of exposure to concentrations that might be attained in human lesions are set out in Table 1. Even with a quinolone concentration of 3.125 mg/liter, a fall of about log10 0.5 was obtained with moxifloxacin and gatifloxacin, while the falls after similar exposures to ciprofloxacin and ofloxacin were only log10 0.054 and 0.073, respectively. The results with model 2 show a similar pattern (Fig. 3), in which ciprofloxacin was least bactericidal while ofloxacin was more bactericidal. The ratio between the concentrations of ofloxacin and ciprofloxacin that killed the same proportion of organisms was about 1:1.8. Levofloxacin was twice as bactericidal as ofloxacin, the corresponding ratio of levofloxacin to ofloxacin being 1:2; moxifloxacin and gatifloxacin had greater, similar bactericidal activities. The falls in counts with the attainable concentrations of 1.56 and 3.125 mg/liter were similar to those in model 1, though they started from a lower CFU count (Table 1). In model 3, ofloxacin had no bactericidal activity even at 56 mg/liter, and isoniazid had negligible activity (Fig. 4). In contrast, moxifloxacin had appreciable activity, especially at lower concentrations. While no change in CFU counts occurred in cultures exposed to any concentration of ofloxacin up to 3.0 mg/liter, there was a fall of 0.279 log10 CFU/ml in the culture exposed to moxifloxacin at 3.0 mg/liter (Table 2). Although the falls in counts with moxifloxacin were less than those found with models 1 and 2, the contrast between the bactericidal activities of ofloxacin and moxifloxacin seemed greater because of the complete absence of any ofloxacin activity. The standard deviations of individual estimates were 0.00949 in model 1, 0.0639 in model 2, and 0.0437 in model 3. Summary estimates of bactericidal activities in the three models are set out in Table 3.
TABLE 1.
MICs and falls in viable counts after exposure to the five quinolones in concentrations attainable in human lesions (models 1 and 2)
| Quinolone | MIC (mg/liter) | Result for model and quinolone concn (mg/liter)
|
|||||
|---|---|---|---|---|---|---|---|
| Model 1 (100-day culture)
|
Model 2 (rifampin-tolerant persisters)
|
||||||
| No. of colonies on 2 plates at 0.0 | Δ Falla at:
|
No. of colonies on 2 plates at 0.0 | Δ Falla at:
|
||||
| 1.56 | 3.125 | 1.56 | 3.125 | ||||
| Ciprofloxacin | 0.500 | 867 | 0.026 | 0.040 | 766 | 0.003 | 0.054 |
| Ofloxacin | 0.707 | 816 | 0.041 | 0.117 | 766 | 0.019 | 0.073 |
| Levofloxacin | 0.354 | 857 | 0.196 | 0.226 | 762 | 0.077 | 0.181 |
| Moxifloxacin | 0.177 | 884 | 0.439 | 0.589 | 760 | 0.222 | 0.466 |
| Gatifloxacin | 0.125 | 812 | 0.368 | 0.498 | 762 | 0.271 | 0.536 |
Δ Fall = (log10 CFU/milliliter after no quinolone exposure) − (log10 CFU/milliliter after exposure to 1.56 or 3.125 mg of quinolone/liter). Standard deviations of Δ fall are 0.0134 in model 1 and 0.0903 in model 2.
FIG. 2.
Viable counts after exposure of 100-day cultures of M. tuberculosis to various concentrations of quinolones (model 1).
FIG. 3.
Viable counts for 100-day cultures of M. tuberculosis subsequently exposed first to 100 μg of rifampin/ml and then to various concentrations of quinolones (model 2).
FIG. 4.
Viable counts for 100-day cultures of M. tuberculosis subsequently exposed to 100 μg of rifampin/ml together with various concentrations of ofloxacin, moxifloxacin, and isoniazid (model 3).
TABLE 2.
Falls in viable counts after exposure to ofloxacin or moxifloxacin concentrations attainable in human lesions (model 3)a
| Quinolone concn (mg/liter) | Δ Fall in viable countsb
|
|
|---|---|---|
| Ofloxacin | Moxifloxacin | |
| 0.5 | −0.035 | |
| 1.0 | −0.002 | 0.131 |
| 1.5 | 0.190 | |
| 2.0 | 0.079 | 0.194 |
| 2.5 | 0.217 | |
| 3.0 | −0.079 | 0.279 |
At a quinolone concentration of 0.0 mg/liter, there were 569 and 511 colonies on the plates for ofloxacin and moxifloxacin, respectively.
Δ Fall = (log10 CFU/milliliter after no quinolone exposure) − (log10 CFU/milliliter after quinolone). The standard deviation was 0.0617.
TABLE 3.
Estimates of bactericidal activity against persisting M. tuberculosis
| Quinolone | Bactericidal endpoint (mg/liter)
|
||
|---|---|---|---|
| MSC50 (model 1) | MDC50 (model 2) | MDC50 (model 3) | |
| Ciprofloxacin | >100 | 30 | |
| Ofloxacin | 52 | 15 | >50 |
| Levofloxacin | 51 | 13 | |
| Moxifloxacin | 2 | 4 | 9 |
| Gatifloxacin | 6 | 3 | |
DISCUSSION
Present interest in quinolones arises for several reasons. First, the results of a randomized clinical trial (RCT) at the Tuberculosis Research Centre, Chennai (Madras), India, suggest that ofloxacin may have sterilizing activity, since a regimen with an initial 3-month phase of ofloxacin-isoniazid-rifampin-pyrazinamide followed by 1 month of twice-weekly rifampin-isoniazid was followed in 81 patients by a 4% relapse rate (15). However, the confidence limits on the relapse rate are wide and there was no 4-month control regimen of standard treatment without ofloxacin. Moxifloxacin and gatifloxacin are recently introduced 8-methoxyfluoroquinolones with greater activities in vitro and in murine tuberculosis than those of ofloxacin (1). Furthermore, in experimental murine tuberculosis, moxifloxacin has been found to have sterilizing activity (6, 9), whereas ofloxacin had none (8). There is a need to investigate all three quinolones, since ofloxacin will come out of patent soon and is very likely to become available cheaply. Levofloxacin, the recently introduced l-isomer of ofloxacin, should act in exactly the same manner as ofloxacin but with twice its activity. Ciprofloxacin seems less suitable for treatment of tuberculosis than ofloxacin since its early bactericidal activity is lower (13, 14).
The experiments that we report here clearly demonstrate that the five quinolones used have very different sterilizing activities against 100-day cultures, particularly in the models in which they act against rifampin-tolerant persisters. In models 1 and 2, ofloxacin had slow but definite bactericidal activity against 100-day cultures, but in model 3, it had no demonstrable activity whatsoever. This might be due to the presence of bacilli in the 100-day cultures which are growing slowly or infrequently. These would be killed by ofloxacin in model 1. In model 2, ofloxacin might have had an opportunity to exert weak bactericidal activity on rifampin-tolerant persistent bacilli as they recovered in rifampin-free culture medium. However, in model 3, there was no opportunity for recovery, so that ofloxacin could have acted only on rifampin-tolerant persisters. The different behaviors in the three models enable us to distinguish between weak bactericidal activity against metabolizing bacilli (albeit slowly or infrequently), and sterilizing activity against true persisters that ofloxacin does not seem to possess. In contrast, moxifloxacin had the greatest activity in all three models, suggesting that it was bactericidal against slowly growing bacilli but also had sterilizing activity against persisters that are tolerant of high rifampin concentrations. The fall in viable counts after exposure to about 3 mg of moxifloxacin/liter was greater in models 1 and 2 (log10 0.5) than in model 3 (log10 0.28), perhaps because model 3 measures only true sterilizing activity. The differences in falls in counts in the three models might also have been influenced by the incubation periods with quinolones, which were 5 days in model 1, 7 days in model 2, and 4 days in model 3. Extension of the treatment period in model 3 would, however, be unlikely to decrease the contrast between the activities of ofloxacin and those of moxifloxacin, since it was just as evident when the cultures were exposed to unrealistically high concentrations. Of the three models, model 3 seems to resemble events in first-line treatment best, since it measures the elimination of rifampin-tolerant persisters in the presence of rifampin, as in patients who would be receiving both drugs.
The important variable that remains to be explored in these models is the effect of longer exposure periods on the fall in viable counts. Exposure for 4 days to moxifloxacin at 3 mg/liter reduced the persister population by only log10 0.279. We do not know whether a much longer exposure period would increase the kill proportionately, though some increase would be expected. On this depends the value of moxifloxacin in shortening treatment. It should be noted that molecules have been found that kill all the surviving bacilli of model 2 within 5 days (Coates and Hu, unpublished data). These could lead to a dramatic decrease in the treatment period.
While the results of the rifampin-tolerant persister models described here agree with evaluations of quinolones in experimental murine tuberculosis, they have never been validated in RCTs. Further RCTs are planned to investigate the possible sterilizing activities of ofloxacin, gatifloxacin, and moxifloxacin (including the OFLOTUB proposal supported by the European Commission and two further studies supported by U.S. government agencies). These studies will incorporate surrogate marker studies which seem to be the only way to demonstrate sterilizing activity in an ethically acceptable manner. We predict from our results that moxifloxacin will be found to have weak but demonstrable sterilizing activity while ofloxacin will not be found to have such activity.
Acknowledgments
Four of the quinolones used in this study were provided by Robert C. Reynolds and Jerry D. Rose of the Southern Research Institute through the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF), N01-AI-95364, a research and development contract with the National Institute of Allergy and Infectious Diseases (Barbara Laughon, Project Officer).
Y. Hu was supported by the Raymond Burton Medical Fund.
REFERENCES
- 1.Alvirez-Freites, E. J., J. L. Carter, and M. H. Cynamon. 2002. In vitro and in vivo activities of gatifloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coates, A., Y. Hu, R. Bax, and C. Page. 2002. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discovery 1:895-910. [DOI] [PubMed] [Google Scholar]
- 3.Global Alliance for Tuberculosis Drug Development. 2001. Tuberculosis scientific blueprint for TB drug development. Tuberculosis 81(Suppl. 1):1-52. [DOI] [PubMed] [Google Scholar]
- 4.Hu, Y., and A. R. M. Coates. 2001. Increased levels of sigJ mRNA in late stationary phase cultures of Mycobacterium tuberculosis detected by DNA array hybridisation. FEMS Microbiol. Lett. 202:59-65. [DOI] [PubMed] [Google Scholar]
- 5.Hu, Y., J. A. Mangan, J. Dhillon, K. M. Sole, D. A. Mitchison, P. D. Butcher, and A. R. M. Coates. 2000. Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J. Bacteriol. 182:6358-6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji, B., N. Lounis, C. Maslo, C. Truffot-Pernot, P. Bonnafous, and J. Grosset. 1998. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:2066-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindani, A., V. R. Aber, E. A. Edwards, and D. A. Mitchison. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939-949. [DOI] [PubMed] [Google Scholar]
- 8.Lalande, V., C. Truffot-Pernot, A. Paccaly-Moulin, J. Grosset, and B. Ji. 1993. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob. Agents Chemother. 37:407-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lounis, N., A. Bentoucha, C. Truffot-Pernot, B. Ji, R. O'Brien, A. Vernon, G. Roscigno, and J. Grosset. 2001. Effectiveness of once-weekly rifapentine and moxifloxacin regimens against Mycobacterium tuberculosis in mice. Antimicrob. Agents Chemother. 45:3482-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchison, D. A. 1992. The Garrod Lecture. Understanding the chemotherapy of tuberculosis—current problems. J. Antimicrob. Chemother. 29:477-493. [DOI] [PubMed] [Google Scholar]
- 11.Mitchison, D. A. 2000. Role of individual drugs in the chemotherapy of tuberculosis. Int. J. Tuberc. Lung Dis. 4:796-806. [PubMed] [Google Scholar]
- 12.O'Brien, R. J., and P. P. Nunn. 2001. The need for new drugs against tuberculosis: obstacles, opportunities, and next steps. Am. J. Respir. Crit. Care Med. 162:1055-1058. [DOI] [PubMed] [Google Scholar]
- 13.Sirgel, F. A., F. J. Botha, D. P. Parkin, B. W. Van de Wal, R. S. Schall, P. R. Donald, and D. A. Mitchison. 1997. The early bactericidal activity of ciprofloxacin in patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 156:901-905. [DOI] [PubMed] [Google Scholar]
- 14.Sirgel, F. A., P. R. Donald, J. Odhiambo, W. Githui, K. C. Umapathy, C. N. Paramasivan, C. M. Tam, K. M. Lam, C. W. Lam, K. M. Sole, D. A. Mitchison, and the EBA Collaborative Study Group. 2000. A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J. Antimicrob. Chemother. 45:859-870. [DOI] [PubMed] [Google Scholar]
- 15.Tuberculosis Research Centre (Indian Council of Medical Research), Chennai. 2002. Shortening short course chemotherapy: a randomised clinical trial for treatment of smear positive pulmonary tuberculosis with regimens using ofloxacin in the intensive phase. Indian J. Tuberc. 49:27-38. [Google Scholar]
- 16.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]