Abstract
C-C chemokine receptor 5 (CCR5) is the primary coreceptor for human immunodeficiency virus type 1 (HIV-1) infection. Native chemokines that bind to CCR5 inhibit HIV-1 infection, albeit weakly, but chemically modified chemokines inhibit infection more efficiently. We have investigated the inhibitory mechanism of three N-terminally modified RANTES variants (AOP-, NNY-, and PSC-RANTES) with the MT-2 human T-cell line stably expressing either native or mutated CCR5. The RANTES analogues showed the same rank order (PSC > NNY > AOP) in their capacity to induce prolonged CCR5 internalization, inhibit surface reexpression, and prevent HIV-1 infection on MT-2 cells expressing wild-type CCR5 or CCR5 with four C-terminal serine phosphorylation sites mutated to alanine. None of the RANTES analogues caused internalization of a C-terminal cytoplasmic domain deletion mutant of CCR5, and each derivative had equal potency in inhibiting HIV-1 infection of MT-2 cells expressing this mutant. We conclude that the C-terminal cytoplasmic residues of CCR5 are necessary for receptor sequestration by RANTES analogues but that the process and the relative activity of each derivative are not dependent upon phosphorylation of the C-terminal serine residues. Two mechanisms of antiviral activity are demonstrated: receptor blockade and receptor sequestration. Potency correlates with the ability to induce CCR5 sequestration but not with receptor binding, suggesting that sequestration may make the greater contribution to antiviral activity.
Entry of human immunodeficiency virus type 1 (HIV-1) into target cells is mediated through binding of HIV-1 envelope glycoprotein to the CD4 receptor and to a coreceptor (1, 11, 14, 15, 19), of which the most important are the two chemokine receptors CCR5 and CXCR4 (26, 40, 44). Chemokines belong to a superfamily of small proteins that play an important role in cell activation, migration, proliferation, and inflammation (46). They deliver a signal by binding to their G protein-coupled receptor, a seven-transmembrane-domain protein which transduces information to intracellular second messengers by coupling to heterotrimeric G proteins and subsequently regulating a variety of effector systems (20).
The chemokines MIP-1α, MIP-1β, RANTES, MCP-2, MCP-3, MCP-4, MCP-1, and eotaxin bind to CCR5 (9, 28, 36). MIP-1α, MIP-1β, and RANTES have been shown to inhibit HIV-1 replication (13). N-terminally modified RANTES analogues that inhibit HIV-1 infection more effectively have been developed (27, 33, 39). These molecules include an N-terminal truncation (RANTES[9-68]) (5), a variant extended by methionine (Met-RANTES) (33), a rationally designed analogue of Met-RANTES (aminooxypentane-RANTES [AOP-RANTES]) (39), and a further-optimized molecule based on AOP-RANTES (Nα-nonanoyl-RANTES [NNY-RANTES]) (27).
Two mechanisms have been proposed to explain the ability of these RANTES derivatives to inhibit HIV-1 infection. One mechanism postulates direct competition between the chemokine and gp120 for the coreceptor binding site (39). The other involves desensitization and internalization of the receptor by full or partial agonists, leading to significant intracellular sequestration of coreceptors (2-4, 24, 37, 42). Unlike native chemokines, AOP- and NNY-RANTES have the additional capacity to inhibit the recycling of internalized CCR5, resulting in remarkably profound and prolonged coreceptor sequestration (24, 37).
The mechanism underlying the potent sequestration effect of these RANTES analogues has yet to be elucidated in detail, although it has become apparent that the signaling properties of AOP-RANTES differ from those of RANTES in a number of different ways (45). Notably, AOP-RANTES has a more potent effect than RANTES on the induction of serine phosphorylation of the C-terminal cytoplasmic domain of CCR5 (30, 45), a process that is known to be important for ligand-induced receptor desensitization and internalization of G protein-coupled receptors (20).
In this study, we have compared the efficiency with which AOP-RANTES, NNY-RANTES, and a novel, further-optimized analogue, l-Thia-Pro2,l-α-cyclohexyl-Gly3-NNY-RANTES (PSC-RANTES), induce internalization and inhibit the reexpression of both wild-type and C-terminally mutated variants of CCR5. Our results show that these modified RANTES derivatives owe their enhanced antiviral activity to an increased capacity to sequester CCR5 within the cell, for which the C-terminal tail of the receptor but not the four previously identified serine residues is essential.
MATERIALS AND METHODS
Generation of CCR5-positive T-cell lines.
The cDNA sequence of CCR5 was amplified from total RNA extracted from human peripheral blood mononuclear cells by reverse transcription-PCR (Stratagene, La Jolla, Calif.), ligated into the PCR-Script Amp SK(+) vector (Stratagene, La Jolla, Calif.), and sequenced.
The C-terminally deleted CCR5 (CCR5Δcyt) was generated with a PCR-based strategy by deleting the last 49 amino acids, corresponding to the C-terminal cytoplasmic domain of CCR5, and inserting a stop codon after residue 303. The CCR5 serine mutant (CCR5-S4A) was generated with a double round of PCR with the Quikchange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.).
CCR5, CCR5-Δcyt, and CCR5-S4A were subcloned into a lentiviral plasmid, with expression driven by the cytomegalovirus promoter, and lentiviral vectors for the different constructs were produced with a third-generation, four-plasmid packaging system (16). The plasmids necessary to produce the lentiviruses were cotransfected into 293T cells, and the medium was collected after 48 h and concentrated by ultracentrifugation at 19,400 rpm in a Beckman SW55 rotor. The virus pellet was resuspended in Hanks' balanced salt solution, and the amount of p24 was determined by enzyme-linked immunosorbent assay (NEN/Perkin-Elmer Life Sciences, Boston, Mass.). All transductions were carried out by exposing MT-2 cells to concentrated lentiviral vectors for 12 h at 37°C. MT-2 is a T-cell line expressing CD4 and CXCR4 but not CCR5, as assessed by flow cytometry and reverse transcription-PCR (data not shown). The MT-2 clones expressing different levels of CCR5 were subcloned by limiting dilution and analyzed by flow cytometry. The MT-2 parental cell line and all the derivatives were maintained in RPMI 1640 culture medium with 10% fetal calf serum (Biowhittaker, Walkersville, Md.) in a 5% CO2 incubator.
Flow cytometry.
A total of 2 × 105 cells were incubated at 37°C with the different chemokines diluted in RPMI 1640, or medium alone as a negative control, in a 100-μl volume. Cells were then cooled to 4°C and washed twice with 10 ml of cold 1× phosphate-buffered saline. The cells were then incubated with the human CCR5 antibody PA12, kindly provided by Progenics Pharmaceuticals, Inc. (Tarrytown, N.Y.), for 30 min at 4°C. Binding of PA12 was detected with phycoerythrin-conjugated donkey anti-mouse immunoglobulin G (Jackson Immunoresearch, West Grove, Pa.) on a FACSCalibur instrument, and the results were analyzed with CellQuest software (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.). Expression of CD4 and CXCR4 was analyzed with commercial antibodies (BD Pharmingen, La Jolla, Calif.).
Virus infection.
Ba-L, an R5 HIV-1 laboratory strain, was propagated in peripheral blood mononuclear cells with phytohemagglutinin and interleukin-2. The different MT-2 clones (106) were incubated with HIV-1 overnight, washed three times with fresh culture medium, and incubated in a 12-well plate. When HIV-1 inhibitors were used, the cells were incubated for 2 h before infection. Replication of HIV-1 was determined in the culture supernatant by p24 enzyme-linked immunosorbent assay.
Cell fusion assay.
R5-tropic envelope-dependent cell fusion assays were performed as previously described (37). Cell fusion is dependent on fusion of HeLa cells expressing the envelope from the HIV-1 strain ADA (31) and HeLa CD4- and CCR5-expressing indicator cells (39).
Chemokines.
AOP-, NNY-, and PSC-RANTES were produced by total chemical synthesis as described previously (47). Met-RANTES was produced as described previously (33).
RESULTS
Generation and characterization of MT-2 cell lines expressing CCR5.
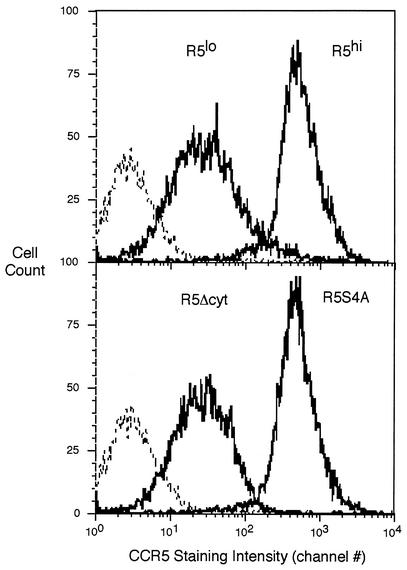
MT-2 cells were transduced with lentiviral vectors carrying either full-length CCR5, a truncated CCR5 (amino acids 1 to 303) lacking the entire C-terminal cytoplasmic domain (CCR5Δcyt), or a mutant CCR5 that has the four C-terminal serine residues mutated to alanine (CCR5-S4A) (Table 1). From the cells transduced with full-length CCR5, two subclones which expressed either low (MT-2-R5lo) or high (MT-2-R5hi) surface levels of CCR5 were derived by limiting dilution (Fig. 1). The level of expression of CCR5 in all the clones was analyzed by flow cytometry and was stable over time. The expression level of CXCR4 and CD4 (data not shown) were not altered after transduction with CCR5 compared to the MT-2 parental cell line.
TABLE 1.
Summary of MT-2 CCR5-expressing cell lines
| Cell line | CCR5 transduced | Level of CCR5 expression | Internalization by RANTES analogues | Infection by R5 HIV |
|---|---|---|---|---|
| MT-2 | No | |||
| MT-2-R5lo | Full length | Low | Normal | Yes |
| MT-2-R5hi | Full length | High | Normal | Yes |
| MT-2-R5Δcyt | 1-303 | Low | No | Yes |
| MT-2-R5S4A | Full lengtha | High | Slow | Yes |
Serines 336, 337, 342, and 349 mutated to alanine.
FIG. 1.
Flow cytometry of MT-2 cells stably transduced with CCR5 (MT-2-R5lo and MT-2-R5hi), CCR5Δcyt (MT-2-R5Δcyt), or serine mutant CCR5 (MT-2-R5S4A). Staining with a CCR5 antibody is shown by a bold line; the negative control (MT-2 cells) is shown by a dashed line.
MT-2 cells were also transduced with CCR5Δcyt (MT-2-R5Δcyt), leading to stable expression of CCR5 on the cell surface (Fig. 1). The CCR5Δcyt truncated protein was also expressed in the cytoplasm of the cells at a level comparable to wild-type CCR5, as detected by flow cytometry of saponin-permeabilized cells and fluorescent microscopy (data not shown). This result shows that the cytoplasmic domain of CCR5 is dispensable for export of the receptor to the cell surface, as expected from some (7, 30) but not all (10, 23, 40) previous reports.
It has been shown previously that the cytoplasmic tail of CCR5 is phosphorylated on four serine residues after ligand binding (30, 45). We generated a mutant CCR5 with the four cytoplasmic serine residues (Ser336, Ser337, Ser342, and Ser349) mutated to alanine to prevent phosphorylation at these critical sites. From the MT-2 cells transduced with this mutant CCR5, a single clone (MT-2-R5S4A) which expressed CCR5 at a level comparable to that by the clone MT-2-R5hi was selected (Fig. 1). This result shows that phosphorylation of the serine residues on CCR5 is not necessary for expression of CCR5 in T cells, in agreement with previous findings with different cell types (10, 43).
Fusion-inhibitory capacity of AOP-, NNY-, and PSC-RANTES.
The inhibitory capacity of two N-terminally modified RANTES derivatives, AOP-RANTES and NNY-RANTES, has been described previously (29, 21). A novel RANTES derivative based on these compounds was recently discovered (J. Wilkens, O. Hartley, D. Thompson, H. Gaertner, R. Fish, C. Pastore, G. Picchio, F. Cerini, N. Heveker, L. Picard, M. Alizon, D. Mosier, S. Kent, and R. Offord, submitted for publication). PSC-RANTES was generated by total chemical synthesis and tested for fusion inhibition between cells expressing gp120 from HIV-1 isolate ADA and target cells expressing CD4 and CCR5 as described before (29). RANTES, AOP-RANTES, NNY-RANTES, and PSC-RANTES had IC50s in the cell fusion assay (mean ± standard deviation) of >1,000,000, 1,200 ± 830, 170 ± 73, and 41 ± 19 pM, respectively. The results are presented graphically as a percent of the control value in Fig. 2.
FIG. 2.
Fusion inhibition by Met-, AOP-, NNY-, and PSC-RANTES. HeLa-P5L and HeLa-Env-ADA cells were exposed to the indicated concentration of modified RANTES, and fusion efficiency was determined by expression of β-galactosidase activity as described before (20).
PSC-RANTES was a potent inhibitor of cell fusion at significantly lower concentrations than NNY- or AOP-RANTES. Most of the prior mechanistic studies have involved AOP-RANTES. The improved potency of NNY- and PSC-RANTES raises the issue of whether these compounds function similarly to AOP-RANTES or invoke novel mechanisms to inhibit HIV-1 gp120-CCR5 interaction.
Internalization and reexpression rate of CCR5 on MT-2-R5 cells.
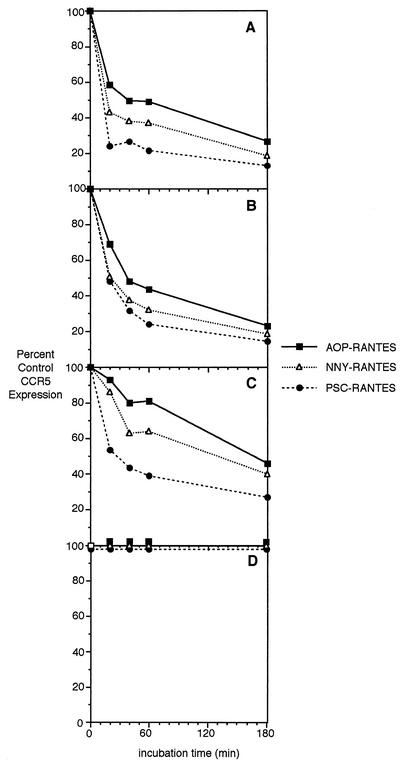
We then tested the ability of the transduced CCR5 to be downregulated by RANTES derivatives. The four cell lines MT-2-R5lo, MT-2-R5hi, MT-2-R5S4A, and MT-2-Δcyt were incubated with 100 nM AOP-, NNY-, or PSC-RANTES, and the expression level of CCR5 was measured by flow cytometry after 20, 40, 60, or 180 min. The antibody used in these experiments (PA12) does not compete for the same binding domain as RANTES (29). As seen in one of three representative experiments presented in Fig. 3A and B, CCR5 was efficiently downregulated in both MT-2-R5lo and MT-2-R5hi cells. The initial internalization rate was slightly faster in the MT-2-R5lo cells. In both cell lines, the efficiency of internalization was PSC > NNY > AOP. In all the experiments performed, CD4 and CXCR4 expression levels were not altered by the presence of the RANTES derivatives (data not shown). Internalization efficiency was slightly lower when a 10 nM concentration of the RANTES analogues were used, showing that the effect is dependent on the concentration of the chemokine used (data not shown).
FIG. 3.
Internalization rate of CCR5 in MT-2-R5lo (A), MT-2-R5hi (B), MT-2-R5S4A (C), and MT-2-R5Δcyt cells (D) during treatment with AOP-, NNY-, or PSC-RANTES. The four MT-2 clones were treated with the different analogues (100 nM), and CCR5 surface expression was analyzed by flow cytometry at different time intervals. The internalization rate is shown as a percentage of the CCR5 expression level in untreated cells.
We next determined if the RANTES derivatives could downregulate expression of the CCR5S4A mutant, in which the previously identified phosphorylation sites (25) in the C-terminal domain had been removed. These experiments address the hypothesis that the different potencies of the RANTES derivatives are due to differences in their capacity to induce serine phosphorylation. Cells from MT-2-R5hi or MT-2-R5S4A were incubated for up to 3 h with AOP-, NNY-, or PSC-RANTES, and expression of CCR5 was analyzed by flow cytometry. As Fig. 3C shows, CCR5S4A was internalized after treatment with AOP-, NNY-, or PSC-RANTES, although the internalization was less efficient than with wild-type CCR5 initially expressed at similar levels (Fig. 3B). This result implies that phosphorylation of CCR5 at the mutated C-terminal serine residues is not essential for internalization of the receptor after ligand binding. Furthermore, the different efficiencies of AOP-, NNY-, and PSC-RANTES in internalizing CCR5 are not dependent on their ability to stimulate serine phosphorylation.
We repeated the internalization experiments, comparing the effects of the natural ligand RANTES on clone MT-2-R5hi and MT-2-R5S4A, to make sure that the results seen before were not due to the modification of the RANTES molecule. Even with the natural ligand RANTES, the serine mutant CCR5 was internalized, albeit at a slower rate than the wild-type CCR5 (data not shown).
To study the role of the C-terminal cytoplasmic domain of CCR5 on its internalization after ligand binding, we analyzed expression of CCR5Δcyt after incubation with 100 nM AOP-, NNY-, or PSC-RANTES. CCR5Δcyt was not internalized from the membrane at any concentration of AOP-, NNY-, or PSC-RANTES tested (up to 100 nM) (Fig. 3D). This result shows that the cytoplasmic domain of CCR5 is essential for ligand-induced internalization in T cells.
Response of CCR5 mutants to continuous exposure to RANTES derivatives.
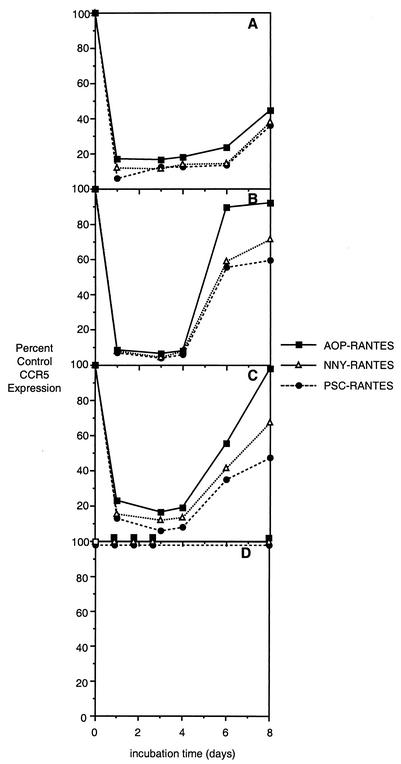
We next incubated the MT-2-R5lo and MT-2-R5hi cells in the continued presence of 10 nM AOP-, NNY-, or PSC-RANTES for 8 days and measured CCR5 surface expression daily to test their efficiency in maintaining CCR5 internalized. The reexpression of CCR5 was dependent upon both the RANTES analogue and the level of CCR5 expression (Fig. 4). In clone MT-2-R5hi, CCR5 levels increased after day 4 (Fig. 4B), while they remained low in clone MT-2-R5lo until day 8 of incubation (Fig. 4A). Treatment with 100 nM RANTES analogues could partially inhibit CCR5 expression for 8 days even in the clone with very high CCR5 expression (data not shown).
FIG. 4.
Extended-incubation experiment with RANTES analogues. MT-2-R5lo (A), MT-2-R5hi (B), MT-2-R5S4A (C), or MT-2-R5Δcyt (D) cells were incubated with 10 nM AOP-, NNY-, or PSC-RANTES for 8 days, and the expression of CCR5 was analyzed by flow cytometry at 1, 3, 4, 6, and 8 days. The reexpression rate is shown as a percentage of the CCR5 expression level in untreated cells.
To study the role of serine phosphorylation in maintaining CCR5 internalization during prolonged exposure to RANTES derivatives, clone MT-2-R5hi or clone MT-2-R5S4A was incubated for 8 days with 10 nM AOP-, NNY-, or PSC-RANTES, and the expression level of CCR5 was analyzed by flow cytometry. In both clones, CCR5 expression was reduced to low levels until day 4 (compare Fig. 4B and C), although the level of expression in MT-2-R5S4A cells was slightly higher than that of wild-type CCR5. These observations are consistent with the internalization results and show that even if unable to activate serine phosphorylation, the three compounds can still block CCR5 expression on the surface. CCR5 expression on MT-2-R5S4A cells returned to normal levels between days 4 and 8 of culture (Fig. 4C) at a rate only slightly slower than that in MT-2-R5hi cells. When MT-2-R5S4A cells were incubated with 100 nM PSC-RANTES, the level of CCR5 remained very low for up to 8 days, confirming that the effect of internalization and inhibition of reexpression is dependent on chemokine concentration.
Continuous incubation of MT-2-R5Δcyt cells with any of the RANTES derivatives at 100 nM for up to 6 days failed to stimulate receptor internalization (Fig. 4D). This result incidentally confirms that binding of antibody PA12 to CCR5 is not inhibited by ligand occupancy.
CCR5 reexpression after ligand removal.
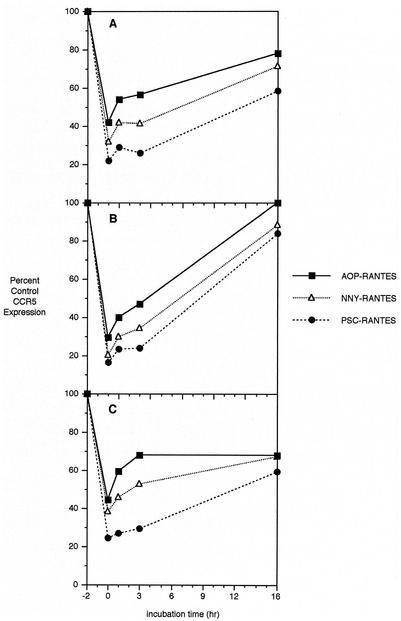
The potency of AOP- and NNY-RANTES has been related to their ability to sequester CCR5 within target cells even after removal of ligand from the extracellular medium (24, 37). To test the ability of RANTES derivatives to inhibit acute reexpression of CCR5, we incubated MT-2-R5lo and MT-2-R5hi cells for 2 h with 10 nM AOP-, NNY-, or PSC-RANTES, then washed them three times with medium, resuspended the cells in fresh medium, and monitored the reexpression of CCR5 by flow cytometry at 1, 3, and 16 h after ligand removal. As shown in one of three representative experiments shown in Fig. 5, the reexpression rate after removal of ligand was higher in clone MT-2-R5hi (Fig. 5B) than in MT-2-R5lo (Fig. 5A), and PSC-RANTES was more potent in inhibiting reexpression than AOP- or NNY-RANTES.
FIG. 5.
CCR5 reexpression rate after ligand-mediated internalization. MT-2-R5lo (A), MT-2-R5hi (B), and MT-2-R5S4A (C) clones were treated with the three analogues (10 nM) and washed three times with and resuspended in fresh medium. CCR5 reexpression was monitored by flow cytometry at 1, 3, and 16 h.
To test whether the ability of the RANTES derivatives to inhibit CCR5 reexpression after ligand removal is dependent on the level of serine phosphorylation induced, similar reexpression experiments were performed with cells from clone MT-2-R5S4A. As shown in Fig. 5C, the reexpression rate in clone MT-2-R5S4A was slower than in clone MT-2-R5hi, although the downregulation was higher for the wild-type CCR5. Both receptor internalization and reexpression appeared to be slowed by the mutation of the four serine phosphorylation sites.
Inhibition of HIV-1 replication in MT-2-R5 cells by RANTES derivatives.
To study the different HIV-1-inhibitory effect of the three RANTES analogues, MT-2-R5lo and MT-2-R5hi cells were incubated with different concentrations of AOP-, NNY-, or PSC-RANTES for 2 h before infection with the R5 HIV-1 isolate BaL. The level of replication was assessed 5 days after infection by a p24 enzyme-linked immunosorbent assay of the cell culture medium. For both clones MT-2-R5lo and MT-2-R5hi, the efficiency of HIV-1 replication inhibition was PSC > NNY > AOP (Fig. 6A and B). In addition, the concentration of RANTES derivatives needed for 50% inhibition (IC50) was proportional to the amount of CCR5 present on the surface, since the IC50 in MT-2-R5lo cells was lower than that in MT-2-R5hi cells for all the RANTES analogues (Table 2). This difference persisted even when we normalized HIV-1 replication rates for the different MT-2-R5 cell lines (Table 2).
FIG. 6.
Infection of MT-2 clones with HIV-1. MT-2-R5lo (A), MT-2-R5hi (B), MT-2-R5S4A (C), or MT-2-R5Δcyt (D) cells were incubated with different concentrations of AOP-, NNY-, or PSC-RANTES for 2 h before infection with the R5 virus BaL. The cells were washed after 24 h and incubated again with the RANTES analogues. The level of replication was assessed 5 days after infection by a p24 enzyme-linked immunosorbent assay of the cell culture medium. The results are shown as percent inhibition compared to the relevant untreated control.
TABLE 2.
Inhibitory concentrations of RANTES derivatives in virus replication assays
| Cell line | RANTES derivative | Mean IC50, pM (SE) | Correcteda IC50 |
|---|---|---|---|
| MT-2-R5lo | AOP-RANTES | 467 (51) | 467 |
| NNY-RANTES | 343 (134) | 343 | |
| PSC-RANTES | 192 (17) | 192 | |
| MT-2-R5hi | AOP-RANTES | 4,540 (445) | 6,124 |
| NNY-RANTES | 2,780 (333) | 3,750 | |
| PSC-RANTES | 1,740 (189) | 2,347 | |
| MT-2-R5S4A | AOP-RANTES | 3,430 (613) | 1,132 |
| NNY-RANTES | 2,230 (452) | 736 | |
| PSC-RANTES | 956 (97) | 315 | |
| MT-2-R5Δcyt | AOP-RANTES | 26 (12) | 497 |
| NNY-RANTES | 20 (6) | 382 | |
| PSC-RANTES | 35 (5) | 669 |
Corrected for p24 antigen concentration (replication efficiency) in untreated control cultures. Virus replication was greatest in MT-2-R5S4A cells and poorest in MT-2-R5Δcyt cells. MT-2-R5lo cells were used as the standard for replication efficiency.
We next determined if the RANTES derivatives AOP-, NNY-, and PSC-RANTES could inhibit infection independently of phosphorylation of the C-terminal serine residues of CCR5 and if different RANTES derivatives have different potency in inhibiting HIV infection in the MT-2-R5S4A serine mutant clone. As seen in Fig. 6C, at a high concentration (100 nM), AOP-, NNY-, and PSC-RANTES completely inhibited replication of the R5 virus BaL in MT-2-R5S4A cells as well as in MT-2-R5hi cells. At lower concentrations, the efficiency of HIV-1 inhibition was PSC > NNY > AOP, as with wild-type CCR5. These results indicate that serine phosphorylation of the C terminus of CCR5 is not essential for the inhibitory capacity of RANTES derivatives. Also, at day 5 after infection, replication was inhibited to the same extent in MT-2-R5S4A as in MT-2-R5hi cells (Fig. 6), while at day 8, the MT-2-R5S4A cells were protected less efficiently (data not shown). These results are consistent with the internalization experiments (Fig. 3), which showed that although the RANTES derivatives were capable of internalizing the mutant receptor, they did so less efficiently than on wild-type CCR5.
RANTES analogues inhibit HIV-1 in MT-2-R5Δcyt cells.
The effect of chemokine inhibition on HIV infection could be due to two different mechanisms, blocking of the HIV binding site or internalization of the receptor before HIV can bind to it. To test to what extent RANTES derivatives can inhibit infection when CCR5 cannot be internalized from the cell surface, we incubated MT-2-R5Δcyt cells with different concentrations of AOP-, NNY-, or PSC-RANTES for 2 h before infection with R5 virus BaL and compared their inhibition efficiency with that measured on cells expressing wild-type CCR5. AOP-, NNY-, and PSC-RANTES were able to fully inhibit HIV infection even without internalization of the receptor (Fig. 6D), showing that their effect includes competition for HIV binding and not solely internalization of CCR5.
Interestingly, AOP-, NNY-, and PSC-RANTES inhibited HIV-1 replication with the same efficiency in MT-2-R5Δcyt cells (Table 2), and the IC50 values were somewhat lower than the corresponding values obtained on cells expressing full-length CCR5. Correction for the lower HIV-1 BaL replication on MT-2-R5Δcyt cells (Table 2) suggests that the potency of each RANTES derivative is in the same range as with MT-2-R5lo cells. The difference in the efficacy of the compounds that is seen in MT-2-R5lo and MT-2-R5hi cells is therefore due to their different ability to internalize and/or inhibit reexpression of the receptor. The initial blockade of coreceptor function appears to be highly efficient but also a highly transient stage in the inhibitory pathway of RANTES analogues and is independent of the different N-terminal RANTES modifications.
DISCUSSION
The discovery of CCR5 as a coreceptor for HIV-1 entry was based on the observation that beta chemokines inhibited infection by macrophage-tropic isolates but not T-cell-line-adapted isolates (13). Native RANTES was the most potent inhibitor of virus infection of T cells, but its activity was modest and variable when macrophages were the targets of infection (39). These observations prompted development of more potent derivatives of RANTES. A number of N-terminal modifications have increased inhibitory capacity; e.g., RANTES 9-68, Met-RANTES, AOP-RANTES, and NNY-RANTES (5, 27, 33, 39). Initial improvements in antiviral potency were associated with increased affinity of the modified RANTES for CCR5, but the relationship between potency and affinity does not hold for the more recent RANTES derivatives.
The ability of AOP- and NNY-RANTES to cause CCR5 internalization and prevent reexpression provides a potential explanation for their increased potency (24, 37), but data supporting a detailed mechanism of action are lacking. The development of the even more potent PSC-RANTES derivative, which binds CCR5 with the same affinity as AOP- or NNY-RANTES (Wilken et al., submitted) but has significantly enhanced fusion inhibitory (Fig. 2) or antiviral activity (Fig. 6), makes resolution of the mechanism of action even more important. It may also be important to examine the activity of these compounds on CCR5 target molecules stably expressed in T cells because the activity of the G protein-coupled receptor has been shown to be cell context dependent (35).
RANTES and AOP-RANTES have been noted to differ in the extent and duration of C-terminal CCR5 phosphorylation stimulated by receptor binding (30, 45). These observations prompted us to examine the importance of the C-terminal region of CCR5 in the absolute and relative activities of AOP-, NNY-, and PSC-RANTES. Wild-type and mutated CCR5s were stably expressed in MT-2 cells following lentivirus vector transduction. Replacing the C-terminal serine targets of phosphorylation with alanine did alter CCR5 internalization and reexpression kinetics modestly (Fig. 4), but it had little impact on the relative or absolute inhibitory capacity of the three RANTES derivatives (Fig. 6). By contrast, deletion of the entire C-terminal domain of CCR5 prevented internalization by any of the RANTES derivatives (Fig. 3 and 4) and equalized their inhibitory capacity (Fig. 6, Table 2). The inhibitory capacity of AOP-, NNY-, and PSC-RANTES is thus predicted by their affinity for CCR5 when coreceptor blockade is the sole mechanism of action. However, the potency of each compound correlated with the kinetics of CCR5 internalization and reexpression on target cells expressing either wild-type or serine-mutated CCR5 (Table 2). Phosphorylation of serine is thus not essential for CCR5 internalization and is not related to the relative potency of the different N-terminal modifications of RANTES.
These results are consistent with several recent observations that suggest that CCR5 internalization is subject to more complex regulation than first appreciated. Kraft et al. (23) found that RANTES induced a slower internalization rate of CCR5 with equivalent serine mutations compared to intact CCR5, in agreement with our findings with AOP-, NNY-, and PSC-RANTES. Total impairment of internalization was achieved only when the tyrosine residue at position 297 was mutated to alanine (23). Tyrosine phosphorylation has also been implicated in the sequestration of other G protein-coupled receptors (22); RANTES and AOP-RANTES as well as R5 HIV-1 envelope induce phosphorylation of CCR5 on tyrosine residues (12, 34). CCR5 with the same four serine mutations and three other substitutions (Tyr339, Thr340, and Thr343 mutated to alanine) was severely impaired for internalization in CEM cells after incubation with MIP-1α or AOP-MIP-1α (10). These observations raise the possibility that CCR5 binds β-arrestin without being phosphorylated in the serine residues, as observed for other G protein-coupled receptors (21). Alternatively, CCR5 may be internalized by novel pathways independently of β-arrestin association (20). The finding that a dominant negative dynamin slowed CCR5 internalization (10) implies that endocytosis is involved.
We were able to obtain relatively high levels of expression of the C-terminal 1-303 truncation mutant of CCR5, in contrast to three recent reports (10, 23, 43). These different results may reflect both the vector used for CCR5 expression and the target cells. We used lentivirus-mediated transduction, which resulted in higher expression levels of all CCR5 constructs than normally obtained by transient transfection. MT-2 cells are human T-lymphotropic virus type 1-transformed T lymphoblastoid cells, and both the cell type and the transformed state may influence the expression of CCR5 and its association with G-proteins, G protein-coupled kinases, and β-arrestins.
We noted that exposure to high concentrations (100 nM) of AOP-, NNY-, or PSC-RANTES led to the internalization of most but not all of cell membrane-associated CCR5 (Fig. 3). Nonetheless, these concentrations of the RANTES derivatives fully inhibited HIV-1 replication (Fig. 6). The residual CCR5 thus was not available or functional for HIV-1 entry. This result could be explained by steric blockade of the envelope binding site by RANTES derivatives, as seen with the C-terminal CCR5 truncation mutant, or by other factors that might block internalization of a small fraction (≈5%) of total CCR5 and also interfere with coreceptor function. Posttranslational modifications of CCR5 are important for coreceptor function (8, 18) and may be rate limiting with the high expression levels of CCR5 observed in these experiments.
We use the terms internalization and reexpression in this report to indicate reduction and subsequent increase in cell surface CCR5. Cell surface-exposed CCR5 was measured by staining with the PA12 antibody, which recognizes an N-terminal CCR5 determinant that is unaltered by ligand binding (29). Indirect staining with a fluorochrome-labeled secondary antibody was used to detect PA12 binding, and all staining procedures were performed at 4°C. to prevent antibody cross-linking and secondary internalization. However, the studies of Signoret et al. (38) with AOP-RANTES suggest that surface expression of CCR5 may be determined by a dynamic process of internalization, rapid receptor recycling, and reinternalization.
When we speak of receptor internalization and reexpression, we are referring to the much slower quasi-steady-state level of CCR5 detectable at the cell surface. As previously noted for AOP-RANTES (24, 38), much of the CCR5 is localized in perinuclear vacuoles after exposure to PSC-RANTES (O. Hartley, unpublished observations). Although one might infer that this CCR5 is unable to be rapidly recycled to the cell surface, the results of Signoret et al. lead us to be cautious about this interpretation. PSC-RANTES was more potent than the other RANTES derivatives in stimulating internalization, slowing reexpression, inhibiting fusion, and blocking infection. These correlations strongly support CCR5 sequestration as an important mechanism of action, but they do not prove this interpretation nor do they rule out additional novel inhibitory mechanisms.
The data in Fig. 2 and 6 show a greater difference between N-terminal modifications of RANTES in their respective potency of inhibition in the cell fusion assay versus the inhibition of infection of MT-2-R5 cells. Several factors may influence these results. The fusion assay is highly dependent upon CCR5 density on HeLa-P5L cells, and the level of CCR5 expression on these cells is substantially lower than on MT-2-R5 cells. Fusion is also a one-time event, by contrast with the virus replication assay, where up to four cycles of infection may occur before the measurement of cumulative p24 capsid antigen levels on day 5 of culture. In addition to these factors that might explain differing sensitivity of the two assays, ADA envelope was used in the fusion assay and HIV-1 BaL was used for infection. Infection with the BaL isolate is known to be more difficult to inhibit with NNY-RANTES than infection with HIV-1 ADA (41). In spite of these several differences in the assay conditions, the relative potency was consistently PSC-RANTES > NNY-RANTES > AOP-RANTES on all CCR5-expressing cell lines except MT-2-R5Δcyt cells.
PSC-, NNY-, and AOP-RANTES were equally and highly potent at inhibiting infection of MT-2-R5Δcyt cells (Fig. 6). The apparent increase in potency (see Table 2) may be surprising given that only one of two mechanisms of action is preserved, receptor blockade. However, comparing the inhibitory capacity between two different cell lines is problematic. Although the amount of CCR5 expressed on MT-2-R5Δcyt cells was matched as closely as possible to that on MT-2-R5lo cells (Fig. 1A), the kinetics of HIV-1 infection are slower on MT-2-R5Δcyt cells (data not shown). It may also be easier to saturate coreceptor binding sites on the static cell surface-exposed CCR5Δcyt molecule than on the recycling full-length CCR5 protein. Binding of native CCR5 ligands may cause a fraction of full-length CCR5 to be internalized and unavailable for immediate interaction with N-terminal modifications of RANTES. Alternatively, intracellular retention of CCR5 may not be a single hit event, but may require multiple binding interactions between molecules like AOP-RANTES and CCR5, as proposed by Signoret et al. (38). Finally, signaling via full-length CCR5 may enhance HIV-1 replication (6, 17, 25, 32) and make inhibition more difficult.
The goal of these studies was to define the mechanisms by which different analogues of the chemokine RANTES inhibit HIV-1 infection and to understand the basis of their relative potency. We found that the novel N-terminally modified RANTES derivative PSC-RANTES induces CCR5 internalization and inhibits its reexpression more efficiently than the previously characterized AOP- and NNY-RANTES. The potency of the three RANTES derivatives in internalization and inhibition of reexpression of CCR5 is reflected in their relative efficacy in inhibiting HIV-1 fusion and replication. We also demonstrate that there are two mechanisms by which these RANTES derivatives inhibit HIV-1 infection, i.e., direct blocking of the CCR5 binding site and coreceptor downmodulation, as originally reported for RANTES by Alkhatib et al. (2). Finally, we show that ligand-induced phosphorylation of CCR5 C-terminal serine residues is not essential for CCR5 downregulation and sequestration following binding of the RANTES analogues and that their different relative potencies are maintained in the absence of serine phosphorylation. The mechanism by which potent HIV-1 inhibitors such as PSC-RANTES cause prolonged intracellular sequestration of CCR5 remains to be determined.
Acknowledgments
This work was supported by NIH grants AI43645, M01-RR00833 (TSRI GCRC), and the Swiss National Science Foundation AIDS Commission (Project 3339-62032-00).
We thank Inder M. Verma for providing the lentiviral vector system.
Footnotes
Publication 15032-IMM from the Scripps Research Institute.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib, G., M. Locati, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1997. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340-348. [DOI] [PubMed] [Google Scholar]
- 3.Amara, A., S. L. Gall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenzana-Seisdedos. 1997. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp Med 186:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aramori, I., S. S. Ferguson, P. D. Bieniasz, J. Zhang, B. Cullen, and M. G. Cullen. 1997. Molecular mechanism of desensitization of the chemokine receptor CCR-5: receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO J. 16:4606-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arenzana-Seisdedos, F., J. L. Virelizier, D. Rousset, I. Clark-Lewis, P. Loetscher, B. Moser, and M. Baggiolini. 1996. HIV blocked by chemokine antagonist. Nature 383:400.. [DOI] [PubMed] [Google Scholar]
- 6.Arthos, J., A. Rubbert, R. L. Rabin, C. Cicala, E. Machado, K. Wildt, M. Hanbach, T. D. Steenbeke, R. Swofford, J. M. Farber, and A. S. Fauci. 2000. CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes. J. Virol. 74:6418-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atchison, R. E., J. Gosling, F. S. Monteclaro, C. Franci, L. Digilio, I. F. Charo, and M. A. Goldsmith. 1996. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science 274:1924-1926. [DOI] [PubMed] [Google Scholar]
- 8.Bannert, N., S. Craig, M. Farzan, D. Sogah, N. V. Santo, H. Choe, and J. Sodroski. 2001. Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines. J. Exp. Med. 194:1661-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanpain, C., I. Migeotte, B. Lee, J. Vakili, B. J. Doranz, C. Govaerts, G. Vassart, R. W. Doms, and M. Parmentier. 1999. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood 94:1899-1905. [PubMed] [Google Scholar]
- 10.Brandt, S. M., R. Mariani, A. U. Holland, T. J. Hope, and N. R. Landau. 2002. Chemokine-mediated block to HIV entry is associated with CCR5 internalization efficiency. J. Biol. Chem. 277:17291-17299. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Z., P. Zhou, D. D. Ho, N. R. Landau, and P. A. Marx. 1997. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J. Virol. 71:2705-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicala, C., J. Arthos, M. Ruiz, M. Vaccarezza, A. Rubbert, A. Riva, K. Wildt, O. Cohen, and A. S. Fauci. 1999. Induction of phosphorylation and intracellular association of CC chemokine receptor 5 and focal adhesion kinase in primary human CD4+ T cells by macrophage-tropic HIV envelope. J. Immunol. 163:420-426. [PubMed] [Google Scholar]
- 13.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 14.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 15.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 16.Dull, T., R. Zufferey, M. Kelly, R. J. Mandel, M. Nguyen, D. Trono, and L. Naldini. 1998. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72:8463-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzan, M., H. Choe, K. A. Martin, Y. Sun, M. Sidelko, C. R. Mackay, N. P. Gerard, J. Sodroski, and C. Gerard. 1997. HIV-1 entry and macrophage inflammatory protein-1beta-mediated signaling are independent functions of the chemokine receptor CCR5. J. Biol. Chem. 272:6854-6857. [DOI] [PubMed] [Google Scholar]
- 18.Farzan, M., T. Mirzabekov, P. Kolchinsky, R. Wyatt, M. Cayabyab, N. P. Gerard, C. Gerard, J. Sodroski, and H. Choe. 1999. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 96:667-676. [DOI] [PubMed] [Google Scholar]
- 19.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson, S. S. 2001. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53:1-24. [PubMed] [Google Scholar]
- 21.Gurevich, V. V., S. B. Dion, J. J. Onorato, J. Ptasienski, C. M. Kim, R. Sterne-Marr, M. M. Hosey, and J. L. Benovic. 1995. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild-type and mutant arrestins with rhodopsin, beta 2-adrenergic, and m2 muscarinic cholinergic receptors. J. Biol. Chem. 270:720-731. [DOI] [PubMed] [Google Scholar]
- 22.Haribabu, B., R. M. Richardson, I. Fisher, S. Sozzani, S. C. Peiper, R. Horuk, H. Ali, and R. Snyderman. 1997. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J. Biol. Chem. 272:28726-28731. [DOI] [PubMed] [Google Scholar]
- 23.Kraft, K., H. Olbrich, I. Majoul, M. Mack, A. Proudfoot, and M. Oppermann. 2001. Characterization of sequence determinants within the carboxyl-terminal domain of chemokine receptor CCR5 that regulate signaling and receptor internalization. J. Biol. Chem. 276:34408-34418. [DOI] [PubMed] [Google Scholar]
- 24.Mack, M., B. Luckow, P. J. Nelson, J. Cihak, G. Simmons, P. R. Clapham, N. Signoret, M. Marsh, M. Stangassinger, F. Borlat, T. N. Wells, D. Schlondorff, and A. E. Proudfoot. 1998. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 187:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marozsan, A. J., V. S. Torre, M. Johnson, S. C. Ball, J. V. Cross, D. J. Templeton, M. E. Quinones-Mateu, R. E. Offord, and E. J. Arts. 2001. Mechanisms involved in stimulation of human immunodeficiency virus type 1 replication by aminooxypentane RANTES. J. Virol. 75:8624-8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michael, N. L., J. A. Nelson, V. N. KewalRamani, G. Chang, S. J. O'Brien, J. R. Mascola, B. Volsky, M. Louder, G. C. White 2nd, D. R. Littman, R. Swanstrom, and T. R. O'Brien. 1998. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 Δ32. J. Virol. 72:6040-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosier, D. E., G. R. Picchio, R. J. Gulizia, R. Sabbe, P. Poignard, L. Picard, R. E. Offord, D. A. Thompson, and J. Wilken. 1999. Highly potent RANTES analogues either prevent CCR5-with human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-with variants. J. Virol. 73:3544-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogilvie, P., G. Bardi, I. Clark-Lewis, M. Baggiolini, and M. Uguccioni. 2001. Eotaxin is a natural antagonist for CCR2 and an agonist for CCR5. Blood 97:1920-1924. [DOI] [PubMed] [Google Scholar]
- 29.Olson, W. C., G. E. Rabut, K. A. Nagashima, D. N. Tran, D. J. Anselma, S. P. Monard, J. P. Segal, D. A. Thompson, F. Kajumo, Y. Guo, J. P. Moore, P. J. Maddon, and T. Dragic. 1999. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J. Virol. 73:4145-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oppermann, M., M. Mack, A. E. Proudfoot, and H. Olbrich. 1999. Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J. Biol. Chem. 274:8875-8885. [DOI] [PubMed] [Google Scholar]
- 31.Pleskoff, O., C. Treboute, and M. Alizon. 1998. The cytomegalovirus-encoded chemokine receptor US28 can enhance cell- cell fusion mediated by different viral proteins. J. Virol. 72:6389-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popik, W., and P. M. Pitha. 1998. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology 252:210-217. [DOI] [PubMed] [Google Scholar]
- 33.Proudfoot, A. E., C. A. Power, A. J. Hoogewerf, M. O. Montjovent, F. Borlat, R. E. Offord, and T. N. Wells. 1996. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J. Biol. Chem. 271:2599-2603. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Frade, J. M., A. J. Vila-Coro, A. Martin, M. Nieto, F. Sanchez-Madrid, A. E. Proudfoot, T. N. Wells, A. C. Martinez, and M. Mellado. 1999. Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J. Cell Biol. 144:755-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohrer, D. K., and B. K. Kobilka. 1998. G protein-coupled receptors: functional and mechanistic insights through altered gene expression. Physiol Rev 78:35-52. [DOI] [PubMed] [Google Scholar]
- 36.Ruffing, N., N. Sullivan, L. Sharmeen, J. Sodroski, and L. Wu. 1998. CCR5 has an expanded ligand-binding repertoire and is the primary receptor used by MCP-2 on activated T cells. Cell. Immunol. 189:160-168. [DOI] [PubMed] [Google Scholar]
- 37.Sabbe, R., G. R. Picchio, C. Pastore, O. Chaloin, O. Hartley, R. Offord, and D. E. Mosier. 2001. Donor- and ligand-dependent differences in C-C chemokine receptor 5 reexpression. J. Virol. 75:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Signoret, N., A. Pelchen-Matthews, M. Mack, A. E. Proudfoot, and M. Marsh. 2000. Endocytosis and recycling of the HIV coreceptor CCR5. J. Cell Biol. 151:1281-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons, G., P. R. Clapham, L. Picard, R. E. Offord, M. M. Rosenkilde, T. W. Schwartz, R. Buser, T. N. Wells, and A. E. Proudfoot. 1997. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276:276-279. [DOI] [PubMed] [Google Scholar]
- 40.Simmons, G., D. Wilkinson, J. D. Reeves, M. T. Dittmar, S. Beddows, J. Weber, G. Carnegie, U. Desselberger, P. W. Gray, R. A. Weiss, and P. R. Clapham. 1996. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J. Virol. 70:8355-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torre, V. S., A. J. Marozsan, J. L. Albright, K. R. Collins, O. Hartley, R. E. Offord, M. E. Quinones-Mateu, and E. J. Arts. 2000. Variable sensitivity of CCR5-tropic human immunodeficiency virus type 1 isolates to inhibition by RANTES analogs. J. Virol. 74:4868-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trkola, A., W. A. Paxton, S. P. Monard, J. A. Hoxie, M. A. Siani, D. A. Thompson, L. Wu, C. R. Mackay, R. Horuk, and J. P. Moore. 1998. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J. Virol. 72:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatesan, S., A. Petrovic, M. Locati, Y. O. Kim, D. Weissman, and P. M. Murphy. 2001. A membrane proximal basic domain and cysteine cluster in the C-terminal tail of CCR5 constitute a bi-partite motif critical for cell surface expression. J. Biol. Chem. 276:40133-40145. [DOI] [PubMed] [Google Scholar]
- 44.Verani, A., E. Pesenti, S. Polo, E. Tresoldi, G. Scarlatti, P. Lusso, A. G. Siccardi, and D. Vercelli. 1998. CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J. Immunol. 161:2084-2088. [PubMed] [Google Scholar]
- 45.Vila-Coro, A. J., M. Mellado, A. Martin de Ana, A. C. Martinez, and J. M. Rodriguez-Frade. 1999. Characterization of RANTES- and aminooxypentane-RANTES-triggered desensitization signals reveals differences in recruitment of the G protein-coupled receptor complex. J. Immunol. 163:3037-3044. [PubMed] [Google Scholar]
- 46.Ward, S. G., and J. Westwick. 1998. Chemokines: understanding their role in T-lymphocyte biology. Biochem. J. 333:457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilken, J., D. Hoover, D. A. Thompson, P. N. Barlow, H. McSparron, L. Picard, A. Wlodawer, J. Lubkowski, and S. B. Kent. 1999. Total chemical synthesis and high-resolution crystal structure of the potent anti-HIV protein AOP-RANTES. Chem. Biol. 6:43-51. [DOI] [PubMed] [Google Scholar]