Abstract
Sex ratio theory predicts that, if prevailing ecological or social circumstances differentially influence the fitness benefits of offspring of each sex, parents should adjust their production accordingly to maximize fitness. For species in which sex is chromosomally determined, such as birds and mammals, a differential effect of maternal condition on the fitness of male and female young is one important route whereby selection is expected to favor a bias in the offspring sex ratio at birth or egg laying. However, despite its central place in sex allocation theory, this hypothesis has rarely been tested in wild populations. We manipulated maternal condition upward and downward in a sexually dimorphic wild bird and examined the effect on offspring survival and on offspring sex ratio. The survival to fledging of male, but not female, young was substantially reduced if they came from less well provisioned eggs produced by females in relatively poor condition. As female condition, and thereby her capacity to produce high quality eggs, declined, she progressively skewed the sex ratio of her eggs toward females; i.e., she produced more of the sex with the higher survival prospects. The decline in the survival of male offspring, and the sex ratio bias, was removed when maternal condition was enhanced. These results provide experimental evidence of an adaptive, facultative adjustment of sex ratio in response to changes in maternal condition in wild birds.
Under specific ecological or social conditions, the fitness benefits of producing daughters and sons may vary differentially. When this is so, facultative manipulation of offspring sex gives parents the potential to fine-tune the number and quality of offspring to prevailing circumstances, thereby maximizing parental fitness (1–3). Experimental data in support of the various hypotheses associated with the facultative adjustment of sex ratios in species in which sex is chromosomally determined are very limited (4–9). One important hypothesis is the “maternal condition advantage.” This hypothesis predicts that females should adjust the sex ratio of their offspring in relation to the effects of their own condition at the time of young production on the offsprings’ fitness (1). However, supporting data are scarce and mainly correlational, particularly in birds and mammals, and experiments so far have been largely confined to captive or domesticated animals, in which it is difficult to measure relevant fitness parameters (4–9).
We experimentally manipulated maternal condition in a wild bird, the lesser black-backed gull (Larus fuscus). Males in this species are larger on average than females (10), grow faster, and are more susceptible to starvation as chicks (11). Maternal body condition has an important effect on egg quality (12, 13), which itself directly influences chicken survival (14). We predicted that (i) when females are in poor condition, and consequently producing low quality eggs, the effect on offspring survival would be greater in male than in female offspring and (ii) females would adjust offspring sex ratios accordingly.
MATERIALS AND METHODS
We manipulated female condition at the time of egg laying by continuous egg removal and supplementary feeding. Gulls usually lay three eggs but can compensate for eggs lost during laying (16). Therefore, when fresh eggs are removed, the gulls can be induced to lay extra eggs. Egg production is costly (17, 18), and this manipulation reduces the condition of the female parent (19) and results in progressively smaller eggs (Fig. 1) containing proportionally fewer nutrients (16). In contrast, supplementary feeding improves both maternal condition and egg quality (12, 13).
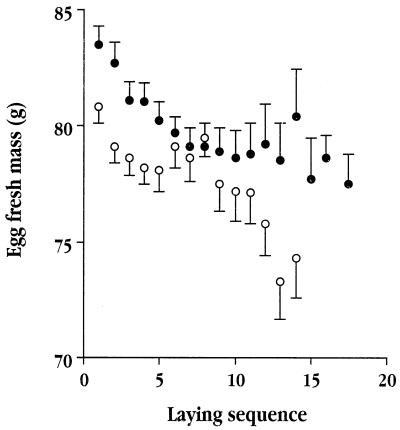
Figure 1.
Changes in mean egg mass (±SE) in relation to position in the laying sequence in female lesser black-backed gulls induced to lay extended clutches. To account for the fact that every female laid several eggs, we calculated separately the slope of linear regressions for each female. The within-female slope for females that were not supplement fed (open circles) averaged −0.413 (±0.096) g per egg laid (n = 57), which is significantly different from 0 (t = 4.29, P < 0.001). The slope for females given supplementary food (solid circles) averaged −0.623 (±0.083) g per egg (n = 57), which is significantly different from 0 (t = 5.53, P< 0.0001). The two groups did not differ in their slopes (t = 1.66, df = 112, P > 0.05), but did in their elevations (t = 3.09, df = 112, P = 0.003). Thus, for any given position in the laying sequence, the eggs of the supplemented birds were larger. (Half of the clutches shown were collected before development for other studies of their chemical composition.)
The field work was carried out in 1996 in a colony on Walney Island, Cumbria, U.K. In study nests, distributed in the center of the colony, we removed every egg within 8 h of its being laid. We induced 62 females, approximately half (32) of which were fed a supplement during the prelaying period, to lay extended clutches. Pairs were randomly allocated to the food supplement group, and these received a bolus of 140 g of baked hen egg beside the nest every night for approximately 3 weeks before the first egg was laid. We previously found that this treatment enhances female condition and egg quality in this species, with no effect on laying date and little effect on clutch size (12, 13). Consequently, as expected, there was no difference in laying date or in the number of eggs laid by the two groups [supplement fed: 9.5 ± 0.57 eggs, mean ± SE, n = 32; unsupplemented: 8.57 ± 0.64, n = 30; t = 1.1, nonsignificant (NS)]. These manipulations, therefore, resulted in a group of females whose condition progressively deteriorated as they laid more eggs (unsupplemented females) and a group whose condition was enhanced during the extended laying sequence (supplemented females). This difference was reflected in the quality of their eggs: egg size declined with laying sequence in the unsupplemented females, but, even at the end of their laying sequences, the supplemented females laid eggs similar in size to those laid by unsupplemented females at the start of laying (Fig. 1). All eggs were cross-fostered to control parents to isolate the effects of maternal condition at the time of laying on offspring survival from effects operating during the rearing period. Removed eggs were therefore swapped for one egg in the nests of foster parent that had completed a normal clutch on the same day. To minimize differences in their quality, we used only foster parents that had themselves laid three eggs. To remove the effects of sibling competition on chick survival, we prevented the foster parents’ own remaining two eggs from hatching by dipping them in mineral oil. All foster parents were equally likely to successfully raise their single chicks, independently of their own laying date (unpublished data). The survival of the experimental chicks was observed until day 35, when close to fledging. Only chicks either known to fledge or found dead were used for the survival analysis; there was no evidence of any male bias in the remaining chicks that disappeared (sex ratio in 38 such chicks was exactly 50:50).
Blood samples, taken under license from the Home Office, were obtained from chicks at the time of hatching, and the sexes of these were determined based on a W chromosome repeat (11). To weight clutches of variable sizes equally, only the sex of three hatchlings per experimental nest was used in the statistical analysis of hatchling sex ratio: the first, the middle, and the last chick of each sequence. If the required egg did not hatch or disappeared during incubation, we used the chick hatched from the egg closest to it instead, as long as it still belonged to the same third of the laying sequence. Where no chick within the correct sector was available, the data cell was left empty. In total, 135 chicks whose sex at hatching was known were included in the sex ratio analysis. To check whether the 51 empty cells could have altered the outcome of the analysis, we ran simulations in which these 51 chicks were allowed to take all possible sex ratio combinations, and all of the significant sex ratio effects reported below remained.
We analyzed sex ratio of the offspring and sex-specific posthatching mortality rates as a function of feeding treatment and laying sequence by using stepwise backward logistic regression, starting with the highest-order interaction. Because all ratios between explained deviance and the df were approximately 1, significance tests were based on the χ2 distribution (20).
RESULTS
Males did not hatch from larger eggs overall (n = 264, t = 0.40, NS). Eggs in the supplemented group were larger than those of unsupplemented birds throughout the laying sequence (Fig. 1). Furthermore, even late in the laying sequence, their eggs were comparable in size to those laid by the unsupplemented birds early in the laying sequence [late eggs (last third of laying sequence) of supplemented females = 79.3 ± 0.97 g, n = 32; early eggs (first third of laying sequence) of unsupplemented females = 80.1 ± 0.96 g, n = 30; t = 0.62, NS)]. Within clutches, egg mass declined at a similar rate for both male and female eggs, and this did not differ between treatments (two-way ANOVA; dependent variable: rate of mass decline per clutch, effect sex: F1,70 = 0.02, NS; effect feeding treatment: F1,70 = 0.71, NS; interaction NS). Hence, the general pattern of allocation of resources into male and female eggs over the laying sequence did not differ between feeding treatments. Male hatchlings were skeletally larger (tarsus length at hatching = 33.55 ± 1.35 mm; females = 32.96 ± 1.29 mm, t = 3.19, n = 240, P < 0.01) but not heavier than females (t = 0.97, n = 238, NS). Thus, males apparently carry smaller reserves at hatching, a situation that adversely affects early survival rate (21).
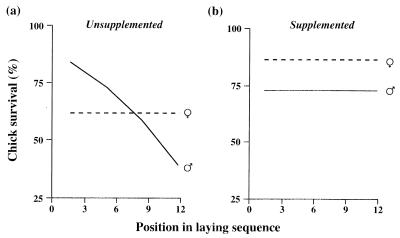
Given the known pattern of posthatching growth and mortality in male chicks in this species (11), we predicted that the experimental reduction in egg quality would influence male offspring more strongly than females. This was found to be the case. The survival of chicks was analyzed in relation to sex, parental feeding treatment, and position in the laying sequence, and the overall results of the logistic regression analysis are given in Table 1. In the unsupplemented group, male survival declined significantly with laying sequence (Fig. 2a; sequence effect on survival of male chicks from unsupplemented group, χ21 = 4.03, P < 0.05, n = 43), whereas that of females did not change with their position in the laying sequence (sequence effect in female chicks from unsupplemented group, coefficient = −0.006 ± 0.011, χ21 = 0.37, df 1, NS, n = 39; overall survival 64.1%, 95% confidence interval = 47.8%, 77.8%).
Table 1.
Results of the multiple logistic regressions in which chick survival was examined in relation to parental feeding treatment, chick sex, and position in the laying sequence (n = 171 chicks), and hatching sex ratio was examined in relation to parental feeding treatment and position in the laying sequence (n = 135 chicks).
| Deviance | χ2 | df | P | |
|---|---|---|---|---|
| Chick survival | ||||
| Null model | 195.02 | 170 | ||
| Final model | 187.40 | 166 | ||
| Sequence | 4.38 | 1 | 0.037 | |
| Feeding treatment | 6.88 | 1 | 0.009 | |
| Sex | 2.40 | 1 | 0.121 | |
| Sex × sequence | 4.12 | 1 | 0.040 | |
| Hatching sex ratio | ||||
| Null model | 185.90 | 134 | ||
| Final model | 176.76 | 131 | ||
| Sequence | 0.82 | 1 | 0.663 | |
| Feeding treatment | 0.51 | 1 | 0.477 | |
| Feeding treatment × sequence | 8.09 | 1 | 0.018 |
Only significant interactions are shown.
Figure 2.
Effect of the decreasing condition of the female parent (manipulated by continuous egg removal) on the survival of male (solid line) and female offspring (dashed line). (a) When parents were given no food supplements during the prelaying period, the survival rate of male chicks declined significantly with position in the laying sequence (logit(survival) = 2.186 − 0.342 (± 0.178) × sequence), whereas that for females remained constant (mean value shown). (b) When parents were given a prelaying food supplement, survival rates were unaffected by position in the laying sequence in either sex; overall survival rates are shown. See text and Table 1 for all analyses.
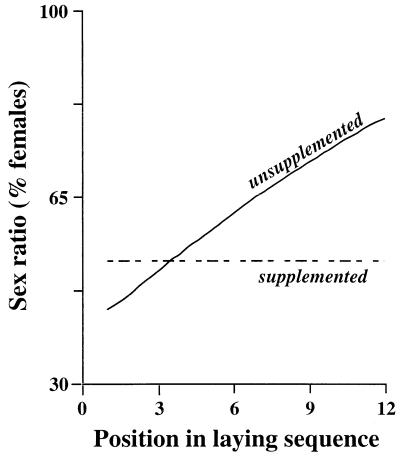
Given that this species is long lived and monogamous (10) and that data from closely related species suggest no sex differences in adult mortality patterns (22), it is very unlikely that a survival disadvantage of the magnitude observed would be offset by any later male fitness benefits. Thus, we predicted that, as more eggs are laid and maternal condition deteriorates, offspring sex ratio should become increasingly skewed toward females. We indeed found that, although the sex ratio was initially unbiased (50 ± 10% females), it became steadily more female biased, reaching 75 ± 9.9% females at the end of laying (Fig. 3; effect of position in the laying sequence on sex in the unsupplemented group, χ21 =4.00, df = 1, NS, n = 67).
Figure 3.
Offspring sex ratio at hatching in relation to position in the laying sequence. That for parents that received no supplementary food became increasingly female biased (solid line, logit(%females) = 0.397 + 0.161(±0.085) × sequence), whereas in the supplement-fed group offspring sex ratio was independent of laying sequences (average sex ratio indicated by dashed line). See text and Table 1 for analysis.
The parental feeding treatment significantly altered offspring survival, removing the decrease in the survival of male offspring with laying sequence (multiple logistic regression analysis for male chick survival from the supplemented group, effect of position in sequence: coefficient = −0.014 ± 0.014, χ21 = 2.01, P > 0.05, n = 37; see Table 1 for overall effects). The survival of male chicks in the supplemented group was 72.8% (95% confidence interval = 56.1%, 85.1%), which is similar to that of males from eggs laid early in the sequence by unsupplemented parents (78.9% for first two eggs, Fig. 2). Female survival again did not change with position in the laying sequence (logistic regression, coefficient = 0.014 ± 0.183, χ21 = 0.65, NS, n = 52; overall female survival 86.5%, 95% confidence interval = 74.0%, 93.5%). Because the survival prospects of the two sexes did not change in this group as more eggs were laid, there should be no progressive bias in offspring sex ratio with laying sequence. As predicted, the average sex ratio remained constant throughout the laying sequence at 53.0 ± 5.9% females (Fig. 3, logistic regression, χ21 = 1.84, NS, n = 68), and the slopes of the relationships for the supplemented and unsupplemented groups differed significantly (Table 1).
The sex ratio bias we observed at hatching in the unsupplemented birds could have arisen as a consequence of differential embryo mortality during incubation or from a difference in the primary sex ratio in the eggs laid. Our data concerning embryo mortality strongly support the latter. First, although hatching success did decline with position in the laying sequence (logistic regression, n = 360, effect of laying sequence, coefficient = −0.126 ± 0.062, χ21 = 4.96, P < 0.05), it did not differ between supplemented and unsupplemented birds (logistic regression, effect of feeding treatment, coefficient = 0.043 ± 0.504, χ21 = 0.51, NS; interaction between treatment and sequence, coefficient = 0.032 ± 0.089, χ21 =0.13, P > 0.05). Second, there was no evidence of any male-biased mortality in 14 sexed dead embryos (nine were females, binomial test P = 0.21).
DISCUSSION
The results of our experiments on lesser black-backed gulls demonstrate that male offspring hatching from less well provisioned eggs are much less likely to survive than are females. Consequently, the fitness value of male eggs laid late in extended laying sequences is very low, because the capacity of females to produce well provisioned eggs declines with increasing egg production. Our data from the unsupplemented treatment group show that females respond to this decline in the survival prospects of male offspring by increasingly skewing the sex ratio of the eggs toward females. The facultative nature of this sex ratio adjustment, and the key role of maternal condition, is further demonstrated in the results from the supplementary fed group. Enhancing maternal condition in this way maintains the capacity of females to produce high quality eggs during extended laying. Although the survival of young of both sexes was improved, most importantly, both the survival disadvantage of males from eggs laid late in the laying sequences and the associated sex ratio bias in production were removed. The absence of sex ratio bias during extended laying in supplemented females is most probably because of their improved egg laying capacity throughout the laying sequence. Although egg mass declines with increasing laying sequence, late eggs of supplemented females are still similar in mass and quality to early eggs of unsupplemented females.
Our experimental procedure ensured that the observed bias in sex ratio produced by the unsupplemented birds was not a consequence of a particular type of female producing more offspring of one or the other sex or any link between sex ratio and number of eggs laid. Females were randomly allocated to treatment groups and each female contributed no more than three eggs to the sex ratio analysis; there was no relationship between sex ratio and clutch size (Spearman rank correlation rs = 0.01, P = 0.9, n = 62 females), as would be expected if females producing small clutches tended to produce mostly males. The observed differences in the survival of male and female offspring were clearly because of egg effects, because all eggs in both treatment groups were reared singly by unsupplemented foster parents. Although the sex ratio was based on hatchling sex, the data suggest that the bias must have occurred at the time of laying, because no sex-biased mortality occurred during incubation.
Sex allocation theory was first developed for species with clear mechanisms of sex ratio manipulation, such as haplo-diploidy or environmentally determined sex (23). Although the Trivers–Willard hypothesis (1) has attracted much attention in recent years in organisms in which sex is chromosomally determined, most studies are correlative and show equivocal results. It is possible that some studies failed to find evidence for adjustment in sex allocation because they examined traits for which differences in allocation into male and female offspring has little or no effect on future reproduction (24, 25). In a correlative study of lesser snow geese (Anser caerulescens), differences in performance between male and female offspring were found to be related to the quality of the mother, but there was no significant bias in the sex ratio at hatching (26, 27). In this study, however, average sex ratio per brood was considered, which may have swamped the subtle effects of a within-brood adjustment of the sex ratio of later laid eggs as reported here. Furthermore, because of many confounding factors in correlative studies, it is difficult to identify the exact conditions that are predictably associated with biased sex ratio. A lack of this knowledge is considered to be a great problem in studies of sex ratio because such conditions need to be identified and then manipulated to test predictions of adaptive adjustment of sex ratios (4).
Few studies of birds have found sex ratio variations that fit the predictions of sex allocation theories, initially thought to be attributable to constraints imposed by the avian sex determination process itself (1–7, 28). However, recent correlative studies in the field have suggested that facultative adjustment of sex ratio in birds in response to local circumstances may be more common than hitherto believed (4, 7). Several circumstances have been predicted to favor the facultative adjustment of offspring sex ratio, but the experimental studies needed to identify the key variables and proximate mechanisms in vertebrates are very rare (4–7). In birds, only one has been conducted in the field involving a species that lays a single egg, the Seychelles warbler (Acrocephalus seychellensis). This species adjusts the primary sex ratio in response to changes in the benefits to be gained by producing daughters, which function as helpers at the nest, or sons, which disperse (29). The maternal condition hypothesis, although considered likely to have the most widely applicable effects on sex ratio, has hitherto been tested experimentally only in captivity (4, 8, 9). We have now shown in our experiments a direct link between maternal condition, offspring survival, and the adaptive adjustment of offspring sex ratio. Maternal condition during egg laying is clearly a very important parameter, which may underlie the sex ratio variations reported in several correlative studies. Variation in this parameter is likely to explain the patchy observations of sex ratio skews in nonexperimental studies, e.g., being sometimes found in a particular group such as gulls and sometimes not (11, 15, 30–32).
Acknowledgments
We thank Andrew Lawson and the Cumbria Naturalists Trust for help in the field, Kate Orr for help in the laboratory, and Kate Lessells, Neil Metcalfe, and Graeme Ruxton for comments on a draft of the manuscript. The work was supported by the Natural Environment Research Council.
ABBREVIATION
- NS
nonsignificant
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Trivers R L, Willard D E. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- 2.Charnov E L. The Theory of Sex Allocation. Princeton: Princeton Univ. Press; 1982. [PubMed] [Google Scholar]
- 3.Clutton-Brock T H. The Evolution of Parental Care. Princeton: Princeton Univ. Press; 1991. [Google Scholar]
- 4.Gowaty P A. Curr Ornithol. 1991;8:141–172. [Google Scholar]
- 5.Emlen S. Trends Ecol Evol. 1997;12:291–292. doi: 10.1016/S0169-5347(97)01119-1. [DOI] [PubMed] [Google Scholar]
- 6.Gowaty P. Nature (London) 1997;385:486–487. [Google Scholar]
- 7.Oddie K. Trends Ecol Evol. 1998;13:130–131. doi: 10.1016/s0169-5347(97)01320-7. [DOI] [PubMed] [Google Scholar]
- 8.Kilner R. Anim Behav. 1998;56:155–164. doi: 10.1006/anbe.1998.0775. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury R B, Blakey J K. Proc R Soc London Ser B. 1998;265:895–899. [Google Scholar]
- 10.Cramp S. Handbook of the Birds of the Western Palearctic. Vol. 4. Oxford: Oxford Univ. Press; 1985. [Google Scholar]
- 11.Griffiths R. Ibis. 1992;134:237–344. [Google Scholar]
- 12.Bolton M, Houston D C, Monaghan P. J Anim Ecol. 1992;61:521–532. [Google Scholar]
- 13.Bolton M, Monaghan P, Houston D C. Can J Zool. 1993;71:273–279. [Google Scholar]
- 14.Bolton M. J Anim Ecol. 1991;60:949–960. [Google Scholar]
- 15.Meathral C E, Ryder J P. Col Waterbird. 1987;10:72–77. [Google Scholar]
- 16.Parsons J. Condor. 1976;78:481–492. [Google Scholar]
- 17.Carey C. In: Avian Energetics and Nutritional Ecology. Carey C, editor. New York: Chapman & Hall; 1996. pp. 324–374. [Google Scholar]
- 18.Monaghan P, Nager R G. Trends Ecol Evol. 1997;12:270–274. doi: 10.1016/s0169-5347(97)01094-x. [DOI] [PubMed] [Google Scholar]
- 19.Monaghan P, Nager R G, Houston D C. Proc R Soc London Ser B. 1998;265:1731–1735. [Google Scholar]
- 20.Crawley M J. GLIM for Ecologists. Oxford: Blackwell; 1992. [Google Scholar]
- 21.Williams T D. Biol Rev. 1994;68:36–59. [Google Scholar]
- 22.Monaghan P, Metcalfe N B. Evolution. 1986;40:1096–1099. doi: 10.1111/j.1558-5646.1986.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 23.Frank A A. Annu Rev Ecol Syst. 1990;21:13–55. [Google Scholar]
- 24.Sikes R S. Behav Ecol Sociobiol. 1996;38:303–310. [Google Scholar]
- 25.Lunn N J, Arnould J P Y. Behav Ecol Sociobiol. 1997;40:351–362. [Google Scholar]
- 26.Cooch E G, Lank D B, Cooke F. J Anim Ecol. 1996;65:439–450. [Google Scholar]
- 27.Cooch E G, Lank D B, Robertson R, Cooke F. J Anim Ecol. 1997;66:189–202. [Google Scholar]
- 28.Williams G C. Proc R Soc London Ser B. 1979;205:567–580. doi: 10.1098/rspb.1979.0085. [DOI] [PubMed] [Google Scholar]
- 29.Komdeur J, Daan S, Tinbergen J, Mateman C. Nature (London) 1997;385:522–525. [Google Scholar]
- 30.Ryder J P. Auk. 1983;100:726–728. [Google Scholar]
- 31.Ryder J P, Termaat B M. Auk. 1987;104:526–528. [Google Scholar]
- 32.Sayce J R, Hunt G L. Auk. 1987;104:33–37. [Google Scholar]