Abstract
Inhalation of fresh water containing the free-living ameba Naegleria fowleri may lead to a potentially fatal infection known as primary amebic meningoencephalitis. Amphotericin B is the only agent with established clinical efficacy in the treatment of primary amebic meningoencephalitis in humans, but therapy with this drug is often associated with adverse effects on the kidneys and other organs, and not all persons treated with amphotericin B have survived. We investigated the in vitro activity and in vivo efficacy of newer therapeutic agents in an attempt to identify other useful agents for treating primary amebic meningoencephalitis. Azithromycin has shown in vitro activity against Acanthamoeba spp. and in vivo activity against experimental toxoplasmosis. In our study, the MIC of azithromycin against N. fowleri was 13.4 μM (10 μg/ml), which was 123 times greater than the MIC of amphotericin B, which was 0.108 μM (0.1 μg/ml). Azithromycin protected 100% of mice infected with N. fowleri at a dose of 75 mg/kg/day for 5 days, whereas amphotericin B protected only 50% of mice at a dose of 7.5 mg/kg/day for 5 days, and all control mice died during the 28-day observation period. We conclude that azithromycin has both in vitro and in vivo activity versus N. fowleri and may be a useful addition to therapy for primary amebic meningoencephalitis.
Primary amebic meningoencephalitis is a rapidly fatal infection caused by the free-living ameba Naegleria fowleri. Victims are usually healthy, young individuals with a recent history of swimming or other water-related activities. N. fowleri gains entry to the nasal cavity during inhalation or aspiration of contaminated water. The ameba then migrates along the olfactory nerves, crosses the cribriform plate, and gains access to the central nervous system. Within the brain, N. fowleri causes extensive inflammation, hemorrhage, and necrosis, leading to death in 3 to 7 days (12).
Mortality among patients with primary amebic meningoencephalitis is greater than 95% (4). This grave prognosis is due to the rapid progression of the disease, the often delayed diagnosis, and the lack of effective therapeutic agents. Since primary amebic meningoencephalitis was identified, a wide range of therapeutic agents against N. fowleri have been evaluated, including many antifungal, antiprotozoal, antibacterial, and antipsychotic agents. Most of these drugs were determined to have little or no efficacy against N. fowleri, either in vitro or in vivo (5-8, 22).
Of the drugs that have been evaluated against N. fowleri, amphotericin B, an antifungal drug, is the only agent with established clinical efficacy. Studies have demonstrated the in vitro and in vivo activity of amphotericin B against various strains of N. fowleri (5-8, 13, 17, 19, 20, 21), and at least seven patients with primary amebic meningoencephalitis have been successfully treated with amphotericin B alone or in combination with other drugs (1, 2, 14, 16, 19, 25).
Although amphotericin B remains the first choice for treatment of primary amebic meningoencephalitis, its use is frequently associated with renal toxicity, manifested as azotemia and hypokalemia. Amphotericin B often causes anemia, and many patients experience chills, fever, nausea, vomiting, and headache. Moreover, not all patients treated with amphotericin B have survived primary amebic meningoencephalitis (21). Therefore, we investigated the activity of newer antimicrobial agents versus N. fowleri in vitro and in a mouse model of primary amebic meningoencephalitis in order to identify other potentially useful agents for treating this infection in humans. Azithromycin, a macrolide antibiotic, was selected for this study on the basis of previous reports that described the in vitro sensitivity of Acanthamoeba spp. to this drug (18) and its activity in experimental toxoplasmosis (3).
This report presents the results of our in vitro and in vivo studies of azithromycin versus N. fowleri and a comparison of its activity with that of amphotericin B.
MATERIALS AND METHODS
Amebae and cultivation.
The Lee (M67) strain of N. fowleri used in this study was originally isolated from a 15-year-old female who died from primary amebic meningoencephalitis in 1968 (12) and has been maintained by 67 passages in mice to retain maximum virulence. The Lee (M67) strain was cultured axenically without agitation in Mix medium (12). Stock cultures of N. fowleri were maintained at 37°C in 25-cm2 polystyrene culture flasks containing 10 ml of medium.
Therapeutic agents.
Amphotericin B aqueous solution, 250 μg/ml (Sigma-Aldrich Inc., St. Louis, Mo.), was diluted with sterile deionized water to obtain the final concentrations used for in vitro studies. For in vivo studies, amphotericin B powder, consisting of 45% amphotericin B, 35% deoxycholic acid sodium, and 20% sodium phosphate (Sigma-Aldrich Inc.), was dissolved in sterile deionized water to provide the amphotericin B concentrations needed to administer the indicated doses to mice. Azithromycin for injection (Zithromax; Pfizer Inc., New York, N.Y.), consisting of powdered azithromycin dihydrate, was dissolved in and diluted with sterile deionized water to provide the concentrations and doses employed in this study.
In vitro studies.
For each drug study, 30 ml of Mix medium was inoculated with 104 amebae/ml from 72-h stock cultures. An aliquot of diluted amphotericin B or azithromycin solution was added to experimental flasks to obtain the required drug concentrations, while control flasks received the same 0.12-ml volume of deionized water. Agents were tested at three concentrations, with each concentration tested in triplicate. Amphotericin B was serially diluted to provide final concentrations of 0.01, 0.1, and 1 μg/ml, while azithromycin was diluted to final concentrations of 1, 10, and 100 μg/ml.
The flasks containing amebae and an experimental agent were Vortex shaken, and 10-ml aliquots were distributed to three culture flasks that were incubated at 37°C. Cell growth was determined daily for a period of 96 h and then again at 168 h with a Coulter counter (model ZF; Coulter Electronics, Inc., Hialeah, Fla.). A 0.2-ml aliquot of each cell suspension was added to 9.8 ml of electrolyte solution consisting of 0.5% (vol/vol) formalin and 0.4% (wt/vol) NaCl in deionized water. Cuvettes were Vortex shaken to separate cell aggregates and then read within 5 min. Four successive counts were obtained for each cuvette. The most deviant count for each cuvette was excluded, and the mean of the remaining nine counts (three flasks of three counts each) was determined, and ameba growth was expressed as amebas per milliliter. Ameba concentrations were compared with Student's t test to determine whether differences between control and treated groups were significant. The MIC of each agent was defined as the lowest concentration that significantly (P < 0.05) inhibited ameba growth compared to control cultures throughout the 7-day culture period as determined by Student's t test.
In vivo studies: cell harvesting and inoculation.
Amebae were harvested for mouse inoculations after 72 h of culture in Mix medium at 37°C. The amebae were centrifuged at 2,000 × g for 10 min, washed, and resuspended in Page saline to provide a final concentration of 2 × 106 amebae/ml. Male 21-day-old CD-1 mice weighing approximately 23 g (Charles River Laboratories, Inc., Wilmington, Mass.) were used in all in vivo experiments. Prior to experimentation, mice were housed in plastic cages for 3 days and given free access to food and water. Mice were inoculated by intranasal instillation of 10 μl of Page saline containing 2 × 104 amebae into a single naris under isoflurane anesthesia (AErrane; Baxter Caribe Inc., Deerfield, Ill.). All animal studies were conducted in accordance with standard animal experimentation guidelines and with the approval of the Animal Care and Use Committee at the Oklahoma State University Center for Health Sciences.
Treatment of experimental amebic meningoencephalitis.
Experimental drug treatments began 72 h after inoculation of amebae and continued once daily for 5 days. For each set of experiments, 40 mice were randomly divided into four groups of 10 mice, with each group receiving a different treatment. The control group received daily 0.1-ml intraperitoneal injections of 0.9% sodium chloride injection (Abbott Laboratories, Chicago, Ill.). The treated groups received 0.1-ml intraperitoneal injections containing the active drug in sufficient concentration to provide the specified dosages of amphotericin B (2.5 or 7.5 mg/kg) or azithromycin (25 or 75 mg/kg). Doses represent the quantity of amphotericin B or azithromycin base administered to mice per kilogram of body weight.
Mortality and mean time to death.
Mice were held for 28 days after inoculation, and the cumulative percent dead was recorded on a daily basis. The mean time to death was also determined for each treatment group. In order to verify the cause of death, brain tissue from dead mice was cultured for amebae with 10 ml of Mix medium containing penicillin (500 U/ml) and streptomycin (500 μg/ml) in 25-cm2 polystyrene culture flasks incubated at 37°C. Amebae were observed microscopically in cultures obtained from brain tissue of all infected mice that died during the 28-day observation period.
Statistical analysis.
The statistical significance of differences between ameba concentrations in treated and control cultures was determined with Student's two-sample t test. The mean time to death of treated and control mice was compared with the Mann-Whitney U test.
RESULTS
In vitro studies.
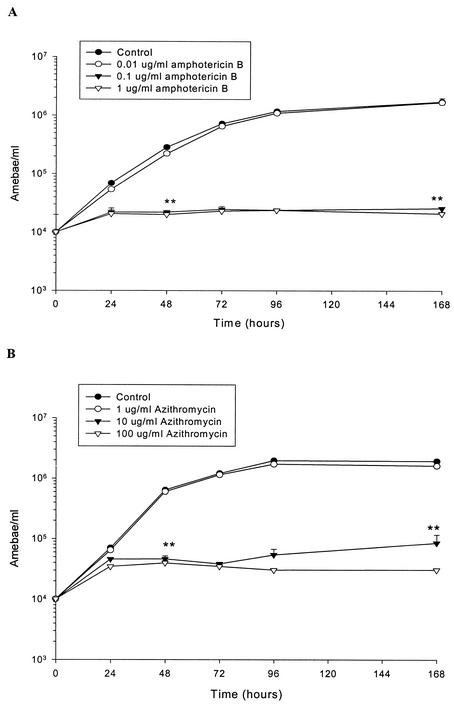
The effect of three log-spaced concentrations of amphotericin B on the growth of the Lee (M67) strain of N. fowleri is shown in Fig. 1. These amphotericin B concentrations were selected on the basis of previous reports of its activity versus N. fowleri (5, 7, 8, 13, 17, 20, 21). Concentrations of 0.1 and 1.0 μg/ml significantly inhibited ameba growth throughout the 7-day incubation period, whereas a concentration of 0.01 μg/ml had limited activity. The 0.1- and 1.0-μg/ml concentrations of amphotericin B inhibited ameba growth by greater than 92% at 48 h of incubation and thereafter, whereas the 0.01-μg/ml concentration produced only 22% inhibition at 48 h, and its inhibitory effect declined at later incubation times. Based on these data, the MIC of amphotericin B versus the Lee strain of N. fowleri was determined to be 0.1 μg/ml (0.108 μM) in this study.
FIG. 1.
Effect of amphotericin B (A) and azithromycin (B) on in vitro growth of the Lee (M67) strain of N. fowleri grown in Mix medium at 37°C. Values are means + standard errors of two experiments performed in triplicate. Error bars that are not visible are smaller than the symbols. Student's t test was used to compare means for treated and control samples at 48 and 168 h. **, significantly different from controls, P < 0.01.
The effect of three concentrations of azithromycin on the Lee strain of N. fowleri is also shown in Fig. 1. These concentrations were derived from a previous report on the effect of this agent on Acanthamoeba and Balamuthia spp. (18). Azithromycin significantly inhibited the growth of N. fowleri at concentrations of 10 and 100 μg/ml, whereas growth in the presence of 1 μg of azithromycin per ml was not significantly different from that of untreated controls. The 10- and 100-μg/ml concentrations produced greater than 90% inhibition of ameba growth at 48 h and thereafter, whereas the 1-μg/ml concentration produced less than 20% inhibition during the 7-day study. These data indicated that the MIC of azithromycin versus the Lee (M67) strain of N. fowleri was 10 μg/ml (13.4 μM) in this study.
In vivo studies.
Amphotericin B and azithromycin were evaluated for their therapeutic effectiveness against the Lee (M67) strain of N. fowleri in a mouse model of primary amebic meningoencephalitis (15). Preliminary experiments were performed to determine the inoculum that would produce 100% mortality in untreated mice. An inoculum of 104 amebae per mouse produced 80% mortality, whereas an inoculum of 2 × 104 amebae produced 100% mortality and was used in all experimental drug studies.
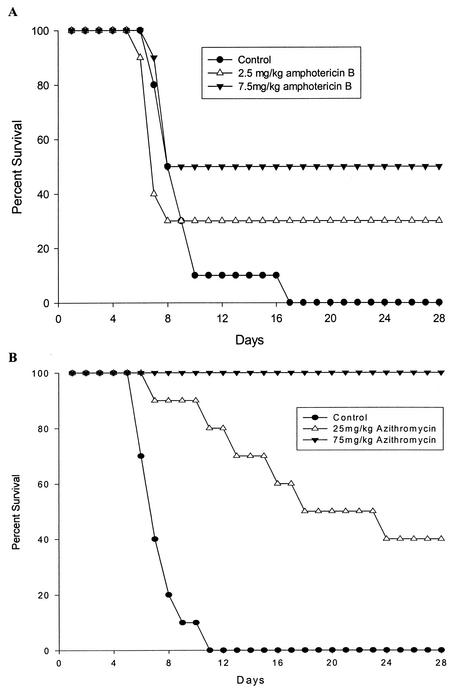
The doses of amphotericin B used in this study were based on previous studies with N. fowleri (5, 9, 23). In our experiments, amphotericin B at 2.5 mg/kg/day protected 30% of mice, with a mean time to death of 7.0 days. A higher dose, 7.5 mg/kg/day, resulted in 50% survival and a mean time to death of 7.8 days (Fig. 2). All untreated control mice died, with a mean time to death of 8.1 days. The mean time to death of amphotericin B-treated mice was not significantly different from that of controls.
FIG. 2.
Survival of mice after inoculation with 2 × 104 N. fowleri, followed by treatment with amphotericin B (A) or azithromycin (B) injected once daily on days to 4 to 8 after infection. Control mice received sterile saline. There were 10 animals per group.
The azithromycin doses used in this study were based on a previous report of the activity of azithromycin in experimental toxoplasmosis (3). Azithromycin at 25 mg/kg/day protected 40% of mice (Fig. 2) and significantly increased the mean time to death, to 14.8 days (P < 0.01). The higher dose of 75 mg/kg/day protected 100% of mice throughout the 28-day observation period. All control mice died, with a mean time to death of 7.5 days in this experiment.
DISCUSSION
Our results indicate that azithromycin has in vitro activity against N. fowleri and protected 100% of mice in an experimental primary amebic meningoencephalitis model. The MIC of azithromycin against N. fowleri in vitro was 13.4 μM (10 μg/ml) in our study, whereas the MIC of amphotericin B was only 0.108 μM (0.1 μg/ml). Thus, amphotericin B was about 123 times more potent on a molar basis than azithromycin in vitro. In contrast, azithromycin was more effective than amphotericin B in an experimental model of primary amebic meningoencephalitis despite its lower in vitro MIC. Azithromycin protected 100% of mice at a dose of 75 mg/kg/day, whereas amphotericin B protected only 50% of mice at a dose of 7.5 mg/kg/day. A lower azithromycin dose of 25 mg/kg/day protected 40% of mice with primary amebic meningoencephalitis and significantly increased the mean time to death.
Amphotericin B is the most potent agent showing activity against N. fowleri, with a MIC ranging from 0.018 to 1.0 μg/ml in various studies (5, 7, 8, 13, 17, 20, 21). The MIC of amphotericin B in our studies (0.1 μg/ml) is close to the median of values reported by other investigators. We found that azithromycin was more effective than amphotericin B in experimental primary amebic meningoencephalitis despite having an MIC that was approximately 123 times greater than that of amphotericin B. The greater in vivo efficacy of azithromycin may be related to the unique pharmacokinetics of this drug, which include a long elimination half-life and high tissue levels (10). Amphotericin B exhibits poor penetration of the blood-brain barrier, whereas azithromycin was widely distributed into human brain tissue following systemic administration to humans (11).
The doses of azithromycin and amphotericin B used in this study were the same doses used by other investigators in experimental primary amebic meningoencephalitis (amphotericin B) or toxoplasmosis (azithromycin). The amphotericin B doses used in mice (2.5 and 7.5 mg/kg/day) are larger than the doses used in the treatment of primary amebic meningoencephalitis in humans (1 to 1.5 mg/kg/day), as reported by John (12). However, Carter (5) stated that the high dose of amphotericin B (7.5 mg/kg) is lower than that required to protect mice from many fungal infections. Based on body surface area rather than weight, this dose is comparable to that customarily used in humans, and the level of amphotericin B in mouse serum with this dose (1.2 μg/ml) is much the same as found in human subjects receiving the usual intravenous doses (5). Carter also showed that 100% of mice survived treatment with amphotericin B at 7.5 mg/kg/day for 10 days. Nevertheless, the high doses of amphotericin B employed in our study may potentially cause kidney toxicity or even contribute to lethality in mice with experimental primary amebic meningoencephalitis.
Likewise, the doses of azithromycin employed in experimental toxoplasmosis (25 to 200 mg/kg) are larger than those required for the successful treatment of human infections. It was reported that 100% of mice with experimental toxoplasmosis survived treatment with 200 mg of azithromycin per kg for 10 days (3). We did not observe any obvious toxicity in mice treated with azithromycin at 75 mg/kg for 5 days.
The protocol used in our mouse model of primary amebic meningoencephalitis employed treatments that began 72 h after inoculation of mice with N. fowleri, whereas most investigators began drug therapy prior to or immediately after inoculation (5, 9, 24). We believe that our protocol more closely resembles the clinical setting, in which patients do not present with severe symptoms until several days after exposure to N. fowleri. This model is therefore more likely to indicate which therapeutic agents might be effective against primary amebic meningoencephalitis in a clinical setting. The only other study of delayed treatment of experimental primary amebic meningoencephalitis found that 37.5% of animals survived after treatment with amphotericin B at 2.5 mg/kg/day for 7 days, which is similar to the 30% survival obtained in our study (23).
Further studies are needed to determine whether azithromycin demonstrates additive, synergistic, or antagonistic effects in combination with amphotericin B in mouse models. Little is known about the mechanisms of action of amphotericin B or other drugs against N. fowleri. Azithromycin inhibits bacterial protein synthesis by binding to the bacterial 50S ribosomal subunit and blocking peptide bond formation and translocation, but its mechanisms of action in Acanthamoeba, Naegleria, and Toxoplasma spp. have not been identified and deserve investigation.
Acknowledgments
We gratefully acknowledge David T. John for providing helpful advice and laboratory facilities.
REFERENCES
- 1.Anderson, K., and A. Jamieson. 1972. Primary amoebic meningoencephalitis. Lancet i:902-903. [DOI] [PubMed] [Google Scholar]
- 2.Apley, J., S. K. Clarke, A. P. Roome, S. A. Sandry, G. Saygi, B. Silk, and D. C. Warhurst. 1970. Primary amoebic meningoencephalitis in Britain. Br. Med. J. 1:596-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo, F. G., R. M. Shepard, and J. S. Remington. 1991. In vivo activity of the macrolide antibiotics azithromycin, roxithromycin and spiramycin against Toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 10:519-524. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, N. D., A. M. Kaplan, R. J. Hopkin, M. A. Saubolle, and M. F. Rudinsky. 1996. Primary amoebic meningoencephalitis with Naegleria fowleri: clinical review. Pediatr. Neurol. 15:230-234. [DOI] [PubMed] [Google Scholar]
- 5.Carter, R. F. 1969. Sensitivity to amphotericin B of a Naegleria sp. isolated from a case of primary amoebic meningoencephalitis. J. Clin. Pathol. 22:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhu, P. 1982. Effect of antimalarial drugs on Naegleria fowleri. Med. J. Aust. 1:13.. [DOI] [PubMed] [Google Scholar]
- 7.Donald, J. J., E. A. Keys, R. T. Cursons, and T. J. Brown. 1979. Chemotherapy of primary amoebic meningoencephalitis (primary amebic meningoencephalitis). N. Z. J. Med. Lab. Technol. March:23-26.
- 8.Duma, R. J., and R. Finley. 1976. In vitro susceptibility of pathogenic Naegleria and Acanthamoeba species to a variety of therapeutic agents. Antimicrob. Agents Chemother. 10:370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante, A. 1982. Comparative sensitivity of Naegleria fowleri to amphotericin B and amphotericin B methyl ester. Trans. R. Soc. Trop. Med. Hyg. 76:476-478. [DOI] [PubMed] [Google Scholar]
- 10.Garey, K. W., and G. W. Amsden. 1999. Intravenous azithromycin. Ann. Pharmacother. 33:218-228. [DOI] [PubMed] [Google Scholar]
- 11.Jaruratanasirikul, S., R. Hortiwakul, T. Tantisarasart, N. Phuenpathom, and S. Tussanasunthornwong. 1996. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob. Agents and Chemother. 40:825-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John, D. T. 1993. Opportunistically pathogenic free-living amebae, p. 143-246. In J. P. Kreier and J. R. Baker (ed.), Parasitic protozoa, 2nd ed., vol. 3. Academic Press Inc., San Diego, Calif.
- 13.Lee, K. K., S. L. Karr, M. M. Wong, and P. D. Hoeprich. 1979. In vitro susceptibilities of Naegleria fowleri strain HB-1 to selected antimicrobial agents, singly and in combination. Antimicrob. Agents Chemother. 16:217-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loschiavo, F., T. Ventura-Spagnolo, E. Sessa, and P. Bramanti. 1993. Acute primary meningoencephalitis from entamoeba Naegleria fowleri. Acta Neurol. (Napoli) 15:333-340. [PubMed] [Google Scholar]
- 15.Martinez, A. J., E. C. Nelson, and R. J. Duma. 1973. Animal model: primary amebic (Naegleria) meningoencephalitis in mice. Am. J. Pathol. 73:545-548. [PMC free article] [PubMed] [Google Scholar]
- 16.Poungvarin, N., and P. Jariya. 1991. The fifth nonlethal case of primary amoebic meningoencephalitis. J. Med. Assoc. Thai. 74:112-115. [PubMed] [Google Scholar]
- 17.Scaglia, M., S. Gatti, A. M. Bernuzzi, C. Cevini, G. Chichino, and E. G. Rondanelli. 1988. An in vitro comparative study on the effect of amphotericin B, econazole, and 5 fluorocytosine on Naegleria fowleri, australiensis, and Naegleria australiensis s.sp. Italica. Microbiologica 11:279-288. [PubMed] [Google Scholar]
- 18.Schuster, F. L., and G. S. Visvesvara. 1998. Efficacy of novel antimicrobials against isolates of opportunistic amebas. J. Euk. Microbiol. 45:612-618. [DOI] [PubMed] [Google Scholar]
- 19.Seidel, J. S., P. Harmatz, G. S. Visvesvera, A. Cohen, J. Edwards, and J. Turner. 1982. Successful treatment of primary amoebic meningoencephalitis. N. Engl. J. Med. 306:346-348. [DOI] [PubMed] [Google Scholar]
- 20.Smego, R. A., and D. T. Durack. 1984. In vitro susceptibility of Naegleria fowleri to ketoconazole, BAYn7133, and allopurinol riboside. J. Parasitol. 70:317-318. [PubMed] [Google Scholar]
- 21.Stevens, A. R., S. T. Shulman, T. A. Lansen, M. J. Cichon, and E. Willaert. 1981. Primary amoebic meningoencephalitis: a report of two cases and antibiotic and immunologic studies. J. Infect. Dis. 143:193-199. [DOI] [PubMed] [Google Scholar]
- 22.Thong, Y. H., B. Rowan-Kelly, C. Shepherd, and A. Ferrante. 1977. Growth inhibition of Naegleria fowleri by tetracycline, rifamycin, and miconazole. Lancet 22:876.. [DOI] [PubMed] [Google Scholar]
- 23.Thong, Y. H., B. Rowan-Kelley, and A. Ferrante. 1979. Delayed treatment of experimental amoebic meningo-encephalitis with amphotericin B and tetracycline. Trans. R. Soc. Trop. Med. Hyg. 73:336-337. [DOI] [PubMed] [Google Scholar]
- 24.Thong, Y. H., B. Rowan-Kelley, and A. Ferrante. 1979. Treatment of experimental Naegleria meningoencephalitis with a combination of amphotericin B and rifamycin. Scand. J. Infect. Dis. 11:151-153. [DOI] [PubMed] [Google Scholar]
- 25.Wang, A., R. Kay, W. S. Poon, and H. K. Ng. 1993. Successful treatment of amoebic meningoencephalitis in a Chinese living in Hong Kong. Clin. Neurol. Neurosurg. 95:249-252. [DOI] [PubMed] [Google Scholar]