Abstract
Data obtained from 318 adult patients treated under the linezolid compassionate-use protocol were used to develop a population model of the pharmacokinetics of intravenous and oral linezolid. All of the patients received 600 mg of linezolid every 12 h, intravenously and/or orally. Blood samples (2 to 10 per patient; median, 4) were obtained and assayed for linezolid by high-performance liquid chromatography. These data and patient covariates were modeled by iterative two-stage analysis, and model discrimination was done by Akaike's information criterion. Of the patient covariates considered (age, sex, ideal body weight, baseline serum albumin, hepatic or renal dysfunction, underlying malignancy, organ transplantation, surgical status, global severity of illness, site of infection, route of administration, and location of care [intensive-care unit, general floor, or outpatient]), only normalized creatinine clearance (CLCR) and body weight explained significant portions of the variance and were incorporated into the pharmacokinetic model. The final model included central and peripheral compartments with parallel capacity-limited (nonrenal) and first-order (renal [CLR]) clearances. Volumes and clearances were normalized to the ideal body weight, and CLR was modeled as proportional to CLCR. Compared to previously studied adult volunteers, intrinsic clearance was ∼60% higher and the maximum rate of metabolism was twice as high in these debilitated patients, resulting in lower area under the time-concentration curve (AUC) values (P < 0.001). The derived 24-h AUC, averaged over the first 7 days of treatment, ranged between 57 and 871 (median, 191) μg/ml · 24 h. Despite these variations, linezolid provided high rates of clinical cure, as well as microbiological success, in the patients treated in the compassionate-use program. The mechanism(s) of these pharmacokinetic differences is unknown and requires further mechanistic study.
Linezolid (Zyvox) is a unique synthetic antimicrobial agent of the oxazolidinone class of antibiotics with activity against all gram-positive organisms, some mycobacteria, and some gram-negative anaerobes (2, 6, 7). Linezolid was approved by the Food and Drug Administration in April 2000 for the treatment of serious infections caused by gram-positive organisms, including those organisms with known multidrug resistance: methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium.
The pharmacokinetics of linezolid following both oral and intravenous (i.v.) administration have been defined in preclinical animal studies and in healthy adult male and female volunteers using a one-compartment linear model (D. J. Stalker, C. P. Wajszczuk, and D. H. Batts, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A115, 1997; D. J. Stalker, C. P. Wajszczuk, and D. H. Batts, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A116, 1997). Absorption of oral linezolid is rapid following administration to humans, with maximum concentrations achieved within 1 to 2 h of dosing. The average absolute bioavailability is 100%, and the drug is 31% bound to plasma protein. The volumes of distribution approximate those of total body water. Nonrenal clearance accounts for ∼65% of total clearance. Renal clearance is low (40 ml/min), and ∼30% of the dose is eliminated unchanged in the urine. Linezolid is metabolized by nonenzymatic chemical oxidation into two inactive metabolites and has not been found to either inhibit or induce any of the major cytochrome P450 isoforms. Linezolid is a weak, reversible, nonselective inhibitor of monoamine oxidase.
In a study conducted by Turnak et al. (M. R. Turnak, A. Forrest, J. M. Hyatt, C. H. Ballow, D. J. Stalker, I. R. Welshman, and J. J. Schentag, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A51, 1998), a two-compartment nonlinear model was used to describe the pharmacokinetics of linezolid. Forty-eight adult volunteers with S. aureus nasal colonization received either 200, 400, or 600 mg of oral linezolid (16 subjects per dose) every 12 h for either 3 or 5 days. The concentrations of linezolid in plasma were shown to increase nonlinearly with increasing doses. Linezolid clearance was modeled as two parallel processes: a first-order renal clearance and a capacity-limited nonrenal process.
The present study was designed to describe the pharmacokinetics of linezolid given i.v. or orally in patients treated under a compassionate-use protocol, including those patients with compromised end organ function and multiple underlying disease states and comorbid conditions.
MATERIALS AND METHODS
Clinical and pharmacokinetic data acquisition.
All patients were enrolled in Pharmacia & UpJohn's protocol compassionate-use program entitled “Linezolid (PNU-100766) given i.v. or orally for compassionate use in patients with significant, multidrug-resistant gram-positive infections” (3). Patients were enrolled from 1 October 1997 to 15 May 2000, and those whose pharmacokinetic sampling and concentration analyses were completed by 31 May 2000 were included in this analysis. Patient inclusion criteria for purposes of this analysis were as follows: males or nonpregnant females at least 13 years of age with signs and symptoms of a significant infectious process; infection due to a multidrug-resistant gram-positive organism which could not be effectively treated with currently marketed conventional antimicrobial agents or patients who were unable to tolerate conventional antibiotic agents. Pediatric patients <13 years of age; patients with renal failure who received peritoneal dialysis, hemodialysis, or hemofiltration; and those patients with inadequate drug concentration data were excluded from the analysis.
Each patient received 600 mg of linezolid twice daily, i.v. and/or orally, at the discretion of the primary investigator. I.v. linezolid was infused over 0.5 to 2 h according to investigator preference. Patients who weighed <40 kg received 10 mg/kg of body weight twice daily. The duration of treatment was up to 3 months with approval from the sponsor. Patient demographics, including sex, age, height, and weight, were collected. Each patient's ideal body weight (IBW) was calculated using standard formulas {male IBW = [50 kg + 2.3(height in inches − 60)] and female IBW = [45.5 kg + 2.3(height in inches − 60)]}. Information regarding the site(s) of infection, hepatic and renal function (aspartate aminotransferase, alanine aminotransferase, total bilirubin, gamma-glutamyl transferase, alkaline phosphatase, lactate dehydrogenase, and serum creatinine), underlying malignancy, surgical status, history of organ transplantation, baseline serum albumin, route of linezolid administration, location of care (intensive-care unit [ICU], general floor, or outpatient), and the patient's overall health status was also collected. The overall baseline health status was assessed by the principal investigator using a McCabe-Jackson scoring system (8) modified as follows: likely survival for <4 days, ≥4 days but <1 month, ≥1 month but <5 years, or ≥5 years. Hepatic function was also categorized by the principal investigator as either normal, abnormal, or end stage. Creatinine clearance (CLCR) was estimated using the equation proposed by Cockcroft and Gault (4) and was normalized to 65 kg.
Pharmacokinetic samples were obtained from patients in one of two ways at the discretion of the study site investigator: by single-interval or split-interval pharmacokinetic sampling. For single-interval sampling, plasma samples were drawn around a single i.v. or oral dose at time zero (immediately prior to drug administration) and at 2, 4, and 8 h after the start of the i.v. infusion or after oral administration. Split-interval pharmacokinetic data sets consisted of peak and trough plasma concentrations drawn around two separate doses. Trough samples were drawn immediately prior to the start of an i.v. infusion or oral-dose administration. Peak samples were drawn 2 h after the start of the i.v. infusion or administration of the oral dose. Study sites were instructed to draw peak samples at the completion of a 2-h infusion and not during the infusion. For all samples, whole blood was drawn into a 5-ml K3EDTA Vacutainer and centrifuged within 1 h to harvest the plasma. The plasma was immediately frozen in an upright position in a −20°C freezer until it was shipped on dry ice via overnight express mail to Pharmacia & Upjohn. Linezolid has been found to be stable in plasma for up to 1 year when stored at or below −20°C (N. K. Hopkins [Pharmacia & UpJohn], personal communication). The analysis of specimens for concentrations of linezolid in plasma was performed by AvTech Laboratories, Inc., Kalamazoo, Mich., using validated high-performance liquid chromatography procedures with a lower limit of quantitation of 0.01 μg/ml and an interday coefficient of variation (CV) of <7%.
Population pharmacokinetic analysis.
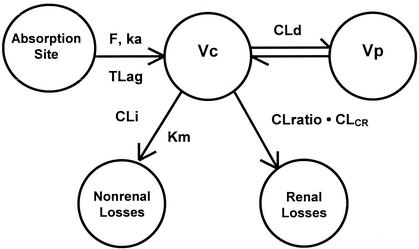
The structural model used to describe linezolid (Fig. 1) is similar to that developed by Turnak et al. (Turnak et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother.) and was chosen on the basis of Akaike's information criterion (1). Changes made to the previous model for the present analysis included the scaling of clearance and distribution volumes to body size and modeling the first-order (renal) clearance as proportional to the estimated CLCR. In modeling the Turnak subjects, neither a linear clearance nor a Michaelis-Menten clearance was adequate to fit the data, and the final model included parallel first-order and Michaelis-Menten eliminations. Because the manufacturer (Pharmacia) had demonstrated renal clearance to be constant with dose ranging, we assumed this linear component of clearance to be renal clearance, assumed that renal clearance was zero when CLCR was zero, and developed a linear function between renal clearance and CLCR based on the measured values in the Turnak subjects.
FIG. 1.
Two-compartment model used to fit linezolid oral and i.v. data. Vc, volume of distribution of the central compartment; ka, absorption rate constant; T lag, lag time before onset of absorption; CLd, distributional clearance; Km, Michaelis-Menten constant; CLi, intrinsic clearance. F, oral bioavailability; CLratio · CLCR, renal clearance as a function of CLCR.
The pharmacokinetic parameters of linezolid were characterized by iterative two-stage analysis (IT2S), a population analysis technique that also provides parameter estimates for each individual in the study sample. The computer program IT2S was based on the methods described by Steimer et al. (9) and was developed using the maximum a posteriori-Bayesian parameter value estimator in the Adapt II, release 4, computer software (5; ADAPT II user's guide: pharmacokinetic/pharmacodynamic systems analysis software, Biomedical Simulations Resource, Los Angeles, Calif.). The pharmacokinetic results from healthy adult volunteers studied by Turnak et al. (Turnak et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother.) were utilized as initial Bayesian priors (see Table 3 for the pharmacokinetic parameters for these subjects). The residual (error) variance model described the observation standard deviation (SD) as linear with the fitted value (y) as follows: SD = SDslope · y + SDintercept, where SDslope and SDintercept are the variance parameters. The values of SDslope and SDintercept were initially estimated based on assay error patterns and were later refined (fitted) based on the data.
TABLE 3.
Mean population pharmacokinetic parametersa
| Parameter | Value
|
P value | |
|---|---|---|---|
| Patients | Volunteers | ||
| Vc (liters/65 kg) | 39.6 (22.7) | 31.77 (31.0) | <0.001 |
| Vss (liters/65 kg) | 65.8 (23.4) | 50.7 (37.6) | <0.001 |
| Km (μg/ml) | 1.46 (68.1) | 1.99 (112) | 0.6 |
| CLi (liters/h/65 kg) | 43.5 (52.5) | 27.2 (93.3) | <0.001 |
| Vmax (mg/h/65 kg) | 53.3 (25.8) | 23.1 (45.2) | <0.001 |
| CLratiob | 0.269 (34.2) | 0.260 (68.9) | 0.4 |
| AUCc (μg/ml · 24 h) | 228 (58.4) | 283 (32.0) | <0.001 |
| CLtavgd (liters/h/65 kg) | 6.85 (50.3) | 4.23 (34.8) | <0.001 |
The mean is the arithmetic or geometric mean; the CV (shown as a percentage in parantheses) is the intersubject CV.
CLratio is the clearance, in liters per hour per 65 kg, by the linear pathway per 1 liter/h/65 kg of CLCR.
24-h AUC for a regimen of 600 mg every 12 h averaged over the first 7 days of treatment.
CLtavg is the calculated average total clearance of linezolid over the first 7 days of treatment.
Plasma concentrations suspected to be outliers were tested as follows. The individual data set was modeled with and without the suspected outlier. If the residual (fitted value minus observed value) was greater than three error SD and if the fitted parameters were substantially different in the two analyses, the sample was declared an outlier and was removed from the study.
The following pharmacokinetic parameters were fitted or derived using data from each subject: volumes of distribution of the central (Vc) and peripheral (Vp) compartments, volume of distribution at steady state (Vss), distributional clearance (CLd), ratio of drug cleared by the linear pathway to the estimated CLCR (CLratio), Michaelis-Menten constant (Km), intrinsic clearance (CLi), and maximum velocity of capacity-limited clearance (Vmax = Km · CLi). The lag time before onset of absorption (TLag) and the absorption rate constant (ka) were estimated for patients receiving oral linezolid. For patients with both oral and i.v. pharmacokinetic samples, an absolute oral bioavailability was estimated. Study patients who were receiving oral linezolid but who did not also receive i.v. linezolid had the absolute oral bioavailability fixed at 1.0 in the analysis. Numeric integration of the fitted model was used to determine the 24-h area under the time-concentration curve (AUC) for each day from 0 to 7, and the average value was used. This 7-day interval was chosen because the majority of patients had measured concentrations in plasma drawn during this time and the parameters derived reflected actual average exposure over the time when bacterial eradication was ongoing. These same parameters were fitted and derived for the healthy adult volunteers studied by Turnak using the same model.
Statistical analysis.
Summary statistics, including the mean, median, SD, and CV, were determined using Systat computer software (SYSTAT: the system for statistics, SPSS, Inc., Chicago, Ill.). General linear modeling was used to determine which patient covariates (age, sex, IBW, CLCR, hepatic function, underlying malignancy, surgical status, history of organ transplantation, baseline serum albumin, site of infection, location of care [ICU, general floor care, or outpatient], and route of administration of linezolid) were significantly associated with the values of certain fitted and derived pharmacokinetic-parameter values (Vss, CLi, Vmax, and Km). Backward stepping with Bonferroni's adjustment of alpha was employed.
RESULTS
Study population.
Patient characteristics are summarized in Table 1. Most subjects had 4 plasma samples (range, 2 to 10). Of the 413 patients with completed linezolid concentration analyses, 318 met the inclusion criteria. Eighty hemodialysis patients and seven pediatric patients were excluded from analysis. Eight patients had data that met outlier criteria or had only a single plasma sample and were excluded from the data set. In the study population, there were 152 females and 166 males with ages ranging from 14 to 88 years (median age, 57). Only 29 patients were treated as outpatients, and 94 had infections or underlying diseases that required treatment in the ICU. The patients in the study had their overall health status categorized by the investigators as follows: 85 patients were likely to survive for ≥5 years, 215 patients were likely to survive for ≥1 month but <5 years, and 18 patients were likely to survive for <1 month. The majority of patients were considered to have normal hepatic function; 64 patients had abnormal function, and 5 patients were considered by the investigators to have end stage hepatic disease. The frequencies for sites of infection were as follows: 119 patients (37%) had bacteremia, 51 patients (16%) had skin and skin structure infections, 49 patients (15%) had intra-abdominal infections or abscesses, 38 patients (12%) had osteomyelitis, 15 patients (4.7%) had endocarditis, 17 patients (5.3%) had pneumonia, 15 patients (4.7%) had urinary tract infections, and 14 patients (4.4%) had other types of infections. Vancomycin-resistant E. faecium (59%) was the most common causative organism in these infections, followed by methicillin-resistant S. aureus (23%). The majority of the patients (73%) received only i.v. linezolid; only 87 patients received any doses of oral linezolid. Eleven patients received both i.v. and oral linezolid.
TABLE 1.
Summary of patient characteristics
| Patient characteristic | Male (n = 166)
|
Female (n = 152)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Median | Mean | CV (%) | Range | Median | Mean | CV (%) | Range | |
| Age (yr) | 56 | 55 | 30 | 14-88 | 58 | 56 | 29 | 16-88 |
| CLCR (ml/min/65 kg)a | 67 | 75 | 50 | 13-175 | 69 | 76 | 49 | 13-175 |
| Total body weight (kg) | 76 | 80 | 28 | 40-200 | 70 | 74 | 30 | 37-142 |
| Calculated IBW (kg)b | 70 | 68 | 13 | 40-85 | 57 | 55 | 14 | 36-77 |
| Baseline serum albumin (g/dl) | 2.5 | 2.6 | 27 | 1.2-4.9 | 2.5 | 2.6 | 29 | 0.9-5.0 |
Estimated by the method of Cockcroft and Gault.
IBW was estimated as follows: male, 50 kg + 2.3(height in inches − 60); female, 45.5 + 2.3(height in inches − 60).
Population pharmacokinetics.
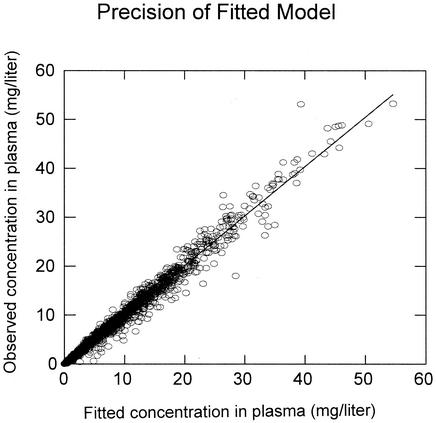
The results of the population pharmacokinetic analysis are shown in Table 2. Although linezolid pharmacokinetics have been characterized by others as having a linear pharmacokinetic model, a Michaelis-Menten or parallel first-order plus Michaelis-Menten model was clearly superior. As shown in Fig. 2, the model fit the data well, with an overall r2 of 0.981. There were no regions of bias; the line of best fit [observed = (1.00 · fitted) + 0.00] did not differ from the line of identity. The fitted SDslope and SDintercept terms were 0.11 and 0.028, respectively. The number of patients with both i.v. and oral data was insufficient to characterize bioavailability in the population, and it was fixed at 1.0 in the analysis.
TABLE 2.
Population pharmacokinetic-parameter values
| Parameter | Mean | CV (%) | Median | Range |
|---|---|---|---|---|
| Vc (liters/65 kg) | 39.6 | 22.7 | 39.3 | 10.0-66.4 |
| Vp (liter/65 kg) | 26.3 | 41.8 | 23.6 | 9.97-90.5 |
| Vss (liters/65 kg) | 65.8 | 23.4 | 63.5 | 27.0-141 |
| CLd (liters/h/65 kg) | 9.09 | 14.9 | 9.17 | 0.98-14.4 |
| CLratioa | 0.269 | 34.2 | 0.275 | 0.100-0.612 |
| Km (μg/ml) | 1.46 | 68.1 | 1.31 | 0.214-12.0 |
| CLi (liters/h/65 kg) | 43.5 | 52.5 | 39.9 | 6.48-246 |
| Vmax (mg/h/65 kg) | 53.3 | 25.8 | 50.3 | 25.8-112 |
| TLag (h) | 0.371 | 97.6 | 0.351 | 0.0-1.91 |
| ka (h−1) | 5.73 | 1.20 | 5.74 | 5.51-5.92 |
| AUC (μg/ml · 24 h) | 228 | 58.4 | 191 | 56.8-871 |
| CLtavg (liters/h/65 kg)b | 6.85 | 50.3 | 6.27 | 1.38-21.1 |
CLratio is the clearance, in liters per hour per 65 kg, by the linear pathway per 1 liter/h/65 kg of CLCR.
CLtavg is the calculated average total clearance of linezolid over the first 7 days of treatment.
FIG. 2.
Observed linezolid concentrations in plasma versus fitted concentrations in plasma. The diagonal is the line of best fit, which did not differ from the line of identity (r2 = 0.981; n = 1,930).
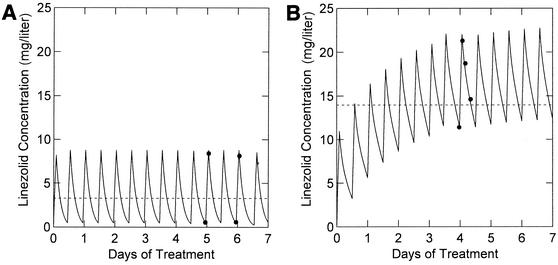
The pharmacokinetic parameters in this target population showed reasonable agreement with the results of Turnak et al. (Turnak et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother.) (Table 3), except for Vss, CLi, and Vmax (P < 0.001). The patients in the present study had much higher nonrenal clearances and Vss: Vmax was twice as high, CLi averaged ∼60% higher, and Vss was 27% higher in these patients. Qualitatively, the increased clearances resulted in lower AUC values in these compassionate-use patients than in healthy adult volunteers given similar regimens (P < 0.001): 8.9% of the patients had AUC values of ≤100 μg/ml · 24 h, 45.0% of the patients had AUC values of >100 but ≤200 μg/ml · 24 h, 37.4% of the patients had AUC values of >200 but ≤400 μg/ml · 24 h, and 8.8% of the patients had AUC values of >400 μg/ml · 24 h. Figure 3 illustrates the difference between a typical patient in the present analysis (Fig. 3A) and a patient whose pharmacokinetic-parameter values were similar to those found in healthy adult volunteers by Turnak et al. (Fig. 3B). The linezolid concentrations, averaged over the first week of treatment, were 3.28 and 13.9 μg/ml in Fig. 3A and B, respectively.
FIG. 3.
Fitted functions in two study patients. The solid circles represent the observed concentrations and the solid lines represent the fitted function for two illustrative patients in this study population. The dashed lines indicate the average concentration during the first 7 days of treatment. (A) Patient pharmacokinetic parameters typical of those found in this study (CLi, 36.6 liters/h/65 kg; Km, 1.8 μg/ml; CLratio, 0.38; CLCR, 82 ml/min; IBW, 75.3 kg). (B) Patient pharmacokinetic parameters similar to those found in healthy adult volunteers by Turnak et al. (CLi, 21.3 liters/h/65 kg; Km, 1.2 μg/ml; CLratio, 0.33; CLCR 62 ml/min; IBW, 70 kg).
None of the remaining patient covariates (age, sex, hepatic function, underlying malignancy, history of organ transplantation, surgical status, baseline serum albumin, site of infection, route of administration, and location of care) explained enough variability in the fitted parameters to justify incorporation into the population model. IBW and total body weight were considered independently and did not differ significantly. Liver function, location of care, and IBW were found to be significant and accounted for 21% of the variance in Vmax. Liver function, which was significant after Bonferroni's adjustment, and CLCR, which was not significant, explained only 3% of the variance in CLi. Although six variables accounted for 13% of the variability of Km, none remained significant after the adjustment of alpha. None of the examined patient covariates explained any of the variance in Vss.
The pharmacokinetic parameters for the patient populations of interest are shown in Table 4. None of the parameters were statistically different, and the magnitude of difference is of no practical importance.
TABLE 4.
Mean pharmacokinetic parameters for targeted patient populationsa
| Parameter | Value
|
||||
|---|---|---|---|---|---|
| ICU patients (n = 94) | Obese patientsb (n = 95) | Oral linezolid therapyc (n = 87) | Elderly patients (n = 74)d | All patients (n = 318) | |
| Vc (liters/65 kg) | 39.8 (25) | 43.9 (18) | 39.3 (19) | 38.2 (21) | 39.6 (23) |
| Vss (liters/65 kg) | 67.7 (24) | 69.7 (18) | 65.1 (25) | 64.2 (22) | 65.8 (23) |
| CLratio | 0.288 (32) | 0.298 (28) | 0.247 (32) | 0.269 (32) | 0.269 (34) |
| Km (μg/ml) | 1.38 (42) | 1.53 (62) | 1.45 (64) | 1.53 (56) | 1.46 (68) |
| CLi (liters/65 kg) | 46.8 (59) | 43.8 (45) | 40.7 (41) | 40.7 (37) | 43.5 (53) |
| Vmax (mg/h/65 kg) | 55.8 (28) | 57.4 (26) | 49.2 (20) | 53.8 (25) | 53.3 (26) |
| AUC (μg/ml · 24 h) | 206 (60) | 210 (56) | 258 (56) | 269 (54) | 228 (58) |
| CLtavg (liters/h/65 kg) | 7.65 (50) | 7.27 (49) | 5.86 (46) | 5.68 (52) | 6.85 (50) |
Patients may be represented in more than one category. The CV (percent) is shown in parentheses.
Patients were categorized as obese if total body weight was >30% above the calculated IBW.
Patients were either started on oral linezolid or switched to oral therapy following initiation of i.v. linezolid.
Patients >70 years of age were considered to be elderly.
We also analyzed the data for factors predictive of total linezolid clearance. Total clearance of linezolid is a function of the concentration in plasma. For each patient, we chose to compute an average total linezolid clearance (the clearance associated with the 7-day average concentration in plasma) using the following formulas: Cavg = AUC0-168/168 h, CLtotal = CLnonrenal + CLR, and CLtotal = (CLi · Km)/(Km + Cavg) + (CLratio · CLCR), where AUC(0-168 h) is the integral of the concentrations in plasma for the interval from 0 to 168 h, Cavg is the average of the drug concentrations over days 1 through 7, CLtotal is the calculated total clearance associated with this average plasma concentration, CLnonrenal is the capacity-limited average clearance, and CLR is the renal clearance; CLratio is the renal clearance per 1 liter/h/65 kg of CLCR. The total average clearance was found to have a mean value of 6.85 liters/h/65 kg (CV = 50%). The relationship between AUC and the average total clearance is illustrated in Table 5.
TABLE 5.
Relationship between AUC and average total clearance
| CLtavga | Value when:
|
|||
|---|---|---|---|---|
| AUC = 100; Cavg = 4.2 μg/ml | AUC = 200; Cavg = 8.3 μg/ml | AUC = 400; Cavg = 17 μg/ml | AUC = 600; Cavg = 25 μg/ml | |
| Minimum | 5.4 | 3.0 | 1.6 | 1.2 |
| 25th percentile | 9.0 | 5.4 | 3.2 | 2.4 |
| Median | 10.1 | 6.3 | 3.9 | 3.0 |
| 75th percentile | 12.0 | 7.6 | 4.8 | 3.7 |
| Maximum | 28.4 | 18.5 | 12.8 | 10.8 |
Average total clearance (in liters per hour per 65 kg) was calculated as the sum of the renal and non renal clearances as follows: CLtavg = Vmax/(Km + Cavg) + CLratio · CLCR, where Cavg is the average linezolid concentration over days 1 through 7; CLratio is defined as the clearance, in liters per hour per 65 kg, by the linear pathway per 1 liter/h/65 kg of CLCR; and CLCR is the normalized estimated creatinine clearance. Note that the median CLtavg decreases by 70% as the AUC increases from 100 to 600 μg/ml·24 h.
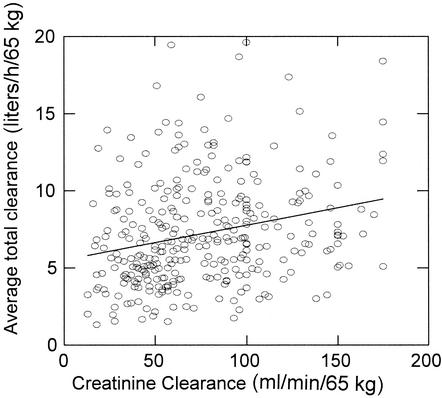
General linear modeling was applied to this derived variable using the same patient covariates described in Materials and Methods. Only liver function, CLCR, location of care, and IBW were found to be significant, and they accounted for 19% of the variance in the average total clearance. In a univariate analysis of total clearance versus age (data not shown), there seemed to be an inverse association. This appears to be due, however, to an age-related decline in CLCR. When age and CLCR (and other covariates) were comodeled versus the average total clearance, only CLCR and weight were significant. Figure 4 is a plot of the normalized total average clearance versus CLCR; the regression equation CLtotal = (5.51 + 0.023 · CLCR)IBW/65 explains only 16% of the variance in the total clearance.
FIG. 4.
Average total clearance versus CLCR. The diagonal is the line of best fit.
DISCUSSION
The pharmacokinetics of linezolid have been previously defined using one-compartment linear models. However, when the data of Turnak et al. (Turnak et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother.) were analyzed, several points became evident. Using a linear model for clearance, we were unable to adequately comodel multiple doses within subjects due to an apparent change in clearance. A pure Michaelis-Menten model of clearance resulted in a significant apparent change in Vmax and Km with dose. Therefore, a model with parallel linear renal clearance and Michaelis-Menten nonrenal clearance was superior by Akaike's information criterion (1) and allowed for direct comparison between patients and healthy adult volunteers. No model, however, has been able to explain the intersubject pharmacokinetic variability in patients treated with linezolid.
Although hepatic functional status, location of care, and IBW were found to be significantly related to Vmax, the cumulative magnitude of their impacts was unimpressive. There was no reason to incorporate any other patient covariates beyond weight and CLCR into the model. The relationship between CLCR and calculated average total clearance was probably partly an artifact of the fixed-dose study design. All patients received 600 mg of linezolid twice daily. For patients with reduced renal clearance, the resulting increased linezolid concentrations in plasma caused saturation of the Michaelis-Menten pathway and a further decrease in the nonrenal clearance. The result was a steeper apparent relationship between total clearance and CLCR. We predict that the relationship will become flatter at a fixed linezolid concentration as opposed to a fixed dose.
The increased Michaelis-Menten clearance in these patients compared to that seen in healthy adult volunteers cannot be easily explained. It is not explained simply by decreased absorption, since most of the patients received i.v. linezolid. Although compliance was not directly assessed, most of the patients were treated in a hospital setting, and it is unlikely that most of the patients missed doses within 24 h of drug concentrations being drawn and that the study site then failed to report the occurrence. The increased intrinsic clearance also cannot be explained by induction of hepatic metabolism because linezolid has not been found to be a substrate for the cytochrome P450 system.
One possible explanation for rapid clearance may lie in the chemical structure and metabolism of linezolid. Linezolid contains a morpholine ring and is susceptible to nonenzymatic chemical oxidation. Oxidative stress may play a role in the increased clearances observed in critically ill patients. Mass balance studies may help to determine if the metabolism of linezolid in these patients is indeed altered. Ultimately, however, the mechanism(s) of this apparent difference in nonrenal elimination and the clinical significance of these findings are unknown and will require further investigation. Theoretically, patients who have a very high clearance of linezolid may be at risk for lower-than-anticipated levels in the blood, and this could lead to treatment failure or possibly the development of resistance. Despite this, linezolid provided high rates of clinical cure, as well as microbiological success, in the debilitated patients treated in this compassionate-use program (3).
REFERENCES
- 1.Akaike, H. 1979. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 66:237-242. [Google Scholar]
- 2.Ashtekar, D. R., R. Costa-Periera, T. Shrinivasan, R. Iyyer, N. Vishvanathan, and W. Rittel. 1991. Oxazolidinones, a new class of synthetic antituberculosis agents; in vitro and in vivo activities of DuP-721 against Mycobacterium tuberculosis. Diagn. Microbiol. Infect. Dis. 14:465-471. [DOI] [PubMed] [Google Scholar]
- 3.Birmingham, M. C., C. R. Rayner, A. K. Meagher, S. F. Flavin, D. H. Batts, and J. J. Schentag. Linezolid in the treatment of multidrug-resistant Gram-positive infections: results of compassionate use experience. Clin. Infect. Dis., in press. [DOI] [PubMed]
- 4.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 5.D'Argenio, D. Z., and A. Schumitzky. 1979. A program package for simulation and parameter estimation in pharmacokinetic systems. Comp. Prog. Biomed. 9:115-134. [DOI] [PubMed] [Google Scholar]
- 6.Jones, R. N., D. M. Johnson, and M. E. Erwin. 1996. In vitro antimicrobial activities and spectra of U-100592 and U-100766, two novel fluorinated oxazolidinones. Antimicrob. Agents Chemother. 40:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaatz, G. W., and S. M. Seo. 1996. In vitro activities of oxazolidinone compounds U-100592 and U-100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 40:799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCabe, W. R., and G. G. Jackson. 1962. Gram-negative bacteremia. Arch. Intern. Med. 110:847-855. [Google Scholar]
- 9.Steimer, J. L., A. Mallet, J. Golmard, and J. Boisieux. 1984. Alternative approaches to estimation of population pharmacokinetic parameters: comparison with the nonlinear mixed effect model. Drug Metab. Rev. 15:265-292. [DOI] [PubMed] [Google Scholar]