Abstract
The aim of this study was to determine whether the time of day ceftriaxone was administered modified its pharmacokinetics. Ceftriaxone was given intraperitoneally at either 0400, 1000, 1600, and 2200 h to Sprague-Dawley rats synchronized under a light-dark cycle of 12 h of light and 12 h of dark. Pharmacokinetic parameters were analyzed for the presence of a 24-h rhythm. Results showed significant daily variations (P < 0.05) in ceftriaxone clearance, with the highest values during the dark phase. It is concluded that time-dependent variations in ceftriaxone pharmacokinetics may affect the therapeutic efficacy of current once-daily dosing schedules.
Ceftriaxone is a broad-spectrum parenteral cephalosporin with potent activity against gram-positive and gram-negative bacteria (19). Ceftriaxone is widely used in humans because of its prolonged half-life, which allows its prescription on a once-a-day basis (4, 17, 20, 28, 32).
Chronokinetics examines the influence of the time of day a drug is administered on drug pharmacokinetics. Most pharmacokinetic studies are performed with one dose in the daytime. However, temporal variations in drug absorption, distribution, hepatic metabolism, and renal excretion have been reported for several drugs (5, 24). Chronokinetic studies are clinically relevant to once-daily drug dosing schedules (5, 12, 23).
The aim of this study was to evaluate possible variations in the disposition of ceftriaxone administered to Sprague-Dawley rats by the intraperitoneal route at different times of the day.
(The results of this study were presented in part at the Millennial World Congress of Pharmaceutical Sciences, San Francisco, Calif., April 2000.)
Four groups (50 to 60 animals/group) of 28-day-old female Sprague-Dawley rats weighing 100 ± 18 g were used throughout the study. Rats were purchased from Bioterio Central (University of Buenos Aires, Buenos Aires, Argentina); they had been kept under a light-dark cycle of 12 h of light (from 0700 to 1900 h) and 12 h of dark at a controlled ambient temperature (22 ± 2°C) from birth. Food and water were freely available. Experiments were conducted in October and November 1999 (spring in the Southern hemisphere). Animal experiments were approved by Secretaría de Ciencia y Técnica, University of Buenos Aires.
Animals were given a single intraperitoneal injection of 100 mg of ceftriaxone (Acantex; Roche, Buenos Aires, Argentina) per kg of body weight either at 0400, 1000, 1600, or 2200 h. At different time intervals after administration of the drug, four to six rats/sampling point were killed by decapitation after cervical dislocation, and blood was collected. Blood samples were allowed to clot, and serum was separated from the blood by centrifugation and frozen (−20°C) until analyzed. Times of drug injection and sampling were expressed in local time.
Ceftriaxone concentrations in serum were determined by a previously described microbiological assay (2). Standard curves of ceftriaxone were prepared from pooled rat serum samples that were run simultaneously with test samples. The quantification limit was 1.17 μg/ml. The correlation coefficient for the regression line of the standard solution was 0.997. The within- and between-day coefficients of variation were less than 10% in the range of observed concentrations (600 to 1.17 μg/ml).
The serum ceftriaxone concentration-time curve for all groups was fitted to a one-compartment model using a nonlinear least-squares regression program (TOPFIT 2.0; Gustav Fischer, Jena, Germany). Pharmacokinetic parameters calculated were as follows: absorption (kabs) and elimination (kel) rate constants, absorption (t1/2abs) and elimination (t1/2el) half-lives, area under the concentration-versus-time curve (AUC), total serum drug clearance (CLT), volume of distribution (V), lag time (Tlag), peak serum concentration (Cmax), and time to reach Cmax (Tmax) (8). Pharmacokinetic parameters were further analyzed by the cosinor method (18) for the presence of a 24-h (circadian) rhythm. This procedure is applicable to longitudinal biologic time series and consists of fitting a cosine curve of constant frequency to sequential data by the least-squares statistical method. Estimated rhythmicity parameters follow: M, mesor (rhythm-adjusted mean); A, amplitude (distance between the mesor and the peak value of the cosine functions); and φ, acrophase (peak value of the fitted cosine function, with midnight [local time] the reference time). The corresponding equation model is Y = (M + A) × cos(0.261t + φ). With this method, the goodness of fit of the cosine curve to the data is indicated by minimizing the sum of squares of the residuals from the analysis (probability of rhythm [PR] value). Rhythm detection is determined by the statistical significance of the amplitude, estimated by testing the null hypothesis that A = 0 (no rhythm exists) by an F test. A PR value of ≥70 and P ≤ 0.05 were considered indicative of rhythm. Relative amplitude was estimated as percent of the mesor (A/M × 100).
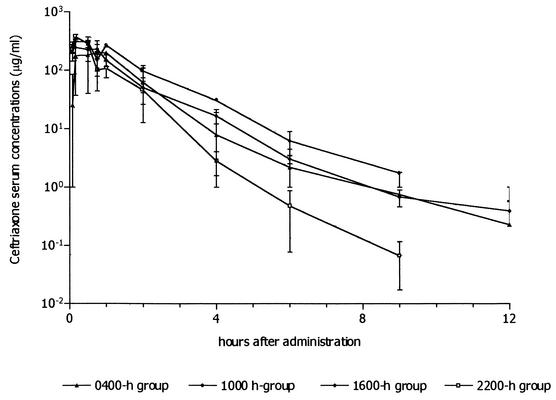
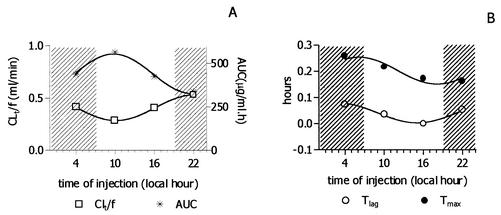
Figure 1 shows the mean concentration-versus-time curve of ceftriaxone in serum after it was administered intraperitoneally at 0400, 1000, 1600, and 2200 h. The pharmacokinetic parameters for each group are presented in Table 1. Cosinor analysis showed significant daily variations in CLT (PR = 100, P = 0.006). PR values for Tlag, AUC, and Tmax indicated a good fit (98, 98, and 88%, respectively), but the P values were 0.14, 0.12, and 0.34, respectively. Mean estimated rhythm parameters (standard deviations given in parentheses) were as follows: for CLT, M = 0.41 (0.0) ml/min, A = −0.12 (0.0) ml/min, and acrophase at 22.08 h; for AUC, M = 439.7 (10.2) μg/ml · h, A = 113.2 (14.5) μg/ml · h, and acrophase at 9.77 h; for Tlag, M = 0.041 (0.004) h, A = 0.03 (0.005) h, and acrophase at 3.05 h; and for Tmax, M = 0.2 (0.01) h, A = 0.05 (0.01) h, and acrophase at 6.11 h. Relative amplitude was higher for Tlag (93%) and lower for AUC and Tmax (25%). Other calculated pharmacokinetic parameters, such as kabs, Cmax, kel, and V, did not exhibit significant rhythmic variations. Figure 2 shows the daily variations observed for CLT, Tlag, AUC, and Tmax.
FIG. 1.
Ceftriaxone concentrations obtained in serum after the intraperitoneal administration (100 mg/kg) to female Sprague-Dawley rats at 0400, 1000, 1600, and 2200 h. Each sampling point represents the mean ± standard deviation (error bar) of data for four to six rats.
TABLE 1.
Pharmacokinetic parameters calculated after the intraperitoneal administration of ceftriaxone (100 mg/kg) to female Sprague-Dawley rats at 0400, 1000, 1600, and 2200 h
| Time (h) of administration | kabs (h−1) | t1/2abs (h) | kel (h−1) | t1/2el (h) | Tlag (h) | V (liter) | CLT (ml/min) | AUC (μg/ml · h) | Tmax (h) | Cmax (μg/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0400 | 19.10 | 0.036 | 0.61 | 1.12 | 0.073 | 0.040 | 0.413 | 436.00 | 0.26 | 240.00 |
| 1000 | 19.70 | 0.035 | 0.64 | 1.08 | 0.035 | 0.027 | 0.285 | 563.00 | 0.21 | 321.00 |
| 1600 | 19.80 | 0.036 | 0.74 | 0.93 | 0.00001 | 0.033 | 0.406 | 423.00 | 0.17 | 276.00 |
| 2200 | 30.60 | 0.022 | 1.28 | 0.54 | 0.054 | 0.025 | 0.532 | 337.00 | 0.16 | 376.00 |
FIG. 2.
Daily profile observed for four pharmacokinetic parameters (CLT, AUC, Tlag, and Tmax) following intraperitoneal administration of ceftriaxone (100 mg/kg) to Sprague-Dawley rats at different times of day. The observed values (symbols) were plotted, and no linear least-squares fitted sine curves for circadian rhythm (frequency = 1/24) were observed. Dark (activity) and light (rest) periods are indicated by the hatched and white backgrounds, respectively.
Several studies have reported temporal variations in the pharmacokinetics of antimicrobial drugs (1, 3, 13, 16, 26, 29-31). Our results show that the total clearance of ceftriaxone varies rhythmically during the day, with its maximum during the dark (activity) period and its minimum during the light (rest) period in rats; however, it must be noted that as intravenous administration was not performed, bioavailability was not included in our CLT calculation. As ceftriaxone is eliminated by biliary and renal excretion in the rat (9, 15), this temporal variation can be attributed, at least partially, to circadian changes in different steps of the kinetic process, such as the biliary and renal functions. Basal bile flow has been reported to exhibit a circadian rhythm with higher values during the active phase of rats (10). The biliar anion carrier system shows its maximum transport capacity during activity (rather than rest) in rats (16, 30). Urinary water, protein and glycosaminoglycan excretions, and glomerular filtration rate (GFR) and renal plasma flow (RPF) show a circadian rhythmicity in rats, with markedly increased values observed during the dark phase in the animals (6, 21, 22). Similar findings were reported for urinary electrolyte (K, P, Mg, Ca, and Na) excretion (21, 25). Cardiac output changes as a function of the diurnal activity and feeding patterns in rats, and renal and biliar blood flow are higher during the activity phase in the rat (7). As GFR, RPF, and renal and biliary excretion follow the same circadian pattern, these factors could be responsible for the time-dependent variation of ceftriaxone clearance.
During the 24 h, CLT acrophase in the dark phase was 180° out of phase with that of AUC, while Tmax and Tlag profiles were very similar, thus confirming the relationship between these two parameters. No rhythm was seen for kel, as this parameter depends on CLT and V, and the latter did not exhibit circadian rhythmicity. Circadian changes in the distribution of the cardiac output may account for the acrophase of Tmax and Tlag observed during the end of the dark period, indicating slower absorption at this time. A period of transitory locomotion activity and sleeping behavior with no feeding activity occurs at the beginning of the light phase and may also exist during the end of the dark phase in the rat. During this period, cardiac output is low and skeletal muscle receives the greatest proportion of this output, while visceral blood flow is low (7). Thus, absorption during this transition may be slower than in other times of day.
Our data are in good agreement with the data of other researchers. In humans, the amount of ciprofloxacin eliminated in urine was greater when the drug was administered at 1000 h than when it was given at 2200 h (26). In rats, when tobramycin was administered at 0200 h (dark period), the CLT was significantly higher and AUC was lower than the values when tobramycin was given at 1400 h (light period) (13). A population pharmacokinetic study of amikacin in humans showed higher values for kel in the morning than in the evening. Ampicillin biliar and renal clearances were significantly higher during the active cycle of rats than during the sleep cycle (16, 30).
Experimental animal models have shown that for antibiotics such as β-lactams that have concentration-independent killing effects in vitro, the time that the antibiotic concentration remains greater than the MIC (T>MIC) is the most important factor for determining the in vivo efficacy (11, 14, 27). Therefore, daily variations in pharmacokinetics may account for impairment in the chemotherapeutic effects. Our results demonstrate that the disposition of ceftriaxone in rats exhibits significant daily changes that could be attributed mainly to enhanced excretion during the active period; thus, T>MIC may be shorter when ceftriaxone is administered during the active phase. This is of great importance when bacteria with low susceptibility are involved in the infectious processes, particularly as ceftriaxone concentrations are lower in tissues than in blood. It can be concluded that the time of administration for ceftriaxone may affect therapeutic efficacy in current once-daily dosing schedules.
Acknowledgments
This work was supported in part by Secretaría de Ciencia y Técnica, Universidad de Buenos Aires, Buenos Aires, Argentina.
REFERENCES
- 1.Beauchamp, D., C. Guimont, L. Grenier, M. LeBrun, D. Tardif, P. Gourde, M. G. Bergeron, L. Thibault, and G. Labrecque. 1997. Time-restricted feeding schedules modify temporal variation of gentamicin experimental nephrotoxicity. Antimicrob. Agents Chemother. 41:1468-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennet, J. V., J. L. Brodie, E. J. Benner, and W. M. M. Kirby. 1966. Simplified, accurate method for antibiotic assay of clinical specimens. Appl. Microbiol. 14:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleyzac, N., B. Allard-Latour, A. Laffont, J. Mouret, R. Jelliffe, and P. Maire. 2000. Diurnal changes in the pharmacokinetic behavior of amikacin. Ther. Drug Monit. 22:307-312. [DOI] [PubMed] [Google Scholar]
- 4.Bourget, P., H. Fernandez, V. Quinquis, and C. Delouis. 1993. Pharmacokinetics and protein binding of ceftriaxone during pregnancy. Antimicrob. Agents Chemother. 37:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruguerolle, B. 1998. Chronopharmacokinetics: current status. Clin. Pharmacokinet. 35:83-94. [DOI] [PubMed] [Google Scholar]
- 6.Cambar, J., and M. Pons. 1998. New tools in renal chronophysiopathology, p. 461-468. In I. Touitou (ed.), Proceedings of the International Congress on Chronobiology. International Congress series 1152. Biological clocks. Mechanisms and applications. Elsevier Science.
- 7.Delp, M. D., R. O. Manning, J. V. Bruckner, and R. B. Armstrong. 1991. Distribution of cardiac output during diurnal changes of activity in rats. Am. J. Physiol. 261:H1487-H1493. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi, M., and D. Perrier. 1982. Farmacocinética. Editorial Reverté, S. A., Barcelona, Spain.
- 9.Gill, C. J., J. J. Jackson, L. S. Gerckens, A. Pelak, R. K. Thompson, J. G. Sundelof, H. Kropp, and H. Rosen. 1998. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-826 L-749,345. Antimicrob. Agents Chemother. 42:1996-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho, K. J., and J. L. Drummond. 1975. Circadian rhythm of biliary excretion and its control mechanisms in rats with chronic biliary drainage. Am. J. Physiol. 229:1427-1437. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen, J. D., N. Frimodt-Moller, and F. Espersen. 1995. Experimental Streptococcus pneumoniae infection in mice for studying correlation of in vitro and in vivo activities of penicillin against pneumococci with various susceptibilities to penicillin. Antimicrob. Agents Chemother. 39:1253-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemmer, B. 1996. The clinical relevance of chronopharmacology in therapeutics. Pharmacol. Res. 33:107-115. [DOI] [PubMed] [Google Scholar]
- 13.Lin, L., L. Grenier, Y. Bergeron, M. Simard, M. Bergeron, G. Labrecque, and D. Beauchamp. 1994. Temporal changes of pharmacokinetics, nephrotoxicity, and subcellular distribution of tobramycin in rats. Antimicrob. Agents Chemother. 38:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutsar, I., A. Ahmed, I. R. Friedland, M. Trujillo, L. Wubbel, K. Olsen, and G. McCracken, Jr. 1997. Pharmacodynamics and bactericidal activity of ceftriaxone therapy in experimental cephalosporin-resistant pneumococcal meningitis. Antimicrob. Agents Chemother. 41:2414-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsui, H., M. Komiya, C. Ikeda, and A. Tachibana. 1984. Comparative pharmacokinetics of YM-13115, ceftriaxone, and ceftazidime in rats, dogs, and rhesus monkeys. Antimicrob. Agents Chemother. 26:204-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesnard-Ricci, B., and C. A. White. 1998. Chronokinetics of active biliary ampicillin secretion in rats. Chronobiol. Int. 15:309-321. [DOI] [PubMed] [Google Scholar]
- 17.Meyers, B. R., E. S. Srulevitch, J. Jacobson, and S. Z. Hirschman. 1983. Crossover study of the pharmacokinetics of ceftriaxone administered intravenously or intramuscularly to healthy volunteers. Antimicrob. Agents Chemother. 24:812-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson, W., Y. Liang Tong, J. K. Lee, and F. Halberg. 1979. Methods for cosinor rhythmometry. Chronobiologia 6:305-323. [PubMed] [Google Scholar]
- 19.Neu, H. C., N. J. Merpol, and K. P. Fu. 1981. Antibacterial activity of ceftriaxone Ro 13-9904, a beta-lactamase-stable cephalosporin. Antimicrob. Agents Chemother. 19:414-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel, I. H., R. E. Weinfeld, J. Konikoff, and M. Parsonnet. 1982. Pharmacokinetics and tolerance of ceftriaxone in humans after single-dose intramuscular administration in water and lidocaine diluents. Antimicrob. Agents Chemother. 21:957-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pons, M., O. Forpomés, S. Espagnet, and J. Cambar. 1996. Relationship between circadian changes in renal hemodynamics and circadian changes in urinary glycosaminoglycan excretion in normal rats. Chronobiol. Int. 13:349-358. [DOI] [PubMed] [Google Scholar]
- 22.Pons, M., A. Schnecko, K. Witte, B. Lemmer, J. M. Waterhouse, and J. Cambar. 1996. Circadian rhythms in renal function in hypertensive TGR mRen-2 rats and their normotensive controls. Am. J. Physiol. 271:R1002-R1008. [DOI] [PubMed] [Google Scholar]
- 23.Reinberg, A. E., G. Labrecque, and M. H. Smolensky. 1991. Chronobiologie et chronotérapeutique, p. 5. Flammarion Medecine-Sciences, Paris, France.
- 24.Ritschel, W. A., and H. Forusz. 1994. Chronopharmacology, a review of drugs studied. Meth. Find. Exp. Clin. Pharmacol. 16:57-75. [PubMed] [Google Scholar]
- 25.Roelfsema, F., D. van der Heide, and D. Smeenk. 1980. Circadian rhythms of urinary electrolyte excretion in freely moving rats. Life Sci. 27:2303-2309. [DOI] [PubMed] [Google Scholar]
- 26.Sarveshwer Rao, V. V., D. Rambhau, B. Ramesh Rao, and P. Srinivasu. 1997. Circadian variation in urinary excretion of ciprofloxacin after a single-dose oral administration at 1000 and 2200 hours in human subjects. Antimicrob. Agents Chemother. 41:1802-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soriano, F., P. García-Corbeira, C. Ponte, N. Fernández-Roblas, and I. Gadea. 1996. Correlation of pharmacodynamic parameters of five beta-lactam antibiotics with therapeutic efficacies in an animal model. Antimicrob. Agents Chemother. 40:2686-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ti, T. Y., L. Fortin, J. H. Kreeft, D. S. East, R. I. Ogilvie, and P. J. Somerville. 1984. Kinetic disposition of intravenous ceftriaxone in normal subjects and patients with renal failure on hemodialysis or peritoneal dialysis. Antimicrob. Agents Chemother. 25:83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinks, A. A., D. J. Touw, H. G. M. Heijerman, M. Danhof, G. P. J. de Leede, and W. Bakker. 1994. Pharmacokinetics of ceftazidime in adult cystic fibrosis patients during continuous infusion and ambulatory treatment at home. Ther. Drug Monit. 16:341-348. [DOI] [PubMed] [Google Scholar]
- 30.White, C. A., R. Pardue, C. Huang, and L. Warren. 1995. Chronobiological evaluation of the active biliary and renal secretion of ampicillin. Chronobiol. Int. 12:410-418. [Google Scholar]
- 31.Yoshiyama, Y., S. Nishikawa, T. Sugiyama, T. Kobayashi, H. Shimada, F. Tomonaga, S. Ohdo, N. Ogawa, and S. Nakano. 1993. Influence of circadian-stage-dependent dosing schedule on nephrotoxicity and pharmacokinetics of isepamicin in rats. Antimicrob. Agents Chemother. 37:2042-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, H. H., Y. P. M. Chan, K. Arnold, and M. Sun. 1985. Single-dose pharmacokinetics of ceftriaxone in healthy Chinese adults. Antimicrob. Agents Chemother. 27:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]