Abstract
Bartonella quintana, the agent of trench fever, has recently been implicated in various diseases, in particular, bacteremia and endocarditis in homeless people. The host cell of Bartonella spp. is believed to be the erythrocyte, and in the present study we demonstrate that B. quintana can be cultured in vitro in human erythrocytes. The bacteria were found to be intraerythrocytic by laser confocal microscopy with Bartonella species-specific monoclonal antibodies. Infections with B. quintana decreased the life span of erythrocytes in culture from 8.6 to 4.8 days. In the culture system we found that most of the antibiotics that we tested (doxycycline, fluoroquinolone compounds, and beta-lactams) were not bactericidal. Gentamicin was bactericidal at 4 μg/ml, as was rifampin, but to a lesser extent. At this concentration, gentamicin has been shown to enter erythrocytes slowly and to reach a peak level of 0.26 μg/ml after 24 h. At 0.26 μg/ml, however, we found that gentamicin was not able to kill extracellular B. quintana, even after 96 h of incubation. We hypothesize that erythrocytes may be a reservoir for B. quintana and that the bactericidal activity of gentamicin that we observed occurs mainly when the bacteria emerge from the erythrocytes and are found extracellularly. It would appear that gentamicin should be administered for at least 5 days to cure patients infected with B. quintana.
Bartonella species are fastidious gram-negative bacteria which belong to the alpha group of the domain Proteobacteria. Of the 16 species characterized, 7 have been associated with human infections. Each Bartonella species is usually highly adapted to a single mammalian reservoir host, with a long-lasting intraerythrocytic bacteremia being the hallmark of infection (2). For two species, Bartonella bacilliformis, the agent of Carrión's disease, and Bartonella quintana, the agent of trench fever, humans are the only known reservoir hosts. B. quintana has recently been implicated in other diseases such as bacillary angiomatosis, chronic lymphadenopathy, bacteremia, and endocarditis (10). B. quintana may cause severe and acute illnesses in homeless patients, but it often produces a chronic, symptomless infection (4).
In vivo, B. bacilliformis infects human erythrocytes and human endothelial cells (2, 24). Bartonella henselae has been shown to infect the erythrocytes of cats (18), and bacteremia may persist for months or years (7, 13). Recently, it has been shown that Bartonella tribocorum occurs in the erythrocytes of rats and does not cause hemolysis (20). We have recently observed B. quintana in the erythrocytes of bacteremic homeless people without hemolysis using species-specific monoclonal antibodies, and we hypothesize that erythrocytes may represent the reservoir of bacteria in such people (17). These different reports suggest that the organisms have a unique strategy that allows them to be preserved as pathogens for efficient transmission by bloodsucking arthropods. The capacity of B. quintana to invade and propagate in erythrocytes suggests that this intracellular location may protect the organisms against antibiotics and the immune response, explaining the relapses of chronic bacteremia. Here we report for the first time the in vitro coculture of B. quintana in human erythrocytes and the bactericidal activities of antibiotics against this bacterium in this new cell culture model. We also studied the pharmacokinetics of gentamicin in erythrocytes using high-performance liquid chromatography (HPLC).
MATERIALS AND METHODS
Bacterial strains.
The B. quintana Oklahoma strain (ATCC 49793) was a gift from D. F. Welch, (Oklahoma City, Okla.). We also used two French isolates of B. quintana obtained in our laboratory from bacteremic homeless people and referred to as UR.BQ.M.TF.99 and UR.BQ.M.TF.106.
Culture of bacterial strains.
The bacteria were grown on Columbia 5% sheep blood agar plates (BioMerieux, Marcy l'Etoile, France). The plates were placed in polyethylene bags and incubated at 37°C in 5% CO2-enriched atmosphere (Genbag CO2 system; BioMerieux) as described previously (19).
Infection and culture in erythrocytes.
Human erythrocytes in EDTA and acid-citrate-dextrose were obtained from blood donors, washed three times in Hanks balanced salt solution without bicarbonate, and resuspended at 108 cells/ml. Bartonella strains cultured on blood agar (6 to 7 days of growth) were inoculated onto human type A-positive erythrocytes in shell vials in a class II biosafety hood in a biosafety level 3-equipped laboratory. After inoculation, the shell vials were centrifuged at 600 × g at 22°C for 1 h and the medium was discarded. Fresh medium (complete RPMI 1640 medium supplemented with 10% fetal calf serum and buffered with 25 mM NaHCO3 and 25 mM HEPES) was added to give a hematocrit of 10% and the shell vials were incubated at 37°C in 5% CO2. Each day the medium was changed and thin blood smears were made. The blood smears were stained with acridine orange, and the percentage of infected erythrocytes was determined. When the percentages of infected erythrocytes and hemolysis were too high, subcultures were made in fresh human erythrocyte cultures as described above. To determine if the bacteria could grow in the cell culture medium without cells being present, they were inoculated and cultivated, as described above, in cell-free culture medium or medium with red blood cell lysates. No growth of the organisms was observed.
Kinetics of B. quintana growth with human erythrocytes.
The numbers of CFU of B. quintana were determined on days 0, 1, 2, 3, 4, and 5 to determine the growth kinetics of the organism. On each day, the shell vials were centrifuged at 1,200 rpm, the cells were washed three times with RPMI and stored at −20°C until they were thawed, and 10-fold dilutions of each sample were made. These were plated onto blood agar plates and incubated for 5 days before the colonies were counted.
Evaluation of erythrocyte invasion by laser confocal microscopy.
Thin smears of erythrocytes that had been infected with B. quintana for 5 days were made. The slides were air dried, fixed with methanol, and stained for 30 min at 37°C with a mouse monoclonal antibody specific for B. quintana (titer of 1/1,600 diluted at 1/400 in phosphate-buffered saline [PBS]) (8, 18). The slides were washed three times with PBS (pH 7.2) and stained with a fluorescein isothiocyanate mouse conjugate (Immunotech, Marseille, France) for 30 min. Red blood cells were counterstained with Evans blue. After three washes in PBS the slides were air dried and examined with a laser scanning confocal microscope at an excitation wavelength of 488 nm and an emission wavelength of 617 nm. Sections were examined at increments of 0.5 μm, and the percentage of infected cells was determined after 500 erythrocytes had been counted.
Antibiotic susceptibility assays. (i) Drugs.
The antibiotics tested were amoxicillin (concentration range, 0.06 to 64 μg/ml; Beecham-Sevigne, Paris, France), ceftriaxone (0.06 to 64 μg/ml; Roche, Neuilly sur Seine, France), gentamicin (0.06 to 4 μg/ml; Dakota Pharm, Creteil, France), telithromycin (0.03 to 4 μg/ml; Hoechst Marion Roussel, Romainville, France), levofloxacin (0.12 to 4 μg/ml; Hoechst Marion Roussel), ciprofloxacin (0.12 to 4 μg/ml; Bayer Pharma, Sebs, France), erythromycin (0.03 to 4 μg/ml; Abbott, Rungis, France), rifampin (0.06 to 4 μg/ml; Cassenne, Puteaux, France), and doxycycline (0.06 to 4 μg/ml; Pfizer, Neuilly, France). The powders were solubilized according to the recommendations of the manufacturers; and stock solutions of 10 mg/ml were prepared, divided into aliquots, and frozen at −80°C until use.
(ii) MICs and MBCs in the extracellular assay.
For MIC determinations, a modified version of the antibiotic agar dilution method of the National Committee for Clinical Laboratory Standards was used with serial twofold dilutions, as described previously (11, 19). The optimum time for visualization of bacterial growth was 5 days. The MIC was defined as the lowest concentration of the antibiotic tested that gave complete inhibition of bacterial growth compared with the growth in a drug-free control. The bactericidal activities of the antibiotics were determined by a broth assay with Schaedler medium supplemented with 5% sheep blood, and twofold serial dilutions of each antibiotic were tested as described previously (19). After incubation for 24 h, 10-fold serial dilutions of the different bacterial suspensions were then plated onto blood agar and reincubated for 5 days before enumeration of colonies. The minimal bactericidal concentration (MBC) was defined as the lowest antibiotic concentration that induced a 99.9% decrease in bacterial inocula following the 24-h incubation period compared with the dose of the primary inoculum.
(iii) MBC determination in human erythrocytes.
Human erythrocytes cultured in 24-well microtiter plates were infected as described above with B. quintana at a concentration equivalent to that of a 0.5 McFarland standard (approximately 106 CFU/ml) for 1 h at 37°C. Antibiotics at twofold serial dilutions as described above were added to different rows on the plates, and drug-free rows with or without bacteria were used as positive and negative controls, respectively. Each concentration of antibiotic was tested in duplicate. On days 0, 1, 2, 3, 4, and 5, two rows of erythrocytes were harvested, centrifuged at 1,200 rpm, washed three times with RPMI 1640 medium, and saved at −20°C. After the cells were thawed, 10-fold serial dilutions of the cells were plated onto blood-enriched agar and the plates were incubated at 37°C in a 5% CO2-enriched atmosphere. After 5 days the colonies were counted and the MBC was determined. The MBC was the minimum concentration of an antibiotic that caused a 99.9% decrease in the bacterial count of the primary inoculum.
Measurement of gentamicin in human erythrocytes by HPLC analysis.
Measurement of the penetration of gentamicin into human erythrocytes in vitro was performed with uninfected erythrocytes. Gentamicin at 3 μg/ml was added to human erythrocytes maintained in a continuous culture in RPMI 1640 medium in culture flasks as described above. The gentamicin-containing medium was changed daily for 4 days. Red blood cells not exposed to gentamicin were used at each time and in each experiment as negative controls. At each time point, one flask of erythrocytes was used to measure the level of gentamicin in the medium and in the erythrocytes. The experiments were performed three times in triplicate to confirm the results.
Sample pretreatment.
Blood samples (flasks of 6 ml) were collected at various times and placed in sterile tubes. Each tube was centrifuged at 2,000 × g for 10 min, and 1 ml of supernatant was transferred to each of three different Centrico centrifugal filter devices with a YM10 membrane (Millipore, Guyancourt, France). Red blood cell pellets were homogenized with 3 ml of PBS (pH 6.5), and after centrifugation at 4,000 rpm for 10 min the supernatant was discarded. This operation was repeated three times before the pellet was homogenized and the red blood cells were transferred to the Centricon centrifugal filter. The reservoir of each Centricon centrifugal filter (supernatant and red blood cell pellet) was filled with 3 ml of distilled water, and the filters were centrifuged at 6,000 rpm at 8°C for 5 h. After centrifugation, 4 ml of distilled water was again added to each reservoir, and the filters were centrifuged for 5 h as described above. This operation was repeated three times, and the final filtrate was lyophilized. Standard solutions of gentamicin were treated in the same way.
Gentamicin samples were analyzed by HPLC by a procedure with a precolumn derivatization with 9-fluorenylmethyl chloroformate (FMOC; Sigma, Ivry, France) to form fluorescent products for detection as described by Stead and Richards (21). Briefly, the residue from the lyophilization was solubilized in 100 μl of 100 mM borate buffer (pH 8.5), and then 100 μl of 2.5 mM FMOC in acetonitrile was added. The mixture was maintained at room temperature for 15 min before HPLC analysis.
The HPLC system comprised a Merck (Lyon, France) model L6200 pump and a Rheodyne model 7125 valve fitted with a 50-μl loop. A Merck Nucleosyl C18 column (diameter, 5 μm; length, 25 cm) was used. Detection was with a Merck model F-1050 fluorometer (excitation wavelength, 260 nm; emission wavelength, 315 nm) connected to a computer (D-2500 Chromato-Integrator; Merck). The mobile phase was acetonitrile-water (80/20; vol/vol), which was degassed prior to use by vacuum filtration through a 0.2-μm-pore-size filter. The FMOC-derivatized gentamicin was separated at room temperature at a flow rate of 1 ml/min.
Controls.
Escherichia coli CIP 53126 and Staphylococcus aureus CIP 103811 (Institut Pasteur, Marnes-la-Coquette, France) were used as controls for antibiotic activity. Either Mueller-Hinton or Columbia agar (BioMerieux, Lyon, France) enriched with 10% horse blood medium was used.
Statistics.
A nonparametric Mann-Whitney U test of the means was performed for comparison of the laboratory data. A difference was considered significant when P was <0.05.
RESULTS
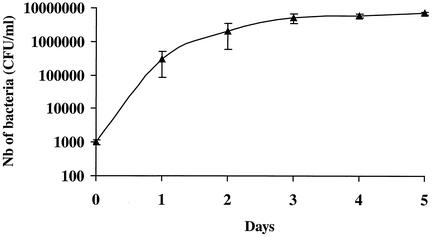
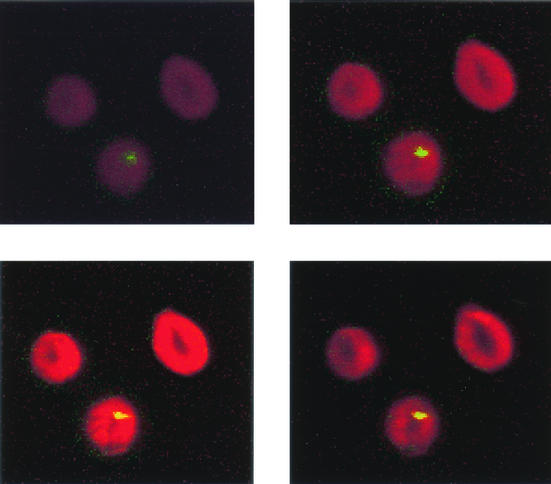
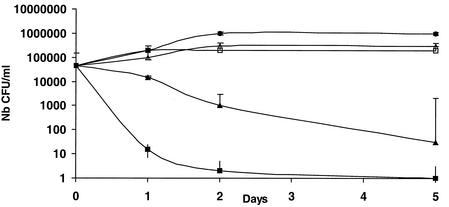
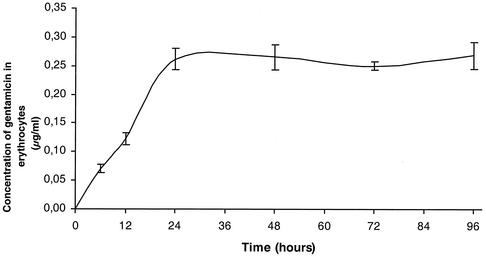
Our attempt to grow B. quintana in human erythrocytes was successful. The bacteria did not grow in cell-free culture medium, but organisms which did not multiply could be seen for 24 h in medium containing cell lysates. Figure 1 shows the kinetics of growth of B. quintana in coculture with human erythrocytes in the five independent experiments that we performed. The proportions of infected erythrocytes ranged from 7.03% ± 0.35% on day 1 to 16.34% ± 1.68% on day 5, and the doubling time in our model was estimated to be 8 h. When fresh erythrocytes were not added to the cultures, all erythrocytes were lysed after 4.8 ± 0.8 days of infection. All cells in uninfected cultures of erythrocytes were found to be lysed significantly later (P < 0.05 by the Mann-Whitney U test), at 8.6 ± 1.5 days, indicating that the life span of erythrocytes was reduced when they were infected with B. quintana. The organisms could be maintained in culture for several weeks by regularly adding fresh erythrocytes to the original culture. Figure 2 shows photographs obtained by laser confocal microscopy of digital sections of human erythrocytes infected with B. quintana and identified with a species-specific monoclonal antibody (9). Bacteria were seen intracellularly or epicellularly, but most had been released from the cells and were seen extracellularly. The fluorescence was more intense in the middles of the erythrocytes, and the number of bacteria per erythrocyte was found to be 1.27 ± 0.04. The MICs and MBCs obtained by the extracellular assay and the red blood cell assay are presented in Table 1. The MBCs found in the two models were similar except for the MBCs of rifampin (Fig. 3). After 24 h of coculture, only gentamicin at 4 μg/ml was bactericidal and caused a 4.6-log decrease in the number of bacteria. After 48 h of incubation, rifampin at 4 μg/ml was also bactericidal and caused a 3.2-log decrease in the number of bacteria. After 5 days of coculture, only gentamicin and rifampin were bactericidal, whereas levofloxacin, doxycycline, erythromycin, telithromycin, amoxicillin, and ceftriaxone had no effect. The levels of gentamicin measured by HPLC in erythrocytes in culture and in the extracellular medium are shown in Fig. 4. Gentamicin entered the erythrocytes slowly, and at concentrations of 3 μg/ml in the culture medium, the intracellular concentration of gentamicin reached a maximum of 0.26 ± 0.03 μg/ml (n = 12 experiments) after 24 h of antibiotic exposure. This concentration remained unchanged, even after 96 h of antibiotic exposure. When we tested the bactericidal effect of gentamicin at 0.3 and 3 μg/ml on B. quintana growing in cell-free liquid Schaedler medium, the drug was not bactericidal at 0.3 μg/ml, even after the organisms were exposed to the antibiotic for 96 h.
FIG. 1.
Kinetics of growth of B. quintana in human erythrocytes as determined by enumeration of colonies after plating onto blood agar. Nb, number.
FIG. 2.
Laser scanning confocal microscopy of digital sections of cultured human erythrocytes infected with B. quintana. Sections were taken in 0.5-μm increments from top to bottom. B. quintana was revealed with a specific monoclonal antibody.
TABLE 1.
MICs and MBCs for the B. quintana strains determined by extracellular assay and red blood cell assay
| Antibiotic | Extracellular assay
|
MBC (μg/ml) by red blood cell assay | |
|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | ||
| Amoxicillin | 0.06 | >64 | >64 |
| Ceftriaxone | 0.25 | >64 | >64 |
| Doxycycline | 0.125 | >4 | >4 |
| Gentamicin | 1 | 2 | 2-4 |
| Levofloxacin | 0.5-1 | >4 | >4 |
| Ciprofloxacin | 0.5-1 | >4 | >4 |
| Rifampin | 0.125-0.25 | >4 | 4 |
| Erythromycin | 0.06-0.125 | >4 | >4 |
| Telithromycin | 0.03-0.06 | >4 | >4 |
FIG. 3.
Bactericidal effects of rifampin at 1 μg/ml (▵) and 4 μg/ml (▴) and gentamicin at 1 μg/ml (□) and 4 μg/ml (▪) on B. quintana in human erythrocytes. •, growth control; Nb, number.
FIG. 4.
Kinetics of penetration of gentamicin into human erythrocytes determined by HPLC. Gentamicin was used at 3 μg/ml in medium that was changed each day.
DISCUSSION
Bartonella species are closely associated with erythrocytes in their natural hosts, with B. bacilliformis occurring in vivo in the erythrocytes of patients with Carrión's disease (1) and B. quintana occurring in erythrocytes of bacteremic homeless people (17, 18). These findings suggest that Bartonella spp. may evade the immune systems of their natural hosts by living within erythrocytes. The natural persistence of B. tribocorum and B. quintana within erythrocytes without causing lysis of the host cells has been proposed as a model for the efficient transmission of bacteria by bloodsucking arthropods (20). Isolation of B. quintana from the blood of patients has been reported several times (4, 5, 15), and the fact that B. quintana resides in the erythrocytes of bacteremic homeless people suggests that human erythrocytes are the natural host cells of the organism (17). In cultured feline erythrocytes infected with B. henselae, less than 1% of the erythrocytes were found to be infected, and there was no evidence of hemolysis (13). On the other hand, the percentage of erythrocytes infected with B. bacilliformis in patients with Oroya fever is high, with up to 100% of cells being infected and severe hemolysis occurring.
In this report we describe for the first time a new cell culture system for B. quintana in human erythrocytes. We successfully cultivated B. quintana in human erythrocytes under conditions similar to those previously described by Trager and Jensen (22) for the culture of Plasmodium falciparum. We found that B. quintana was unable to survive in cell-free medium or in medium containing red blood cell lysates. Organisms were seen in the erythrocytes by laser scanning confocal microscopy, but most organisms were seen in the medium, which was in accordance with the percentage of infected erythrocytes that we found. The presence of free B. quintana in the medium may have been due to the release of the organisms by cell budding, leading to cell membrane lesions or erythrocyte death. Human erythrocytes had significantly shortened life spans when they were infected with B. quintana, and the cultures were lost if fresh erythrocytes were not added. The detection was specific, since we have used a previously described monoclonal antibody specific for B. quintana (8). We found that erythrocytes can be infected with more than one bacterium, but we were not able to detect as many as five bacteria, as previously reported in vivo in homeless people (17). This may explain why more CFU of Bartonella are found when organisms are isolated from blood which has been frozen and thawed, destroying erythrocytes and maybe releasing the bacteria (3). Of note is the fact that trench fever caused by B. quintana (quintana fever) lasts for 5 days (12), and in our erythrocyte model, lysis of erythrocytes occurred after 5 days. The data from our culture system also support the natural cycle recently proposed by Schülein et al. (20) for B. tribocorum in rats.
We used the new cell culture model to evaluate the bactericidal activities of antibiotics against B. quintana within erythrocytes. While Bartonella spp. are susceptible to almost all antibiotics (11), diseases due to the pathogens are often difficult to cure, and after antibiotic withdrawal, relapses are frequent, suggesting that the antibiotics used for treatment are not curative (12). The capacity of B. quintana to invade and propagate in human erythrocytes may enable them to avoid exposure to antibiotics and immune responses. Indeed, the bactericidal activities of antibiotics in our culture system were poor, and the only bactericidal effects seen were with gentamicin after 24 h of exposure and, to a lesser extent, with rifampin after 48 h of exposure. Similar results demonstrating the general lack of bactericidal activity of antibiotics against Bartonella have been published by our team; however, we have found that aminoglycosides act against extracellular bacteria (19) and intracellular bacteria (14). Data on the treatment of infections with B. quintana are scarce, but the use of an aminoglycoside seems crucial in the successful treatment of Bartonella endocarditis (16). Our results apparently disagree with those obtained with experimental animal models and Bartonella grahamii, B. tribocorum, and B. henselae (6, 13, 20). In those models gentamicin did not kill intraerythrocytic Bartonella, but the incubation time used was only 2 or 3 h and aminoglycosides are only slowly concentrated within cells by pinocytosis (9, 23). Human red blood cells are not capable of pinocytosis, and thus, the mode of entry into red blood cells is unknown; the mode of entry has not been studied before. Moreover, in these models the blood of experimentally infected animals was exposed to gentamicin for 2 h before the blood was washed and before the erythrocytes were lysed and plated on blood agar to determine the number of viable intracellular bacteria. The total number of B. henselae in the blood of a cat at the time of peak bacteremia was 1.8 × 105 CFU/ml, and after exposure to gentamicin for 2 h it was 2.2 × 104 CFU/ml (13). This decrease in the proportion of viable bacteria by about 1 log after 2 h suggests that only 12% of bacteria were inside erythrocytes. We have previously shown that the bactericidal activities of aminoglycosides against B. quintana in an extracellular model can be seen only after 24 h of antibiotic exposure (19), and our present results are also consistent with those reported previously (6, 13, 20). The intraerythrocytic location of B. quintana may protect the bacteria from antibiotics; therefore, we measured the level of gentamicin in erythrocytes incubated with a concentration of gentamicin similar to that which would be found in the serum of a person receiving treatment. Gentamicin entered human erythrocytes slowly, probably by passive diffusion, and reached a peak level of 0.26 μg/ml. This concentration was similar to that obtained in patients treated with gentamicin (unpublished data) and was not able to kill B. quintana even after 5 days of exposure. Our hypothesis is that gentamicin (at concentrations achievable in human serum) is bactericidal for bacteria once they are released from the erythrocytes. The lytic cycle occurred in our model after 5 days, and we therefore suggest that gentamicin should be given to infected patients for at least 5 days to enable cure. The clinical manifestations of trench fever in people may be due to the cyclical release of bacteria into the sera of patients without evidence of hemolysis (17), since only a small proportion of infected erythrocytes are destroyed.
In conclusion, we have shown that B. quintana can be cultured in human erythrocytes and that infections accelerate the natural course of erythrocyte death. Only gentamicin and rifampin were bactericidal in our cell culture model, and they appeared to kill the bacteria when they were released into the extracellular medium. We suggest that patients infected with B. quintana be treated with gentamicin for at least 5 days. Clinical studies should be performed to investigate the efficacy of this treatment. The physiopathology of the disease and the mechanism of the invasion process in red blood cells should be investigated in the future to explain the natural course of the infection.
Acknowledgments
We thank Patrick Kelly for review of the manuscript, Robert Pistoresi for technical assistance, and Pierre Berger for statistical analysis.
REFERENCES
- 1.Benson, L. A., S. Kar, G. McLaughlin, and G. M. Ihler. 1986. Entry of Bartonella bacilliformis into erythrocytes. Infect. Immun. 54:347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breitschwerdt, B., and D. Kordick. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner, S. A., J. A. Rooney, P. Manzewitsch, and R. L. Regnery. 1997. Isolation of Bartonella (Rochalimae) henselae: effects of methods of blood collection and handling. J. Clin. Microbiol. 35:544-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouqui, P., B. La Scola, V. Roux, and D. Raoult. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N. Engl. J. Med. 340:184-189. [DOI] [PubMed] [Google Scholar]
- 5.Drancourt, M., J. L. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N. Engl. J. Med. 332:419-423. [DOI] [PubMed] [Google Scholar]
- 6.Koesling, J., T. Aebischer, C. Falch, R. Schulein, and C. Dehio. 2001. Cutting edge: antibody-mediated cessation of hemotropic infection by the intraerythrocytic mouse pathogen Bartonella grahamii. J. Immunol. 167:11-14. [DOI] [PubMed] [Google Scholar]
- 7.Kordick, D. L., and E. B. Breitschwerdt. 1995. Intraerythrocytic presence of Bartonella henselae. J. Clin. Microbiol. 33:1655-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang, Z., and D. Raoult. 2000. Species-specific monoclonal antibodies for rapid identification of Bartonella quintana. Clin. Diagn. Lab. Immunol. 7:40-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ly, T. M. C., and H. E. Müller. 1990. Ingested Listeria monocytogenes survive and multiply in protozoa. J. Med. Microbiol. 33:51-54. [DOI] [PubMed] [Google Scholar]
- 10.Maurin, M., R. J. Birtles, and D. Raoult. 1996. A review of bartonellae and their infections. p. 587-610. In J. Kazar and R. Toman (ed.), Rickettsiae and rickettsial diseases. Veda, Bratislava, Slovakia.
- 11.Maurin, M., S. Gasquet, C. Ducco, and D. Raoult. 1995. MICs of 28 antibiotic compounds for 14 Bartonella (formerly Rochalimaea) isolates. Antimicrob. Agents Chemother. 39:2387-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurin, M., and D. Raoult. 1996. Bartonella (Rochalimaea) quintana infections. Clin. Microbiol. Rev. 9:273-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehock, J. R., C. E. Greene, F. C. Herardini, T. W. Hahn, and D. C. Ause. 1998. Bartonella henselae invasion of feline erythrocytes in vitro. Infect. Immun. 66:3462-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso, D., M. Drancourt, and D. Raoult. 1995. Lack of bactericidal effect of antibiotics except aminoglycosides on Bartonella (Rochalimaea) henselae. J. Antimicrob. Chemother. 36:101-108. [DOI] [PubMed] [Google Scholar]
- 15.Raoult, D., M. Drancourt, A. Carta, and J. A. Gastaut. 1994. Bartonella (Rochalimaea) quintana isolation in patient with chronic adenopathy, lymphopenia, and a cat. Lancet 343:977.. [DOI] [PubMed] [Google Scholar]
- 16.Raoult, D., P. E. Fournier, F. Vandenesch, J. L. Mainardi, S. J. Eykyn, J. Nash, E. James, C. Benoit-Lemercier, and T. J. Marrie. 2002. Outcome and treatment of Bartonella endocarditis. Arch. Intern. Med., in press. [DOI] [PubMed]
- 17.Rolain, J. M., C. Foucault, R. Guieu, B. La Scola, P. Brouqui, and D. Raoult. 2002. Bartonella quintana in human erythrocytes. Lancet 360:226-228. [DOI] [PubMed] [Google Scholar]
- 18.Rolain, J. M., B. La Scola, Z. Liang, B. Davoust, and D. Raoult. 2001. Immunofluorescent detection of intraerythrocytic Bartonella henselae in naturally infected cats. J. Clin. Microbiol. 39:2978-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolain, J. M., M. Maurin, and D. Raoult. 2000. Bactericidal effect of antibiotics on Bartonella and Brucella spp.: clinical implications. J. Antimicrob. Chemother. 46:811-814. [DOI] [PubMed] [Google Scholar]
- 20.Schülein, R., A. Seubert, C. Gille, C. Lanz, Y. Hansmann, Y. Piemont, and C. Dehio. 2001. Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J. Exp. Med. 193:1077-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stead, D. A., and R. M. Richards. 1996. Sensitive fluorimetric determination of gentamicin sulfate in biological matrices using solid-phase extraction, pre-column derivatization with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 675:295-302. [DOI] [PubMed] [Google Scholar]
- 22.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 23.Tulkens, P., and A. Trouet. 1978. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured fibroblasts. Biochem. Pharmacol. 27:415-424. [DOI] [PubMed] [Google Scholar]
- 24.Zbinden, R., M. Höchli, and D. Nadal. 1995. Intracellular location of Bartonella henselae cocultivated with Vero cells and used for an indirect fluorescent-antibody test. Clin. Diagn. Lab. Immunol. 2:693-695. [DOI] [PMC free article] [PubMed] [Google Scholar]