Abstract
Background
The mature mouse egg contains the full complement of maternal proteins required for fertilization, the transition to zygotic transcription, and the beginning stages of embryogenesis. Many of these proteins remain to be characterized, therefore in this study we have identified highly abundant egg proteins using a proteomic approach and found that several of these proteins also appear to localize to the egg surface. Characterization of such molecules will provide important insight into the cellular events of fertilization and early development.
Methods
In order to identify some of the more abundant egg proteins, whole egg extracts were resolved on coomassie-stained two-dimensional (2D) PAGE gels. Several highly abundant protein spots were cored and microsequenced by tandem mass spectrometry (TMS), and determined to be molecular chaperone proteins. Concurrent experiments were performed to identify oolemmal proteins using 2D avidin blotting. Proteins spots that appeared to be surface labeled by biotinylation were correlated with the initial coomassie-stained reference gel. Surprisingly, some of the surface labelled proteins corresponded to those abundant chaperone proteins previously identified. To confirm whether these molecules are accumulating at the oolemmal surface in eggs, we performed immunofluoresence on live, zona-free eggs using antibodies to HSP70, HSP90, GRP94, GRP78, calreticulin and calnexin.
Results
The putative surface-labeled proteins identified by biotinylation included the molecular chaperones HSP70 (MW 70 KDa, pI 5.5), HSP90a (MW 85 KDa, pI 4.9), GRP94 (MW 92 KDa, pI 4.7), GRP78 (MW 72 KDa, pI 5.0), Oxygen regulated protein 150 (ORP150; MW 111 KDa, pI 5.1), Calreticulin (MW 48 KDa, pI 4.3), Calnexin (MW 65 KDa, pI 4.5), and Protein disulfide isomerase (PDI; MW 57 KDa, pI 4.8). Immunofluoresence results showed that antibodies to HSP90, GRP94, GRP78 and calreticulin were reactive with oolemmal proteins. We were unable to confirm surface localization of HSP70 or calnexin by this method.
Conclusions
We report here the identification of nine highly abundant molecular chaperones in the mouse egg proteome. In addition, we present preliminary data suggesting that these molecules localize to the oolemma of the mature mouse egg.
Background
The egg is a transcriptionally inactive cell and as such is a storehouse of maternal proteins and mRNA required for fertilization and the initiation of zygotic development. However, many of the proteins comprising the mature egg proteome have yet to be identified. Identification and molecular characterization of such proteins will provide much insight into the regulation of fertilization and early embryogenesis. The surface of the egg consists of an extracellular matrix, or zona pellucida, and plasma membrane, or oolemma, which sits beneath. The three proteins that comprise the zona pellucida (ZP1, ZP2 and ZP3) and their roles in sperm-binding are well characterized [1]. In contrast, little is known about the surface proteins of the egg plasma membrane. Almeida et al. [2] demonstrated the presence of α6β1 and αvβ3 integrins at the egg surface by indirect immunofluorescence and PCR, and showed involvement of the α6β1 integrin in sperm-egg fusion by peptide and antibody inhibition assays. Currently, however, the functional significance of egg surface integrins is unclear. Data from Zhu and Evans [3] substantiates the involvement of α4/α9 integrin and α6 integrin in sperm-egg binding, while other results have been contradictory ([4,5], see Primakoff and Myles, [6] for review). There is now compelling evidence demonstrating that CD9, a tetraspan membrane protein, is present on the oolemma and essential for sperm-egg fusion, possibly by organizing functional multimolecular complexes in the egg [7]. Glycosyl-phophatidyniositol (GPI)-anchored proteins have also been described on the oolemma and implicated in sperm-egg fusion; removal of GPI-anchored protein from the egg plasma membrane results in greatly reduced fertilization rates without affecting sperm-zona pellucida binding [8]. Other egg surface molecules are the adhesion molecules NCAM, VCAM-1, ICAM-1 and ECAD [9], and the selectins [10]. Additionally, the IgG receptor [11], complement receptors C1q [12], CD35 and CD11b, [13] and the Fc gamma receptors [14] exhibit oolemmal expression in a variety of mammalian species.
Molecular chaperones bind to nascent proteins in the endoplasmic reticulum (ER), promote proper protein folding, and prevent the aggregation of nonnative and misfolded proteins. Most are constitutively expressed at low levels in almost all cell-types, but a number are upregulated in response to cellular stresses and these are known as the heat shock proteins (HSPs). A number of molecular chaperones are retained in the ER, due to a conserved signal sequence at the C-terminal end of the protein, (KDEL), which binds to a receptor in the Golgi apparatus [15]. More recently, however molecular chaperones bearing the ER retention signal have been localized to the surface of different cell types. Calnexin, Calreticulin, GRP94 (glycoprotein 96) and GRP78 (BiP) have been identified on the cell surface of immature thymocytes [16,17]. Cell surface expression of GRP94 is found in lymphocytes and lymphoid-like cells from several vertebrate species and is phylogenetically conserved [18]. Calnexin has been found on the surface of pro-B cells [19], as well as in a variety of mammalian culture cells [20] and a number of HSPs are found expressed on the cell surface in human and murine tumor cells [21-24]. Calreticulin, GRP78 and PDI were described on the plasma membrane of different cell types [25-28]; GRP78, GRP94, and PDI, have been found on the surface of rat exocrine pancreatic cells [29,30], and GRP78 cell surface expression was recently shown in human endothelial cells [31].
A number of reports have characterized constitutive expression of HSP70 and HSP90 in both immature oocytes and ovulated eggs from various species [32-36]. HSP70 and HSP89 are constitutively synthesized in the immature, preovulatory mouse oocyte, although the expression is not heat-inducible [37]. Levels of HSP70 mRNA decrease following oocyte maturation and ovulation [38], and HSP70 is one of the first genes to be transcribed at the onset of zygotic gene activation in the early 2-cell mouse embryo [39,40]. Interestingly, while previous investigators did not localize HSP70 to the oolemma, they did demonstrate that the presence of anti-HSP70 antibody reduces bovine sperm-egg binding in vitro. indicating a possible role for HSP70 in fertilization [41]). An egg-surface sperm receptor, identified on sea urchin oocytes, bears a sperm-binding domain with sequence similarity to HSP70 and its recombinant protein binds sperm and inhibits fertilization in vitro [42]. Among the other molecular chaperones described here, calreticulin has been found in oocytes from a number of species [43]. Interestingly, Munoz-Gotera et al. [44] showed that calreticulin localizes to cortical granules in guinea pig oocytes and is released into the perivitelline space following activation. PDI has been identified in sea urchin eggs [45], though localized only to the ER. Calnexin, GRP78, GRP94, and ORP150 have not been previously identified in oocytes of any species.
In order to identify maternal proteins present in the ovulated mouse egg, we performed 2D gel electrophoresis of proteins extracted from 2850 whole mature eggs and cored those protein spots that appeared to be most abundant. Concurrently, we surface labelled zona-free eggs with biotin and performed 2D avidin blotting to attempt to identify surface proteins. Proteins that appeared to be surface labelled were then cross-referenced between corresponding silver-stained 2D gels of egg extracts and compared to microsequence data from the coomassie-stained reference gel. We report here the preliminary identification of nine highly abundant HSPs and related chaperones in the mature mouse egg that also appear to be surface labelled. To support the hypothesis that these molecular chaperones localize to the egg surface, we performed indirect immunofluorescence analysis and found that antibodies to HSP90α, GRP94, GRP78 and Calreticulin specifically bind to proteins on the oolemma.
Methods
All experiments requiring the use of animals were performed according to USDA-condoned protocols and received prior approval by the University of Virginia IACUC committee.
Egg isolation
Mature, metaphase II-arrested mouse eggs were collected from superovulated 24-gram ICR female mice (Harlan Breeders, Indianapolis; Hilltop Labs, Philadelphia) primed with 7 IU PMSG 48 hr and 7 IU HCG 15 hr prior to egg collection. Cumulus – oocyte complexes were collected from oviducts of superovulated ICR strain females, in medium-199 (M199, Gibco-BRL (Invitrogen), Carlsbad, CA). Cumulus cells were removed by treating eggs for 3 min with 1 mg/ml hyaluronidase (Sigma, St. Louis, MO) in M199; eggs were washed through four 50-ml drops of M199 medium covered with mineral oil using a pulled , heat-polished, Pasteur pipette (employed in all experiments). Zonae pellucidae were removed by treating the eggs for 3 min with 10 μg/ml α-chymotrypsin (Sigma, St. Louis, MO) followed by mechanical shearing. Eggs were washed in M199 medium and incubated in M199 medium overlaid with light mineral oil for 3 h at 37°C in a 5% CO2 incubator to allow for recovery before use.
2D Electrophoresis
The eggs were extracted in Celis lysis buffer (see [46]) containing 2% (v:v) NP-40, 9.8 M urea, 100 mM dithiothreitol (DTT), 2% ampholines (pH 3.5–10), and protease inhibitors for 30 min at room temperature, according to the protocol described in [47]. Isoelectric focusing of the egg extracts (IEF) was performed using the Mini-PROTEAN II tube cell apparatus according to the manufacturer's protocol (Bio-Rad, Richmond, CA) with ampholines of pH 3.5–5 (30%), 3.5–10 (40%), 5–7 (20%), and 7–9 (10%), (Pharmacia Biotech, Uppsala, Sweden). The tube gels were placed on 12% minislab gels and the focused proteins were separated in the second dimension at 20 mA per gel. The gel was stained with coomassie (BioRad, Hercules, CA) for 30 min., washed and abundant protein spots (as determined by intensity of staining) were cored and submitted for microsequencing by TMS.
Protein microsequencing
Peptide sequencing was performed by the W.M. Keck Foundation Center for Biomedical Mass Spectrometry at the University of Virginia. Surface-labeled egg proteins were identified on 2D streptavidin immunoblots, and corresponding silver-stained protein spots were cored from a 1.5 mm-thick 2D SDS-PAGE gel that had been run in tandem. The gel core was diced into small pieces, destained with 1:1 v/v 30 mM potassium ferricyanide and 100 mM sodium thiosulfate, reduced in 10 mM DTT, and alkylated in 50 mM iodoacetamide in 0.1 M ammonium bicarbonate. After reagents were removed, the gel pieces were incubated with 12.5 ng/μl trypsin in 50 mM ammonium bicarbonate overnight at 37°C. Digested peptides were extracted from the gel fragments in 50% acetonitrile, 5% formic acid and evaporated to 25 μl for microsequencing. Peptides were analyzed by mass spectrometry using a capillary HPLC column interfaced to a Finnigan MAT TSQ7000 electrospray ionization-tandem quadrupole mass spectrometer. Selected peptides were mass analyzed using capillary liquid chromatography, yielding a collision-activated dissociation (CAD) spectra from which the amino acid sequence could be determined. Amino acid sequences were entered into and searched against the protein and DNA databanks using Sequest software available from GenBank.
Surface labeling
This procedure is explained in detail in Coonrod et al, 1999 [8]. Briefly, after collection zona-free eggs were washed six times in BWW medium (Irvine Scientific, Santa Ana, CA) containing 100 μg/ml polyvinylalcohol (PVA, Sigma, St. Louis, MO), biotinylated with 2 mg/ml Sulfo-NHS biotin (Pierce, Rockford, IL) in BWW/PVA for 7 min at room temperature, and washed six times in BWW/PVA. Biotinylated eggs were extracted in Celis buffer and run on a 2D gel as described above. The proteins were then electroblotted to nitrocellulose membranes at 125 mA for 45 min. The nitrocellulose membranes were blocked in PBS with 0.1% Tween and 5% dried milk for 30 min at room temperature, washed 1 × in PBS/0.1% Tween, and probed with 20 mg/ml streptavidin-HRP (Pierce, Rockford, IL) for 30 min at room temperature. The blots were washed 3 × 15 min in PBS/0.1% Tween and developed using enhanced chemiluminescence (Amersham Corp, Buckinghamshire, UK) for 5 min. An identical number of eggs were collected and remained untreated prior to protein extraction, and proteins from these eggs were separated according to the same protocol outlined above. This gel was silver-stained according to Hochstrasser et al. [48].
Indirect immunofluorescence with zona-free Eggs
Antibody Reagents: All antibodies used in these experiments were purchased from Affinity Bioreagents, Golden, CO and all were diluted in M199 media to desired concentration. Anti-GRP94 (clone 9G10) is a rat monoclonal IgG antibody used at 50 μg/ml. Purified rat IgG was diluted to 50 μg/ml for use as a negative control. Anti-calnexin (clone AF18), Anti-HSP90 (clone 3B6), and anti-HSP70 (5A5) are mouse monoclonal IgG antibodies, and were used at a 1:100 dilution. Purified mouse IgG was diluted 1:100 for use as a negative control. The secondary antibodies used were cy3-conjugated anti-rat or mouse antibody at 1:200. Anti-calreticulin (cat. # PA3-900) and anti-GRP78 (cat. # PA1-014) are rabbit polyclonal antibodies and were used at a 1:200 dilution. Normal rabbit serum was used at 1:200 as a negative control. The secondary antibody used was a cy3-conjgated anti-rabbit antibody at 1:200. Hoechst chromatin stain was used at 10 μg/ml in PBS in order to determine which, if any, eggs had been activated or were undergoing degeneration as a consequence of manipulation.
Zona-free eggs were prepared as described above and allowed to recover for 3 h at 37°C in a 5% CO2 incubator before use. After the recovery period, eggs were washed through 4 × 50 μl wash drops of M199 media (see [8] for recipe). Live eggs were then incubated with primary antibody or control IgG as noted. The eggs were subsequently washed in M199 media and incubated in the appropriate secondary antibody for 1 h at 37°C in a 5% CO2 incubator, to determine the localization of primary antibody reactivity. Eggs were then washed in M199 media and incubated in 10 μg/ml Hoechst chromatin stain at 37°C for 10 min. Staining of the live eggs was visualized using ultra violet light and a fluorescent filter on a light microscope (Standard 18 model, Zeiss Microimaging, Thornwood, NY).
Results
2D Electrophoresis and TMS identification of abundant egg proteins
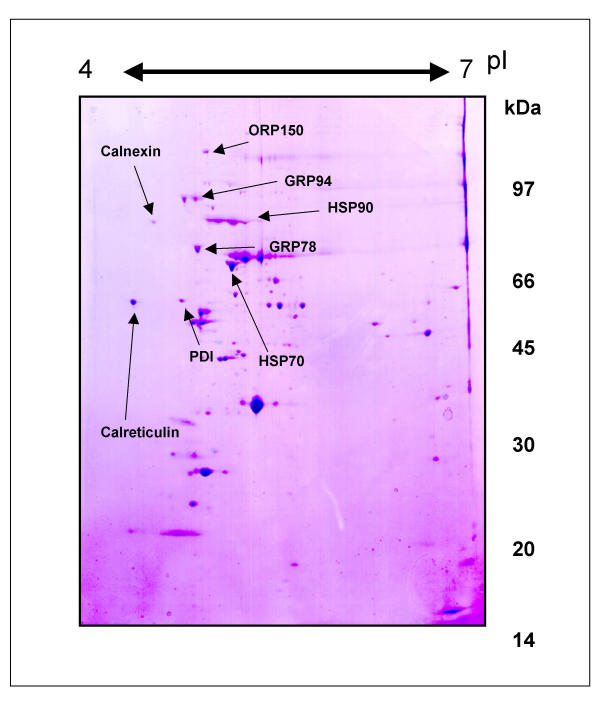
2D gel electrophoresis of whole egg extract from 2850 mature, metaphase II-arrested, untreated mouse eggs was performed in order to identify some of the more abundant proteins of the egg proteome. The 2D gel repertoire of whole, untreated eggs is presented in Fig. 1. Abundant, coomassie stained egg protein spots were cored from the gel and submitted for TMS analysis. Of the spots submitted, a subset matched known chaperones/HSP proteins; these are HSP70, HSP90α, GRP78, GRP94, Calreticulin, Calnexin, PDI, and ORP150, as indicated by labelled arrows in Fig. 1, displayed in Table 1.
Figure 1.
2D Elecrophoresis of whole egg extracts identifies abundant egg proteins. 2D electrophoresis of 2850 zona-free mouse egg proteins, extracted with Celis buffer and stained with coomassie. Black arrows indicate protein spots that were cored for TMS. Each spot is labelled with the resulting protein identification. Horizontal axis shows isoelectric point and vertical axis shows molecular weight (kDa).
Table 1.
Oolemmal surface-labeled Chaperone/HSPs Identified by TMS. List of putative oolemmal proteins identified by 2D electrophoresis and TMS, with predicted molecular weight and isoelectric point, genbank accession number, expression profile with associated references and presence or absence of ER-retention sequence (KDEL).
| Protein | MW | pI | Acc. # | Hsp or chaperone | Previously described on cell surface | Previously described in oocyte | ER-retention motif |
| HSP 70 | 70.0 | 5.46 | P17879 | HSP | [22-24] | [32-35,38-40] | - |
| HSP 90 (HSP 86) | 84.7 | 4.93 | P07901 | HSP | [23] | [36] | - |
| GRP 94 (gp96) | 92.5 | 4.74 | P08113 | HSP | [16,18,21,30] | - | +KDEL |
| GRP 78 (BiP) | 72.4 | 5.07 | P20029 | HSP | [16,28,30,31] | - | +KDEL |
| ORP 150 | 11.1 | 5.11 | AAB05672 | HSP | - | - | + KNDEL |
| Calreticulin | 48.0 | 4.33 | P14211 | Chaperone | [16,25,26,28] | [43,44] | +KDEL |
| Calnexin | 6.50 | 4.48 | AAA62450 | Chaperone | [16,17,19-22] | - | + KPRRE |
| PDI | 57.1 | 4.80 | P09103 | Chaperone | [27-29] | [45] | +KDEL |
Biotinylation and avidin blotting identifies surface-labeled egg proteins
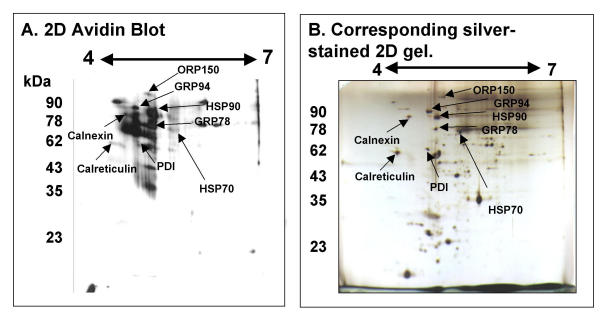
Separation of surface-labeled biotinylated egg proteins followed by avidin blotting was utilized to identify surface-labeled proteins. For these experiments, proteins from 800 zona-free biotinylated mouse eggs were extracted. The extracts from the treated eggs were separated by 2D gel electrophoresis, electroblotted to nitrocellulose membranes, and probed with streptavidin-HRP. Biotinylated proteins were visualized using enhanced chemiluminescence. Another 800, untreated zona-free eggs were extracted, separated by 2D electrophoresis and the gel was silver-stained. The 2D gel repertoire of biotin-labeled oolemmal surface proteins is presented in Fig. 2A. The corresponding silver stained 2D gel of untreated eggs (from which protein spots were cored for TMS) is presented in Fig. 2B. To ensure that plasma membrane proteins were biotinylated and cytoplasmic proteins were not, the blot shown in Fig. 2B was stripped of streptavidin-HRP and re-probed with antibodies to tubulin and actin (two known cytoskeletal proteins). The antibodies to the cytoplasmic markers only recognized two spots on the blot and these spots correspond to the expected mass and pI of tubulin and actin (data not shown). No surface-labeling of these cytoskeletal proteins occurred, validating the surface-specificity of the labeling method. Additionally, 2D blots of non-biotinylated oocytes were probed with streptavidin-HRP. Two proteins in the unlabeled eggs bound streptavidin, corresponding to endogenous non-specific avidin binding proteins not included in the repertoire of surface-labeled proteins (data not shown, see figures in Coonrod et al., [46]).
Figure 2.
2D Electrophoresis, biotinylation and avidin blotting identifies surface-labeled oolemmal proteins. 2D electrophoresis of 800 zona-free mouse egg proteins, surface labeled with 2 mg/ml Sulfo-NHS biotin, and separated by 2D electrophoresis. Blotted with streptavidin-HRP and visualized by ECL (A). Corresponding 2D electrophoretic gel of 800 unlabeled zona-free mouse eggs visualized by silver staining. Labelled black arrows indicate previously identified protein spots (B). Horizontal axis of each shows isoelectric point and vertical axis shows molecular weight (kDa).
Immunofluorescence with antibodies to surface-labeled chaperones/HSPs
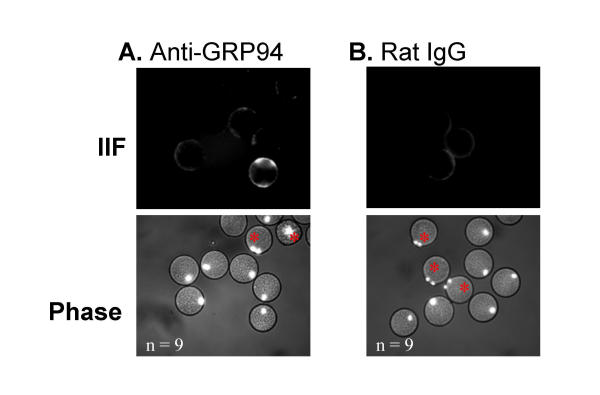
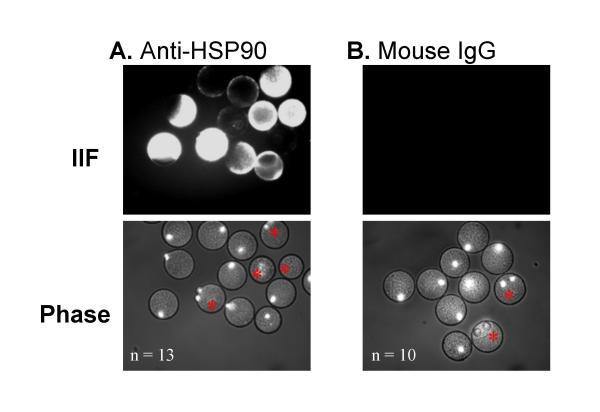
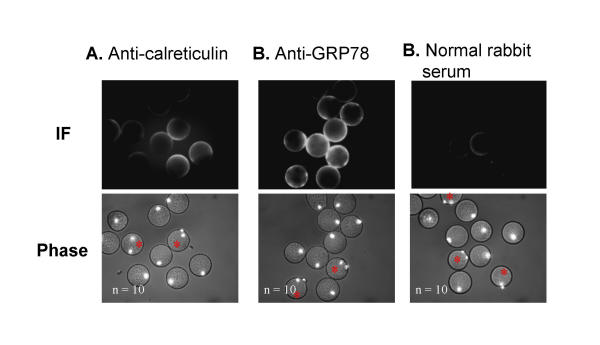
In order to provide additional evidence for surface localization, antibodies to five of the eight molecular chaperones identified by surface labelling were used in immunofluorescence experiments. Hoechst chromatin stain was utilized to determine whether eggs had parthenogenetically activated (activated eggs are mitotic and exhibit two chromatin poles rather than one, as indicated by an asterisk in Figs 3,4,5). When zona-free mature eggs were incubated with anti-GRP94, the antibody specifically bound to isolated regions of the plasma membrane in several eggs; however, the majority of eggs did not show any reactivity (Fig. 3A). In contrast, incubating in rat IgG alone did not result in any antibody reactivity (Fig. 3B). It is not clear from these preliminary results what common feature this subpopulation of positively staining eggs shared in common, but there does not appear to be an association between activation status and the degree of positive staining. Anti-HSP90 antibodies reacted very strongly with the egg plasma membrane of the majority of those eggs examined (Fig. 4A). Incubating eggs in mouse IgG alone did not result in any reactivity (Fig. 4B). Intriguingly, HSP90 staining was primarily localized to the microvillar region of normal eggs (the region opposite the pole containing the metaphase chromatin) whereas the staining became delocalized and more intense following activation. Anti-calreticulin antibodies bound to the plasma membrane in approximately half of the eggs, and no association with activation status was observed (Fig. 5A). Anti-GRP78 antibodies bound to the plasma membrane in the majority of the eggs examined (Fig. 5B). Incubating eggs in normal rabbit sera alone did not result in any reactivity (Fig. 5C). Neither anti-HSP70 nor anti-calnexin antibodies resulted in positive immunostaining in any of the eggs examined (data not shown). A more rigorous quantitative analysis of the surface localization of these molecular chaperones to the oolemma is now required.
Figure 3.
Immunofluorescent localization of GRP94. Immunofluorescence using rat monoclonal antibodies to GRP94 on live, zona-free mouse eggs (A). Compare to control egg population incubated with rat IgG. Secondary antibody used for both is cy3-conjugated anti-rat antibody (B). Red asterisks mark activated eggs.
Figure 4.
Immunofluorescent localization of HSP90. Immunofluorescence using mouse monoclonal antibodies to HSP90 on mature, zona-free mouse eggs (A). Compare to control egg population incubated with mouse IgG. Secondary antibody used for both is cy3-conjugated anti-rat antibody. n = 10 (B). Red asterisks mark activated eggs.
Figure 5.
Immunofluorescent localization of Calreticulin and GRP78. Immunofluorescence using rabbit polyclonal antibodies to (A) calreticulin, and (B) GRP78, on mature, zona-free mouse eggs. Compare to control egg population incubated with normal rabbit sera. (C). Secondary antibody used for both is cy3-conjugated anti-rabbit antibody. Red asterisks mark activated eggs.
Discussion
It has been estimated that individual mammalian cells express from 5,000, to 10,000 proteins at any given point in time [49]. Analysis of the 2D gel shown in Fig. 1 shows from ~3000 eggs, only several hundred proteins can be resolved by coomassie-staining, and therefore these proteins are likely some of the most abundant proteins in the ovulated mouse egg. In this study we have found that a subset of these highly abundant proteins are molecular chaperones. It will be of interest to establish the functional role of these proteins in the egg and early embryo. HSP70 is one of the first genes to be expressed following zygotic gene activation in the two-cell mouse embryo [38-40] and appears to play a role in developmental processes in a number of species [50-53]. Endogenous HSP90 has also been implicated in developmental regulation in the mouse [52], and GRP78 is described as having a stress-response function during avian development [54]. Activation of the zygotic genome initiates an accumulation of embryonic transcripts in the early two-cell embryo, and this likely requires machinery to ensure the accurate translation and folding of nascent proteins. The HSPs and chaperones found in the mature egg may represent a maternal stockpile of such folding machinery.
Another preliminary finding of this study is that several molecular chaperones appear to be localized to the egg surface. Although HSPs were originally identified as a family of proteins that are upregulated in response to cell stress, most are also endogenously expressed under physiological conditions. Constitutive expression of a number of HSPs and molecular chaperones has been characterized in immature oocytes and mature mouse eggs [38-40], though none as yet have been localized to the oolemma. Members of the chaperone/HSP protein families, including those containing an ER retention signal (e.g. KDEL, KKXX) have been described on the cell surface in a wide variety of cell types [16-23,27,26,28,30,31], though this phenomenon has not been previously described in oocytes or eggs. How these proteins are escaping ER retention is not yet known. It is possible that in association with another protein or protein complex, the chaperone's KDEL sequence is sterically prevented from binding with ER retention receptors, and this allows the protein to be transported to the plasma membrane. Another hypothesis is that alternative folding of the chaperone protein itself masks the retention sequence, allowing it to escape the ER. Further studies are needed to confirm the surface localization of these molecules and to determine which, if any, of these potential mechanisms are being employed.
The functional significance of surface expression of ER chaperones in different cell types has yet to be determined. A sperm receptor identified on the surface of sea urchin oocytes has an extracellular domain that shows sequence similarity to HSP70 and appears to be required for fertilization [42]. Additionally, members of the HSP family are known to be involved in the regulation of apoptosis (see [55] for review). HSP70 and GRP78 both have anti-apoptotic activity, whereas HSP90 appears to have both pro-apoptotic and anti-apoptotic activities, depending on the specific stimuli received. Early bovine embryos cultured in the presence of HSP70 antibodies exhibit increased apoptosis and reduced embryo viability [41]. However, it is still controversial as to whether the unfertilized egg is actively apoptotic or undergoes necrosis in the reproductive tract. During development, selective cell death by apoptosis becomes crucial, and chaperone molecules in the egg may play a role in later apoptotic events. Future experiments need to be performed before the significance of findings presented here can be addressed, both at the functional and mechanistic level. In general, the molecular chaperones are proving to play a wide variety of roles in diverse cellular processes and this list will likely grow as our understanding of these molecules increases.
Conclusions
Using 2D electrophoresis and TMS, we have identified eight molecular chaperone proteins that are abundantly expressed in the mature mouse egg. Of these proteins, GRP 94, GRP 78, Calreticulin and HSP 90 can be surface-labeled by biotin and appear to be localizing to the egg surface based on preliminary immunofluoresence data. These results provide preilimary evidence for the presence of these molecular chaperones on the egg surface.
Acknowledgments
Acknowledgements
This original work was supported by NIH grants HD 29099 and HD 38353.
Contributor Information
Meredith E Calvert, Email: mec4s@virginia.edu.
Laura C Digilio, Email: lcb2a@virginia.edu.
John C Herr, Email: jch7k@virginia.edu.
Scott A Coonrod, Email: sc9b@virginia.edu.
References
- Wassarman P, Chen J, Cohen N, Litscher E, Liu C, Qi H, Williams Z. Structure and function of the mammalian egg zona pellucida. J Exp Zool. 1999;285:251–8. doi: 10.1002/(SICI)1097-010X(19991015)285:3<251::AID-JEZ8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Almeida EA, Huovila AP, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG, et al. Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell. 1995;81:1095–104. doi: 10.1016/s0092-8674(05)80014-5. [DOI] [PubMed] [Google Scholar]
- Zhu X, Evans JP. Analysis of the roles of RGD-binding integrins, alpha(4)/alpha(9) integrins, alpha(6) integrins, and CD9 in the interaction of the fertilin beta (ADAM2) disintegrin domain with the mouse egg membrane. Biol Reprod. 2002;66:1193–202. doi: 10.1095/biolreprod66.4.1193. [DOI] [PubMed] [Google Scholar]
- Evans JP, Kopf GS, Schultz RM. Characterization of the binding of recombinant mouse sperm fertilin beta subunit to mouse eggs: evidence for adhesive activity via an egg beta1 integrin-mediated interaction. Dev Biol. 1997;187:79–93. doi: 10.1006/dbio.1997.8611. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Georges-Labouesse E, Primakoff P, Myles DG. Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J Cell Biol. 2000;149:1289–96. doi: 10.1083/jcb.149.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P, Myles DG. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science. 2002;296:2183–5. doi: 10.1126/science.1072029. [DOI] [PubMed] [Google Scholar]
- Chen MS, Tung KS, Coonrod SA, Takahashi Y, Bigler D, Chang A, Yamashita Y, Kincade PW, Herr JC, White JM. Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc Natl Acad Sci U S A. 1999;96:11830–5. doi: 10.1073/pnas.96.21.11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod SA, Naaby-Hansen S, Shetty J, Shibahara H, Chen M, White JM, Herr JC. Treatment of mouse oocytes with PI-PLC releases 70-kDa (pI 5) and 35- to 45-kDa (pI 5.5) protein clusters from the egg surface and inhibits sperm-oolemma binding and fusion. Dev Biol. 1999;207:334–49. doi: 10.1006/dbio.1998.9161. [DOI] [PubMed] [Google Scholar]
- Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD. Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum Reprod. 1995;10:1571–8. doi: 10.1093/humrep/10.6.1571. [DOI] [PubMed] [Google Scholar]
- Fusi FM, Montesano M, Bernocchi N, Panzeri C, Ferrara F, Villa A, Bronson RA. P-selectin is expressed on the oolemma of human and hamster oocytes following sperm adhesion and is also detected on the equatorial region of acrosome-reacted human spermatozoa. Mol Hum Reprod. 1996;2:341–7. doi: 10.1093/molehr/2.5.341. [DOI] [PubMed] [Google Scholar]
- Wiley LM, Obasaju MF. Immunofluorescent localization of immunoglobulins on the cell surface of mouse oocytes and preimplantation embryos. J In Vitro Fert Embryo Transf. 1986;3:319–25. doi: 10.1007/BF01133393. [DOI] [PubMed] [Google Scholar]
- Fusi F, Bronson RA, Hong Y, Ghebrehiwet B. Complement component C1q and its receptor are involved in the interaction of human sperm with zona-free hamster eggs. Mol Reprod Dev. 1991;29:180–8. doi: 10.1002/mrd.1080290214. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Abbott AF, Jack RM. The role of complement component C3b and its receptors in sperm-oocyte interaction. Proc Natl Acad Sci U S A. 1993;90:10051–5. doi: 10.1073/pnas.90.21.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson RA, Fusi FM, Fleit HB. Monoclonal antibodies identify Fc gamma receptors on unfertilized human oocytes but not spermatozoa. J Reprod Immunol. 1992;21:293–307. doi: 10.1016/0165-0378(92)90032-Y. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–96. doi: 10.1146/annurev.genet.27.1.437. [DOI] [PubMed] [Google Scholar]
- Wiest DL, Bhandoola A, Punt J, Kreibich G, McKean D, Singer A. Incomplete endoplasmic reticulum (ER) retention in immature thymocytes as revealed by surface expression of "ER-resident" molecular chaperones. Proc Natl Acad Sci U S A. 1997;94:1884–9. doi: 10.1073/pnas.94.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest DL, Burgess WH, McKean D, Kearse KP, Singer A. The molecular chaperone calnexin is expressed on the surface of immature thymocytes in association with clonotype-independent CD3 complexes. Embo J. 1995;14:3425–33. doi: 10.1002/j.1460-2075.1995.tb07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Menoret A, Cohen N. Cell surface expression of the endoplasmic reticular heat shock protein gp96 is phylogenetically conserved. J Immunol. 1999;163:4133–9. [PubMed] [Google Scholar]
- Nagata K, Nakamura T, Kitamura F, Kuramochi S, Taki S, Campbell KS, Karasuyama H. The Ig alpha/Igbeta heterodimer on mu-negative proB cells is competent for transducing signals to induce early B cell differentiation. Immunity. 1997;7:559–70. doi: 10.1016/s1074-7613(00)80377-5. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Ohno H, Takase K, Ochiai T, Saito T. Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J Biol Chem. 2000;275:35751–8. doi: 10.1074/jbc.M007476200. [DOI] [PubMed] [Google Scholar]
- Altmeyer A, Maki RG, Feldweg AM, Heike M, Protopopov VP, Masur SK, Srivastava PK. Tumor-specific cell surface expression of the-KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer. 1996;69:340–9. doi: 10.1002/(SICI)1097-0215(19960822)69:4<340::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–9. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51:613–9. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- Botzler C, Schmidt J, Luz A, Jennen L, Issels R, Multhoff G. Differential Hsp70 plasma-membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int J Cancer. 1998;77:942–8. doi: 10.1002/(SICI)1097-0215(19980911)77:6<942::AID-IJC25>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Goicoechea S, Orr AW, Pallero MA, Eggleton P, Murphy-Ullrich JE. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J Biol Chem. 2000;275:36358–68. doi: 10.1074/jbc.M005951200. [DOI] [PubMed] [Google Scholar]
- White TK, Zhu Q, Tanzer ML. Cell surface calreticulin is a putative mannoside lectin which triggers mouse melanoma cell spreading. J Biol Chem. 1995;270:15926–9. doi: 10.1074/jbc.270.27.15926. [DOI] [PubMed] [Google Scholar]
- Essex DW, Chen K, Swiatkowska M. Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood. 1995;86:2168–73. [PubMed] [Google Scholar]
- Xiao G, Chung TF, Pyun HY, Fine RE, Johnson RJ. KDEL proteins are found on the surface of NG108-15 cells. Brain Res Mol Brain Res. 1999;72:121–8. doi: 10.1016/S0169-328X(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Akagi S, Yamamoto A, Yoshimori T, Masaki R, Ogawa R, Tashiro Y. Localization of protein disulfide isomerase on plasma membranes of rat exocrine pancreatic cells. J Histochem Cytochem. 1988;36:1069–74. doi: 10.1177/36.8.3292644. [DOI] [PubMed] [Google Scholar]
- Takemoto H, Yoshimori T, Yamamoto A, Miyata Y, Yahara I, Inoue K, Tashiro Y. Heavy chain binding protein (BiP/GRP78) and endoplasmin are exported from the endoplasmic reticulum in rat exocrine pancreatic cells, similar to protein disulfide-isomerase. Arch Biochem Biophys. 1992;296:129–36. doi: 10.1016/0003-9861(92)90554-a. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Fradelizi D, Triantafilou K. Major histocompatibility class one molecule associates with glucose regulated protein (GRP) 78 on the cell surface. Hum Immunol. 2001;62:764–70. doi: 10.1016/S0198-8859(01)00269-5. [DOI] [PubMed] [Google Scholar]
- Bienz M. Xenopus hsp 70 genes are constitutively expressed in injected oocytes. Embo J. 1984;3:2477–83. doi: 10.1002/j.1460-2075.1984.tb02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M, Gurdon JB. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982;29:811–9. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Billoud B, Rodriguez-Martin ML, Berard L, Moreau N, Angelier N. Constitutive expression of a somatic heat-inducible hsp70 gene during amphibian oogenesis. Development. 1993;119:921–32. doi: 10.1242/dev.119.3.921. [DOI] [PubMed] [Google Scholar]
- Kawarsky SJ, King WA. Expression and localisation of heat shock protein 70 in cultured bovine oocytes and embryos. Zygote. 2001;9:39–50. doi: 10.1017/S0967199401001058. [DOI] [PubMed] [Google Scholar]
- Coumailleau P, Billoud B, Sourrouille P, Moreau N, Angelier N. Evidence for a 90 kDa heat-shock protein gene expression in the amphibian oocyte. Dev Biol. 1995;168:247–58. doi: 10.1006/dbio.1995.1077. [DOI] [PubMed] [Google Scholar]
- Curci A, Bevilacqua A, Mangia F. Lack of heat-shock response in preovulatory mouse oocytes. Dev Biol. 1987;123:154–60. doi: 10.1016/0012-1606(87)90437-4. [DOI] [PubMed] [Google Scholar]
- Manejwala FM, Logan CY, Schultz RM. Regulation of hsp70 mRNA levels during oocyte maturation and zygotic gene activation in the mouse. Dev Biol. 1991;144:301–8. doi: 10.1016/0012-1606(91)90423-z. [DOI] [PubMed] [Google Scholar]
- Bensaude O, Babinet C, Morange M, Jacob F. Heat shock proteins, first major products of zygotic gene activity in mouse embryo. Nature. 1983;305:331–3. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- Christians E, Campion E, Thompson EM, Renard JP. Expression of the HSP 70.1 gene, a landmark of early zygotic activity in the mouse embryo, is restricted to the first burst of transcription. Development. 1995;121:113–22. doi: 10.1242/dev.121.1.113. [DOI] [PubMed] [Google Scholar]
- Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7:829–37. doi: 10.1093/molehr/7.9.829. [DOI] [PubMed] [Google Scholar]
- Foltz KR, Partin JS, Lennarz WJ. Sea urchin egg receptor for sperm: sequence similarity of binding domain and hsp70. Science. 1993;259:1421–5. doi: 10.1126/science.8383878. [DOI] [PubMed] [Google Scholar]
- Parys JB, McPherson SM, Mathews L, Campbell KP, Longo FJ. Presence of inositol 1,4,5-trisphosphate receptor, calreticulin, and calsequestrin in eggs of sea urchins and Xenopus laevis. Dev Biol. 1994;161:466–76. doi: 10.1006/dbio.1994.1045. [DOI] [PubMed] [Google Scholar]
- Munoz-Gotera RJ, Hernandez-Gonzalez EO, Mendoza-Hernandez G, Contreras RG, Mujica A. Exocytosis of a 60 kDa protein (calreticulin) from activated hamster oocytes. Mol Reprod Dev. 2001;60:405–13. doi: 10.1002/mrd.1103. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Lebeche D, Kaminer B. ERcalcistorin/protein disulfide isomerase (PDI). Sequence determination and expression of a cDNA clone encoding a calcium storage protein with PDI activity from endoplasmic reticulum of the sea urchin egg. J Biol Chem. 1994;269:23112–9. [PubMed] [Google Scholar]
- Coonrod SA, Wright PW, Herr JC. Oolemmal proteomics. J Reprod Immunol. 2002;53:55–65. doi: 10.1016/S0165-0378(01)00102-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen HH, Van Damme J, Puype M, Gesser B, Celis JE, Vandekerckhove J. Microsequencing of proteins recorded in human two-dimensional gel protein databases. Electrophoresis. 1991;12:873–82. doi: 10.1002/elps.1150121107. [DOI] [PubMed] [Google Scholar]
- Hochstrasser DF, Merril CR. 'Catalysts' for polyacrylamide gel polymerization and detection of proteins by silver staining. Appl Theor Electrophor. 1988;1:35–40. [PubMed] [Google Scholar]
- Celis JE, Gesser B, Rasmussen HH, Madsen P, Leffers H, Dejgaard K, Honore B, Olsen E, Ratz G, Lauridsen JB, et al. Comprehensive two-dimensional gel protein databases offer a global approach to the analysis of human cells: the transformed amnion cells (AMA) master database and its link to genome DNA sequence data. Electrophoresis. 1990;11:989–1071. doi: 10.1002/elps.1150111202. [DOI] [PubMed] [Google Scholar]
- Angelier N, Moreau N, Rodriguez-Martin ML, Penrad-Mobayed M, Prudhomme C. Does the chaperone heat shock protein hsp70 play a role in the control of developmental processes? Int J Dev Biol. 1996;40:521–9. [PubMed] [Google Scholar]
- Giudice G, Sconzo G, Roccheri MC. Studies on heat shock proteins in sea urchin development. Dev Growth Differ. 1999;41:375–80. doi: 10.1046/j.1440-169X.1999.00450.x. [DOI] [PubMed] [Google Scholar]
- Loones MT, Chang Y, Morange M. The distribution of heat shock proteins in the nervous system of the unstressed mouse embryo suggests a role in neuronal and non-neuronal differentiation. Cell Stress Chaperones. 2000;5:291–305. doi: 10.1379/1466-1268(2000)005<0291:tdohsp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio E, Valenciano AI, Segundo C, Sanchez N, de Pablo F, de la Rosa EJ. Programmed cell death in the neurulating embryo is prevented by the chaperone heat shock cognate 70. Eur J Neurosci. 2002;15:1646–54. doi: 10.1046/j.1460-9568.2002.01998.x. [DOI] [PubMed] [Google Scholar]
- Muscarella DE, Rachlinski MK, Bloom SE. Expression of cell death regulatory genes and limited apoptosis induction in avian blastodermal cells. Mol Reprod Dev. 1998;51:130–42. doi: 10.1002/(SICI)1098-2795(199810)51:2<130::AID-MRD2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–42. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]