Abstract
Antigen exposure via airway epithelia is often associated with a failure to prime or with the preferential priming of Th2 cells. We previously reported that the intranasal delivery of a Th1-inducing antigen promoted Th2-dominated responses, rather than the expected Th1 responses. Thus, we proposed that when pulmonary T cell priming is induced, the lung microenvironment might intrinsically favor the generation of Th2 types of responses. To establish a potential mechanism for such preferential priming, we examined the initial interactions between antigens and resident antigen-presenting cells (APCs) within the lung. We show that intranasally delivered antigens are preferentially taken up and can be presented to antigen-specific T cells by a resident population of CD11cbright APCs. Most of these antigen-loaded APCs remained within lung tissues, and migration into secondary lymphoid organs was not crucial for T cell priming to occur within the pulmonary tract. Furthermore, these pulmonary APCs demonstrated a marked expression of IL-6 and IL-10 within hours of antigen uptake, suggesting that resident tissue APCs have the capacity to promote Th2 T cell differentiation in situ.
Introduction
The innate immune microenvironment within different organs and tissues has the potential to impact significantly on T cell priming and differentiation. Thus, different tissue sites may be predisposed to the generation of specific types of immune responses, due to their intrinsic microenvironment. For example, mucosal tissues, such as the pulmonary and gastrointestinal tracts, are often prone to the induction of Th2-dominated responses. A striking example of this is observed in human allergic asthma, where immune responses are selectively dominated by Th2-type effector cells (1). In fact, in almost all models where T cell priming was successfully initiated via the pulmonary tract, the outcome was a predominance of Th2 immune responses (2–4). In one of these models of pulmonary T cell priming, developed within our laboratory, an infectious pathogen (Leishmania major) is delivered intranasally to C57BL/6 mice. The findings were particularly striking because delivery of L. major to C57BL/6 mice via most other routes of immunization is associated with priming for Th1 CD4+ T cells (5). These data support the hypothesis that the lung microenvironment intrinsically favors priming for the differentiation of Th2 CD4+ T cells. Alternatively, the lung might be predisposed to “disfavor” priming for Th1-type responses.
The type of antigen-presenting cell (APC) and the way in which antigen is presented to a population of naive T cells play an important role in determining what type of T cell response is initiated (6). In the lung, several potential APCs exist, including alveolar macrophages, tissue monocytes, epithelial cells, and dendritic cells. The ability of these cells to take up and present antigen during the initiation of an immune response in situ, and their role in regulating T cell effector responses, are not well understood. In the current paper we have examined the early immunologic events following the intranasal delivery of two different antigens, both with the capacity to induce preferential Th2 T cell priming, to establish how their uptake affects APCs within the pulmonary microenvironment. Our results show that (a) immunogens are preferentially taken up and presented to antigen-specific CD4+ T cells by a restricted population of resident APCs, (b) the majority of antigen-loaded APCs remain within lung tissues rather than migrating into draining lymph nodes, (c) migration of APCs into draining lymph nodes is not necessary for pulmonary T cell priming to occur, and (d) in situ uptake of immunogens induces a rapid upregulation in IL-6 and IL-10 cytokines within resident lung APCs.
Methods
Animals.
All mice were used at 6–8 weeks of age. C57BL/6 mice were purchased from the National Cancer Institute (Frederick, Maryland, USA). A breeding pair of lymphotoxin-α (LTα) knockout mice (on a mixed 129 × C57BL/6 background) were originally obtained from David Chaplin (Washington University, St. Louis, Missouri, USA) and have since been backcrossed for more than eight generations onto a C57BL/6 background. For some experiments, LTα knockout mice were splenectomized 2 weeks prior to immunization. Splenectomies were performed by staff of the Veterinarian Care Services at Yale University.
Antigens.
L. major promastigotes of the WR309 substrain were maintained at 23°C in Schneider’s Drosophila medium (GIBCO BRL; Invitrogen Corporation, Grand Island, New York, USA) supplemented with 20% FCS and 50 μg/ml gentamicin. For some experiments, L. major parasites were labeled with CFSE (5- and 6-carboxyfluorescein diacetate succinimidyl ester; Molecular Probes Inc., Eugene, Oregon, USA) using a protocol slightly modified from that of Lyons and Parish (7). Briefly, parasites were washed three times in PBS and resuspended at 5 × 107 per milliliter in prewarmed PBS containing 10 μM CFSE. The mixture was then incubated in six-well plates at 32°C for 30 minutes. The labeling reaction was quenched by washing the parasites once with PBS containing 0.1% BSA. Labeled parasites were then washed two more times with PBS and recounted before delivery. In some studies, FITC-labeled ovalbumin (FITC-OVA; Molecular Probes Inc.) was used. Unlabeled OVA protein and LPS (from Escherichia coli serotype 055:B5) were purchased from Sigma-Aldrich (St. Louis, Missouri, USA).
Sensitization protocols.
For studies in which priming and effector responses to L. major were induced in vivo, mice were given an intranasal administration of 107 L. major parasites in 50 μl PBS (with or without 0.4 μg LPS) or a subcutaneous delivery of 5 × 106 parasites in 10 μl PBS in both hind feet. All animals were challenged 1–4 weeks later with an intranasal delivery of 107 parasites (without LPS) to induce effector responses in the pulmonary tract. Responses were measured 4 days after challenge. The intranasal priming/challenge regimen using OVA protein has been described in detail previously (2). For experiments examining uptake and presentation of L. major by APCs, mice received 107 parasites (either unlabeled or CFSE-labeled) in 50 μl PBS by intranasal administration. FITC-OVA was administered by intranasal delivery at a dose of 1 mg per 50 μl PBS with or without 0.4 μg LPS. For experiments in which the course of L. major infection was monitored, mice received 2 × 106 L. major promastigotes in 10 μl PBS in the right hind foot, and at weekly intervals footpad thickness of the infected right versus the uninfected left foot was measured using spring-loaded calipers.
Bronchoalveolar lavage and differential staining.
At various times after delivery of antigen, mice were sacrificed by exposure to CO2 and a cannula was inserted into the trachea. Cells from the lung airways were collected by infusing three 1-ml washes of cold PBS. Cytospin preparations of the bronchoalveolar lavage (BAL) cells (up to 1 × 105 per slide) were generated for individual mice and then stained using a Wright-Giemsa stain. Differential counts were performed on 200 cells based on morphology and staining characteristics.
In vitro restimulation of lung tissue cells to generate effector cytokines.
Following the BAL procedure, animals were perfused via the right ventricle with 20 ml ice-cold PBS, and lungs were isolated and pooled from three to five animals per group. The lungs were finely chopped and then incubated at 37°C for 20 minutes in 5 ml per lung of a digestion mixture consisting of Click’s medium, 10% FCS, 150 U/ml collagenase, and 20 μg/ml DNase I type IV from bovine pancreas. After digestion, tissues were placed on ice for 2 minutes to quench the reaction before being pushed through a metal strainer, filtered through nylon mesh, and centrifuged over a Ficoll gradient to remove dead cells. Cell suspensions of lung tissue were cultured in 48-well plates at a final concentration of 1 × 106 cells per milliliter with T cell–depleted splenocytes (also at 1 × 106 per milliliter) prepared from naive C57BL/6 mice and varying doses of L. major lysate or OVA protein. All cultures were set up in Click’s medium supplemented with 5% FCS. Supernatants were collected after 4 days of culture for cytokine analysis. The supernatants were then assayed for IFN-γ, IL-4, and IL-5 using ELISA mini-kits purchased from Endogen Inc. (Woburn, Massachusetts, USA). IL-13 was measured using an ELISA kit purchased from R&D Systems Inc. (Minneapolis, Minnesota, USA).
Staining for FACS analysis and sorting.
Suspensions of lung tissue cells were stained in PBS containing 5% FCS and 0.01% sodium azide, and all staining was performed on ice. To reduce nonspecific binding, cells were preincubated with a cocktail of anti-FcR and mouse Ig for 20 minutes prior to staining with specific Ab’s (mouse Ig only was used for blocking when staining for CD14 and FcR). The following Ab’s were used: biotinylated anti-CD11c (BD Biosciences, San Jose, California, USA); phycoerythrin-conjugated (PE-conjugated) anti-CD11c, anti-CD14, anti-FcR, anti–B7-1, anti–B7-2, anti-CD40, and anti–I-Ab MHC class II (BD PharMingen); and FITC- or PE-conjugated anti-CD11b and PE-conjugated anti–F4/80 (Caltag Laboratories Inc., Burlingame, California, USA). Anti-DEC205 (NLDC145) was purified and biotinylated in house. RED670-conjugated streptavidin was purchased from Invitrogen Corp. Appropriate irrelevant Ab’s (PE-conjugated anti-CD4 or biotinylated anti-CD4) and PE-conjugated isotype control Ab’s (rat IgG or hamster IgG) were used throughout.
Lung tissue and lymph node APCs for T cell stimulation.
Cell suspensions were generated from lung tissue or lymph nodes 18 hours after intranasal delivery of L. major parasites. The cells were stained with a combination of anti-CD11c and anti-CD11b Ab’s, and sort gates were set to isolate populations of CD11cbright CD11bdim versus CD11cdim CD11bbright cells (see Figure 2a). After sorting, the populations were irradiated with 20 Gy from a cesium source. These antigen-loaded APCs were then mixed at twofold dilutions starting at 1 × 104 with a fixed number (2 × 105 per well) of “filler” APCs. The filler APCs were prepared from the spleen of naive C57BL/6 mice by Ab-mediated complement lysis of T cells followed by mitomycin C treatment. Leishmania-specific CD4+ T cells were generated from popliteal and inguinal lymph nodes isolated from C57BL/6 mice that had received a subcutaneous infection with 2 × 106 L. major promastigotes 4 weeks earlier. The CD4+ T cells were purified from lymph node cell suspensions by immunomagnetic depletion of CD8+, MHC class II+, and FcR+ cells. CD4+ T cells (2 × 105 per well) and APCs were set up in triplicate in 96-well plates in Click’s medium supplemented with 5% FCS, and 4 days later the cultures were pulsed for 18 hours with 3H-thymidine to measure T cell proliferation.
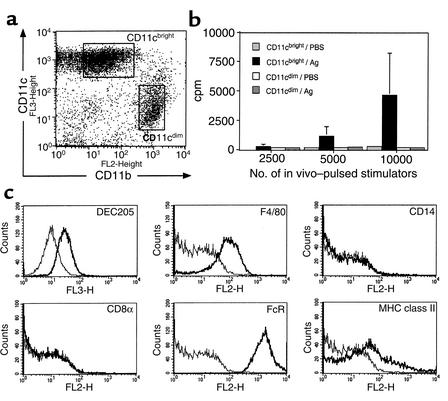
Figure 2.
Phenotypic and functional characterization of lung tissue APCs. C57BL/6 mice received an intranasal dose of L. major parasites in PBS, or PBS alone, and 18 hours later the mice were sacrificed and perfused with PBS and their lungs were removed to generate cell suspensions. (a) Lung tissue cells were stained with a combination of biotinylated anti-CD11c and PE-conjugated anti-CD11b, followed by RED670-conjugated streptavidin. FACS dot plot shows a representative profile of CD11c versus CD11b staining of lung tissue cells (after gating on total live cells) following either PBS or L. major administration. Boxes show the FACS-sort regions usually set for the isolation of CD11cbright (CD11bdim) versus CD11cdim (CD11bbright) populations. (b) Sorted populations of antigen-loaded CD11cbright and CD11cdim cells were irradiated and set up in culture at various numbers in the presence of L. major–specific CD4+ T cells. Bar graph shows the proliferation (mean ± SEM of triplicate wells) of the responder T cells after 4 days of culture in the presence of stimulators from mice that had received an intranasal delivery of either PBS alone or antigen (Ag) (L. major parasites). (c) Lung cells were stained with a combination of biotinylated anti-CD11c and PE-conjugated anti–F4/80, -CD14, -CD8α, -FcR, or –I-Ab, followed by RED670-streptavidin. DEC205 staining was performed using PE–anti-CD11c plus biotinylated anti-DEC205 and RED670-streptavidin. Histogram plots show positive staining (heavy lines) relative to staining with irrelevant Ab’s after gating on CD11cbright cells.
Histology.
Freshly isolated whole lungs and mediastinal lymph nodes were incubated overnight at 4°C in periodate-lysine-paraformaldehyde (PLP) fixative. The following day, fixed organs were rinsed three times in cold 0.1 M phosphate buffer (pH 7.2) and then soaked sequentially in cold 10% and 20% sucrose (both prepared in 0.1 M phosphate buffer). Fully soaked tissues were embedded in cryomolds containing Tissue-Tek OCT compound (VWRInternational Inc., Bridgeport, New Jersey, USA) and frozen in 2-methyl butane over dry ice. Sections (7 μM) of frozen tissue were then cut by cryostat and examined by fluorescence microscopy for the presence of FITC+ cells.
RNase protection assay.
Suspensions of lung tissue cells were incubated in six-well tissue culture plates in Click’s medium containing 5% FCS at 37°C for 2 hours to enrich for adherent cells. Nonadherent cells were then removed by gentle washing with prewarmed Click’s medium/5% FCS, and the remaining adherent cells (>85% CD11cbright) were lysed directly in the wells using TRIzol reagent (GIBCO BRL; Invitrogen Corp.).Cytokine RNA levels were analyzed by RNase protection assay using a customized RiboQuant template (BD Biosciences) designed to detect the following mRNA: IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-12p35, IL-12p40, IL-15, and IFN-γ. Five micrograms of total RNA was used in each reaction. Transcript levels for the GAPDH housekeeping gene were used as a control for equal loading. RNA extracted from activated dendritic cells was used as a positive control for the detection of cytokine mRNA. These dendritic cells were generated from bone marrow cells cultured for 10 days in the presence of 200 U/ml rGM-CSF, followed by overnight stimulation with 1 μg/ml LPS.
Results
Intranasal delivery of a Th1-type immunogen preferentially induces Th2 T cell priming.
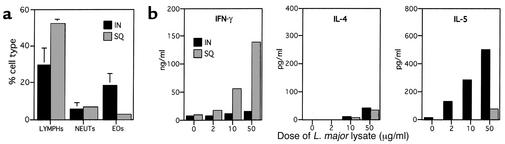
L. major pathogens are usually associated with the induction of Th1-dominated responses when delivered to C57BL/6 mice (5). However, in previous studies, we established that the intranasal delivery of these pathogens leads to the preferential induction of Th2-type effector responses instead (3). Figure 1 shows a comparison of the pulmonary effector responses induced in C57BL/6 mice primed either by an intranasal or by a subcutaneous route of parasite delivery. As previously shown, C57BL/6 mice primed via an intranasal route demonstrated Th2-dominated effector responses upon challenge, characterized by the presence of eosinophils within BAL fluid (Figure 1a) and the production of low levels of IFN-γ and high levels of IL-5 following in vitro restimulation of lung tissue cells (Figure 1b). In contrast, the effector responses observed in subcutaneously primed mice were characterized by an absence of eosinophilic infiltrates within BAL (Figure 1a), as well as high levels of IFN-γ and low IL-5 production (Figure 1b), consistent with a Th1-like phenotype. Low levels of IL-4 were generated by both groups. The low levels of IL-4 detected within the Th2 cultures were almost certainly due to reutilization of the cytokine by proliferating cells, since the induction of Th2 priming following intranasal antigen delivery has previously been shown to require IL-4 (2). However, IL-5 and IL-13 cytokines were reproducibly detected at high levels in these Th2 cultures. Taken together, these data show that the type of T cell priming induced by the intranasal delivery of L. major, in contrast to subcutaneous delivery, is Th2-dominated.
Figure 1.
Delivery of a Th1-type immunogen to lung favors priming for Th2 responses. C57BL/6 mice were given an intranasal (IN) delivery of 107 L. major or a subcutaneous (SQ) delivery of 5 × 106 parasites into each hind foot. Both groups were challenged 28 days later with an intranasal dose of 107 L. major to induce effector responses. Four days after challenge, the mice were sacrificed and their BAL cells recovered, followed by perfusion with PBS and isolation of lung tissue. (a) Cytospins were prepared from BAL cells recovered from individual mice. Bar graph shows the mean (± SE) proportions of lymphocytes (LYMPHs), neutrophils (NEUTs), and eosinophils (EOs) recovered from BAL of individual (n = 4) intranasally or subcutaneously primed mice. Macrophages make up the remaining percentage of cells recovered by BAL and have been omitted for clarity. (b) Lung tissue cells from pooled mice from each group were isolated by collagenase/DNase treatment and set up in culture with varying doses of L. major soluble lysate and splenic APCs to induce antigen-specific cytokine production. Bar graphs show the mean IFN-γ, IL-4, and IL-5 production of duplicate ELISA wells after 4 days of culture.
Intranasally delivered antigen is preferentially taken up and presented to CD4+ T cells by a restricted population of resident APCs.
We next examined the initial interactions between antigens and APCs, to establish how these might contribute to preferential Th2-type priming in the pulmonary tract. Using a combination of Ab’s to CD11c (N418) and CD11b (MAC-1), two distinct populations were identified within lung tissues: (a) CD11cbright CD11bdim, and (b) CD11cdim CD11bbright (Figure 2a shows a typical FACS profile). To establish which of the two populations might be important for the uptake and presentation of antigen, L. major parasites were delivered intranasally to C57BL/6 mice, and 18 hours later the populations were FACS-sorted using the sort regions depicted in Figure 2a. When the two populations of antigen-loaded APCs were examined for their ability to stimulate antigen-specific CD4+ T cells, only the CD11cbright population induced significant levels of T cell proliferation (Figure 2b). Further phenotypic analysis of the CD11cbright CD11bdim population showed the cells to express DEC205 and F4/80 antigens and to be CD8α– and CD14– (Figure 2c). The CD11c+ F4/80+ phenotype (8), in conjunction with DEC205 expression (9), is suggestive of a dendritic cell–like population similar to epidermal Langerhans cells. This is further supported by a high expression of FcR and low levels of MHC class II (Figure 2c).
Phenotypic changes after uptake of antigen by resident pulmonary APCs.
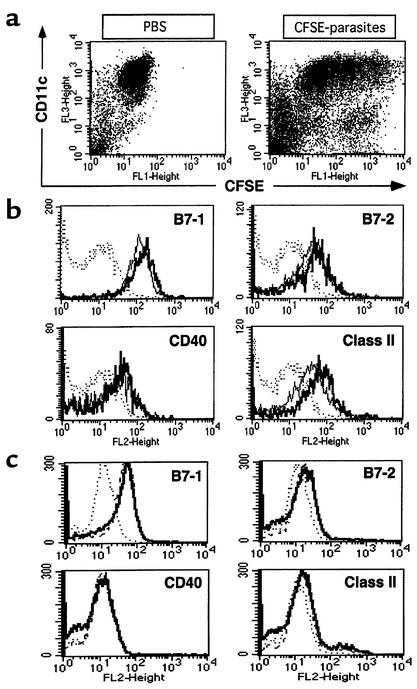
We next examined phenotypic markers usually associated with the activation and/or maturation status of APCs on the CD11cbright population of cells both before and after intranasal delivery of antigen. To identify those cells that had taken up antigen, L. major parasites were labeled with a fluorescent dye, CFSE (7), before intranasal delivery. CFSE-labeled parasites could be readily identified as FL1-positive events and were located predominantly within the population of CD11cbright cells, and to a lesser extent in CD11cdim cells (Figure 3a). As shown in Figure 3b, although we observed some increase in the expression of most activation markers on CFSE+ cells, these changes were small. Comparable results were observed following the intranasal delivery of another immunogen, FITC-OVA (Figure 3c). Cells were monitored for up to 7 days following antigen delivery, but no further changes in phenotype were observed. Taken together, there is evidence of some APC activation following antigen uptake; however, the small changes in activation markers suggest that the APCs are not undergoing any significant maturation and/or differentiation in situ.
Figure 3.
Uptake of antigen does not alter the immature phenotype of resident pulmonary APCs. C57BL/6 mice were given an intranasal administration of CFSE-labeled L. major parasites or FITC-OVAin PBS, or PBS alone, and 18 hours later their lungs were isolated and cell suspensions were generated for FACS staining. The cells were stained with a combination of biotinylated anti-CD11c and PE-conjugated anti–B7-1, anti–B7-2, anti-CD40, anti–MHC class II, or a relevant isotype control, followed by RED670-conjugated streptavidin. (a) Dot plots show the distribution of CD11c versus CFSE (after gating on live cells) on lung cells isolated from mice that had received either PBS or CFSE-labeled parasites. Lung cells were analyzed to compare the activation/maturation markers on the CD11cbright cells that had taken up CFSE–L. major (b) or FITC-OVA (c) antigen. Analysis gates were set on CFSE+/FITC+ cells versus CFSE–/FITC– cells to compare the phenotype of cells that had taken up versus cells that had not taken up antigen. Histograms show the expression of different surface markers on CFSE+/FITC+ (heavy lines) versus CFSE–/FITC– cells (thin lines) after gating on CD11cbright cells. The staining with relevant isotype control Ab’s is also shown (dotted lines). The staining profile for CD11cbright cells that received intranasal PBS only colocalized with that of the CFSE–/FITC– populations (data not shown).
Migration of antigen-loaded APCs into draining lymph nodes is limited.
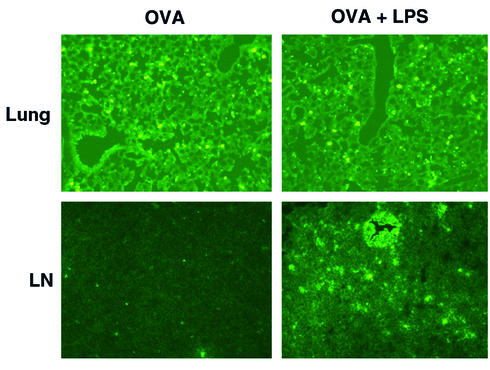
Based on studies examining tissue APCs from other sites, notably skin dendritic cells and monocytes, we thought it likely that the majority of pulmonary APCs taking up antigen would be induced to migrate out of lung tissues and into draining lymph nodes. Thus, mice received an intranasal delivery of fluorescently labeled immunogen, and at daily intervals (up to 7 days) their lungs and lung-draining lymph nodes (mediastinal) were examined for the presence of antigen-loaded APCs using a histologic approach. As a positive control for our capacity to detect APC migration, a separate group of animals received an intrana-sal delivery of fluorescent immunogen plus LPS, a proinflammatory agent known to promote APC emigration from tissues, including mucosal sites (10). Figure 4 shows sections of lung tissue and lymph nodes at 48 hours (peak detection) after intranasal delivery of FITC-OVA. Surprisingly, while the uptake of FITC-OVA within lung tissues was equivalent with or without codelivery of LPS, the presence of FITC+ APCs within draining lymph nodes was markedly lower in the absence of LPS. Confirmation that some APC migration was occurring in the absence of LPS was obtained using a functional approach in which CD11c+ cells were isolated from draining lymph nodes 48 hours after intranasal delivery of OVA and used as APCs for OVA-specific T cell proliferation. While the stimulation by these APCs was reproducibly detectable, it was extremely low (3,091 ± 1,619 cpm). Taken together, these data suggest that in the absence of a secondary proinflammatory signal, such as LPS, only a small number of antigen-loaded APCs migrate into draining lymph nodes from pulmonary tissues. Instead, the majority of these APCs remain within tissues and fail to emigrate.
Figure 4.
Migration of antigen-loaded pulmonary APCs into draining lymph nodes is limited. Mice were given an intranasal delivery of 1 mg FITC-OVA, with or without 0.08 μg LPS. At daily intervals for up to 7 days, mice from each group were sacrificed and perfused, and their intact lungs and mediastinal lymph nodes were then isolated and incubated overnight in PLP fixative. After several soaking cycles in sucrose, the fixed tissues were embedded and frozen in Tissue-Tek and cut into 7-μM sections by cryostat. These were examined by fluorescence microscopy for the presence of FITC+ cells. Panels show representative sections of lung tissue and lymph nodes (LN) from animals given FITC-OVA alone (OVA) or FITC-OVA with LPS (OVA + LPS). Data are from the 48-hour time point.
T cell priming can occur in the lung in the absence of APC migration to draining lymph nodes.
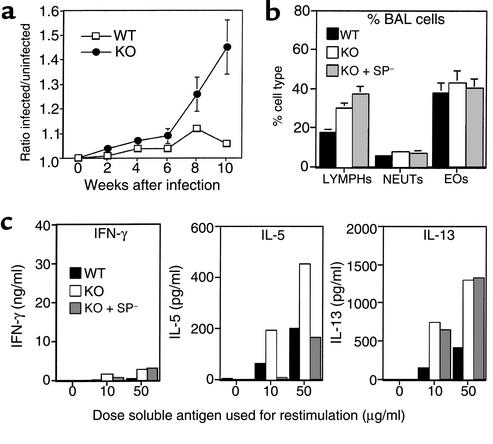
The limited migration of antigen-loaded APCs into draining lymph nodes during the course of pulmonary Th2-type priming led us to ask whether APC migration into secondary lymphoid organs is absolutely necessary for priming to occur, and whether the small numbers of antigen-loaded APCs present within draining lymph nodes are crucial for the initiation of T cell differentiation. Thus, we examined the capacity of lymphotoxin-α (LTα) knockout mice to become primed to antigen delivered via the airways. These mice are devoid of lymph nodes (including lung-draining lymph nodes) and Peyer’s patches (11, 12) and therefore provided an ideal noninvasive means to establish the importance of APC migration in draining lymph nodes during pulmonary T cell priming. Importantly, these lymph node–deficient mice have been shown to be severely impaired in their ability to become primed to a variety of different immunogens following either a subcutaneous or an intraperitoneal route of antigen delivery (12, 13). In support of this, we observed a failure of these mice to generate effective antiparasite T cell responses following a subcutaneous delivery of L. major parasites, resulting in their inability to clear infection (Figure 5a).
Figure 5.
Th2 T cell priming can occur in the lung in the absence of APC migration to draining lymph nodes. (a) Groups of C57BL/6 and LTα knockout mice were given a subcutaneous delivery of L. major promastigotes in the right hind foot, and at weekly intervals their right and left footpad thickness was measured using spring-loaded calipers. Plot shows the mean ratio ± SE (n = 10) of infected to uninfected foot measurements in C57BL/6 (WT) versus lymph node–deficient (KO) mice. (b) Groups of C57BL/6 and LTα knockout mice, with or without splenectomy, were given an intranasal administration of L. major parasites and challenged 7 days later with a second intranasal dose of parasites to induce effector responses. Four days after challenge, the mice were sacrificed and their BAL cells recovered, followed by perfusion with PBS and isolation of lung tissue. Bar graph shows the mean (± SE) proportions of lymphocytes (LYMPHs), neutrophils (NEUTs), and eosinophils (EOs) recovered from BAL of individual WT, LTα knockout (KO), and splenectomized LTα knockout (KO + SP–) mice (n = 5 per group). Macrophages make up the remaining percentage of cells recovered by BAL and have been omitted for clarity. (c) Lung tissue cells from mice in b were isolated by collagenase/DNase treatment and set up in culture with varying doses of L. major–soluble lysate and splenic APCs to induce antigen-specific cytokine production. Bar graphs show the mean IFN-γ, IL-5, and IL-13 production of duplicate ELISA wells after 4 days of culture.
To establish the potential of lymph node–deficient mice to become primed via an intranasal route, groups of knockout and wild-type mice received an intranasal priming dose of L. major parasites, and 7 days later all mice were challenged with a second dose of parasites to induce effector responses. To exclude the spleen as a potential site for the priming observed in knockout mice, the same experimental regimen was also carried out in LTα knockout animals that had been splenectomized 2 weeks prior to infection. The effector responses induced following intranasal priming with L. major showed a marked presence of lymphocytes and eosinophils within BAL fluid, with comparable results between wild-type and knockout groups (Figure 5b). When suspensions of lung tissue cells were restimulated in vitro with soluble L. major antigen, cultures from wild-type and both knockout groups showed a comparable profile of cytokines, consisting of a marked production of Th2-derived cytokines (IL-5 and IL-13) and negligible IFN-γ (Figure 5c).
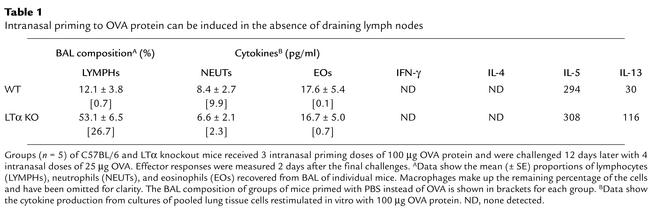
Similar findings were observed with an alternative intranasal priming regimen in which OVA protein was used. We have previously demonstrated that this intranasal regimen generates OVA-specific Th2 T cell priming that is potent and long-lived (2), unlike other intranasal OVA regimens that are reported to induce T cell tolerance (14). Table 1 summarizes our findings using intranasal delivery of OVA in lymph node–deficient versus wild-type mice. As with our findings with L. major, the absence of draining lymph nodes during intranasal priming to OVA protein had virtually no impact on the induction of BAL eosinophilia, or the selective generation of IL-5 and IL-13 cytokines by OVA-restimulated lung cells. Taken together, these findings suggest that the initial APC/antigen interactions that occur within tissues may contribute to the outcome of pulmonary T cell priming.
Table 1.
Intranasal priming to OVA protein can be induced in the absence of draining lymph nodes
Antigen uptake preferentially stimulates IL-10 and IL-6 production by resident pulmonary APCs.
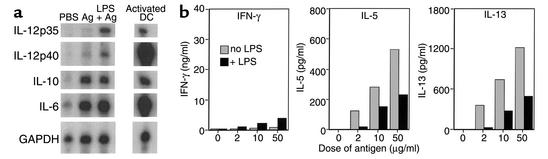
To address the issue of how resident lung APCs might contribute to Th2 differentiation within the pulmonary tract, we next examined the profile of cytokines generated by these APCs following antigen uptake in situ. Mice received an intranasal delivery of L. major as an immunogen (or PBS alone), and 18 hours later suspensions of lung tissue cells were prepared and the CD11cbright APCs enriched to greater than 85% using an adherence protocol. Total RNA was extracted from these enriched APCs and analyzed for expression of cytokine mRNA by RNase protection assay. The most striking feature of the data was the very marked upregulation of IL-10 and IL-6 mRNA following uptake of antigen (Figure 6a). Importantly, both of these cytokines are associated with promotion of the differentiation of Th2 cells, although the mechanism may be via the inhibition of Th1 responses (15, 16). As expected, intranasal codelivery of LPS with L. major induced a major upregulation of both IL-12p35 and IL-12p40 mRNA; however, levels of IL-10 and IL-6 were virtually unaffected. In fact, when we examined the type of T cell differentiation induced within mice that had received a codelivery of L. major and LPS (Figure 6b), we observed a decrease in all Th2-type cyto-kines but only a small increase in IFN-γ, suggesting a mixed (Th0) response rather than a switch to Th1. Taken together, these data suggest that, in the absence of a strong proinflammatory signal, APCs within the pulmonary microenvironment may have an inherent bias to generate Th2-promoting cytokines, which in turn would explain the predominance in Th2 effector responses often observed within the pulmonary tract. In addition, these findings provide further evidence that events in the local tissue environment are likely to play an important role during pulmonary T cell priming and differentiation.
Figure 6.
Antigen uptake in the lung promotes IL-10 and IL-6 expression by resident APCs. (a) Mice were given an intranasal delivery of PBS, 107 L. major (Ag), or 107 L. major plus 0.4 μg LPS (LPS + Ag), and 18 hours later their lungs were isolated to generate cell suspensions. Pulmonary CD11cbright APCs were enriched by adherence and lysed, and RNA was extracted. Activated dendritic cells (DCs) were generated from bone marrow cells cultured in the presence of rGM-CSF, followed by stimulation with 1 μg/ml LPS. Five micrograms of each RNA sample was analyzed by RNase protection assay using a RiboQuant multiprobe kit. Protected fragments were separated on a 5% denaturing polyacrylamide gel and visualized by autoradiography. The figure shows transcript levels of the indicated cytokines after a 16-hour exposure. GAPDH data are from a 2-hour exposure. (b) Groups (n = 4) of C57BL/6 mice were given an intranasal administration of L. major parasites, with or without 0.4 μg LPS, and challenged 7 days later with a second intranasal dose of parasites (without LPS) to induce effector responses. Four days after challenge, the mice were sacrificed and their lung tissue cells were pooled for each group and set up in culture with varying doses of L. major–soluble lysate and wild-type splenic APCs to induce antigen-specific cytokine production. Bar graphs show the mean IFN-γ, IL-5, and IL-13 production after 4 days of culture.
Discussion
The goal of the current studies was to examine the early immunologic events following intranasal delivery of two different immunogens previously shown to induce selective Th2 priming in the pulmonary tract, to establish how the uptake of antigen might affect APCs and in turn influence T cell priming within the lung microenvironment. We found that a resident population of CD11cbright cells were the principal APCs to become loaded with antigen in the lung following intranasal delivery, and that this population was phenotypically most analogous to an immature subset of dendritic cells, without an obvious segregation into either the myeloid or lymphoid lineage of dendritic cells. However, we cannot rule out the possibility that these cells represent a unique subset of airway dendritic cells or a population of incompletely committed precursor cells of monocytic origin. It is important to note that many of the previous studies examining pulmonary dendritic cells/APCs were intentionally focused on the subset of APCs expressing the highest levels of MHC class II (17), providing a likely explanation for the apparent disparity between our phenotypic findings and those of others. However, in studies where pulmonary APCs were examined as a whole population (18), the same phenotype as in our current studies was reported (CD11c+ CD11b– F4/80+ DEC205+ MHC class IIlow).
One striking aspect of our findings was the limited migration of antigen-loaded APCs from pulmonary tissues into draining lymph nodes. While Pauwels and coworkers (17) reported migration of OVA-loaded lung APCs into draining lymph nodes, they did not examine the status of these APCs within pulmonary tissues, making it difficult to conclude the extent of emigration. However, as in our current studies, Coffman and coworkers (19) reported the preferential retention, rather than emigration, of antigen-loaded pulmonary APCs within tissues. Interestingly, the presence of IL-10 has recently been demonstrated to impair the trafficking of antigen-loaded dendritic cells in vivo (20), which fits well with our findings of a rapid upregulation in IL-10 expression by pulmonary tissue APCs after antigen uptake in situ. The relatively limited migration of antigen-loaded APCs into draining lymph nodes in response to intranasally delivered antigens led us to question the importance of such migration to the successful induction of T cell priming within the lung microenvironment. We found that the absence of lung-draining lymph nodes did not decrease the ability of mice to become primed via the airways, suggesting a more important role for antigen/APC/T cell interactions within local tissues. This is in marked contrast to the findings showing a defect in priming in lymph node–deficient mice following immunization via other routes of antigen delivery (12, 13) (Figure 5a). Our findings resemble those recently reported for another mucosal tissue, the intestinal tract, where gut-associated lymphoid tissue rather than secondary lymphoid organs was shown to be the primary site for T cell priming following oral delivery of immunogens (21). Thus, T cell priming within the pulmonary tract may be more dependent on localized lymphoid organs, such as bronchus-associated lymphoid tissue, or tertiary lymphoid organs that are known to form within tissues following the initiation of certain types of inflammatory responses (22). Both of these alternative lymphoid organs would provide a means whereby a mucosal surface, such as the pulmonary tract, is able to localize the majority of its immunologic responses, thereby minimizing the potential of repeated systemic sensitization to inhaled exogenous immunogens. However, the exact location of the initial T cell/APC interactions within the pulmonary tract in the absence of classical secondary lymphoid organs remains to be established.
Of major significance is the observation that pulmonary tissue APCs preferentially expressed Th2-promoting cytokines, including IL-10 and IL-6, within a few hours of antigen uptake. These findings provide evidence for the potential of the lung microenvironment to favor the generation of Th2-dominated immune responses. Indeed, while the codelivery of LPS promoted the expression of IL-12 by antigen-loaded APCs, levels of IL-10 and IL-6 expression were virtually unchanged and a major switch to Th1-type responses was not observed. Taken together, these data support the idea that a bias in the generation of Th2 responses is likely to predominate within this mucosal site, and that Th1-type immunity will only be induced in response to very specific types of immunogens, including live bacteria (e.g., Streptococcus and Mycobacterium) and viruses (e.g., influenza). This idea fits well with the recent findings of several groups showing that a failure to signal strongly via toll-like receptors on APCs is associated with a default Th2 outcome (23). Thus, APCs within the pulmonary tract may have a significantly greater threshold of activation through toll-like receptors than do APCs in other tissues, providing an explanation for their apparent major default to Th2 types of differentiation.
Acknowledgments
This work was supported by NIH grants AI-39158 (to S.L. Constant), CA-16885 (to N.H. Ruddle), and HL-56389 and AI-26791 (to K. Bottomly). The authors thank Claudia Swanson and Shira Ovadia for splenectomies and Heather MacLeod and Cheryl Bergman for technical assistance.
Footnotes
Stephanie L. Constant’s present address is: Department of Microbiology and Tropical Medicine, George Washington University, Washington, DC, USA.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: antigen-presenting cell (APC); lymphotoxin-α (LTα); 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE); ovalbumin (OVA); bronchoalveolar lavage (BAL); phycoerythrin (PE); periodate-lysine-paraformaldehyde (PLP).
References
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Herrick CA, MacLeod H, Glusac E, Tigelaar RE, Bottomly K. Th2 responses induced by epicutaneous versus inhalational protein exposure are differentially dependent on IL-4. J Clin Invest. 2000;105:765–775. doi: 10.1172/JCI8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Constant SL, Lee KS, Bottomly K. Site of antigen delivery can influence T cell priming: pulmonary environment promotes preferential Th2-type differentiation. Eur J Immunol. 2000;30:840–847. doi: 10.1002/1521-4141(200003)30:3<840::AID-IMMU840>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Jones HP, et al. The pulmonary environment promotes Th2 cell responses after nasal-pulmonary immunization with antigen alone, but Th1 responses are induced during instances of intense immune stimulation. J Immunol. 2001;167:4518–4526. doi: 10.4049/jimmunol.167.8.4518. [DOI] [PubMed] [Google Scholar]
- 5.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 6.Constant SL, Bottomly K. The induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 7.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 8.Hume DA, Robinson AP, MacPherson GG, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983;158:1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraal G, Breel M, Janse M, Bruin G. Langerhans’ cells, veiled cells, and interdigitiating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roake JA, et al. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Togni P, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. [PubMed] [Google Scholar]
- 12.Banks T, et al. Lymphotoxin-α-deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 13.Fu Y-X, et al. Lymphotoxin-α (LTα) supports development of splenic follicular structure that is required for IgG responses. J Exp Med. 1997;185:2111–2120. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 15.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl S, et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 17.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byersdorfer CA, Chaplin DD. Visualization of early APC/T cell interactions in the mouse lung following intranasal challenge. J Immunol. 2001;167:6756–6764. doi: 10.4049/jimmunol.167.12.6756. [DOI] [PubMed] [Google Scholar]
- 19.Julia V, et al. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 20.Demangel C, Bertolino P, Britton WJ. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur J Immunol. 2002;32:994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 22.Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res. 1999;19:119–125. doi: 10.1007/BF02786481. [DOI] [PubMed] [Google Scholar]
- 23.Kelsall BL, Biron CA, Sharma O, Kaye PM. Dendritic cells at the host-pathogen interface. Nat Immunol. 2002;3:699–702. doi: 10.1038/ni0802-699. [DOI] [PubMed] [Google Scholar]