The magnitude and diversity of T cell responses reflects the outcome of a competition between positive signals initiated by T cell receptors (TCRs) and negative signals originating from an array of cell surface receptors. Fine control of this balance is essential for mobilizing protective immune responses while concurrently limiting potential immunopathologic complications, such as autoimmunity and destruction of bystander cells by toxic cytokines or cytolysis.
A rapidly accumulating body of literature in a variety of viral infection and tumor systems documents that conventional αβ TCR CD8+ T cells express receptors originally shown to impair NK activity (1). Do these inhibitory NK receptors (iNKRs) operate similarly to dampen CD8+ T cell functions? In many, but not all, cases, when these receptors are blocked by mAbs or transgenically expressed, effector activities (i.e., cytotoxicity and cytokine production) of antigen-specific CD8+ T cells are enhanced or diminished, respectively (2). Taken together, the data support the concept that CD8+ T cells, like NKs, express iNKRs to restrain their “lethal” behavior. iNKRs also regulate noneffector CD8+ T cell functions, including protection from TCR-driven apoptosis and the promotion of memory T cell homeostasis (3).
iNKRs and their ligands
iNKRs fall into two structurally distinct groups. The first consists of type I transmembrane proteins with immunoglobulin (Ig) domains: these include killer cell Ig-like receptors (KIR) and Ig-like transcripts (ILT)/leukocyte Ig-like receptors (LIR). The second group includes type II transmembrane proteins containing C-type lectin-like domains: these include the Ly49 homodimers, and heterodimers of CD94 covalently associated with either the inhibitory NKG2A or activatory NKG2C or NKG2E isoforms. Of the panoply of iNKRs expressed by NK and T cells, only the CD94/NKG2 receptors are conserved between mice and humans, indicating that these receptors evolutionarily predate the other iNKR families. In general, the ligands for the Ig-like iNKRs and Ly49 receptors are classical MHC class I molecules. CD94/NKG2 receptors stand out as an exception. Both the inhibitory and activatory CD94/NKG2 receptors recognize the nonclassical MHC class Ib molecule human leukocyte antigen (HLA)–E or its murine ortholog, Qa-1, loaded with a nonapeptide derived from the leader sequence of certain classical MHC class I heavy chains (5, 6). Thus, CD94/NKG2 receptors can survey a broader range of MHC class I molecules than can those iNKRs whose ligand specificity is constrained by class I MHC polymorphisms.
CD94/NKG2A receptors inhibit antiviral and anti-tumor CTLs
Recent evidence points toward CD94/NKG2A receptors as a dominant iNKR expressed by activated CD8+ T cells. In response to infection by mouse polyoma virus or lymphocytic choriomeningitis virus, most virus-specific CD8+ T cells upregulate CD94/NKG2A receptors (7–9). In the polyoma virus model, clearance of infectious virus is paralleled by an increase in the proportion of antiviral CD8+ T cells expressing CD94/NKG2A receptors, which act to limit their cytotoxic activity (7). Polyoma virus is a potent oncogenic pathogen that establishes persistent infection in mice; therefore CD94/NKG2A plays a critical role in balancing excessive CTL lysis of widespread chronically infected cells against the need for effective CTL surveillance for virus-transformed cells. This is in line with the proposal that iNKRs help maintain peripheral tolerance of autoimmune T cells (10). It is perhaps also not surprising that CD94/NKG2A is stably expressed at high levels by virus-specific memory CD8+ T cells in persistent, but not acutely cleared, viral infections (7, 8). Deleterious consequences of an imbalance in iNKR activation is evident in the finding that premature CD94/NKG2A expression on antiviral CD8+ T cells is associated with delayed viral clearance and susceptibility to polyoma virus-induced tumors (7).
Like persistent virus infections, tumors provide a venue for chronic antigen presentation to T cells. Because tumors frequently overexpress self-proteins that serve as targets for anti-tumor T cells, the host might unwittingly engage iNKRs to preserve peripheral T cell tolerance. High proportions of CD8+ T cells that infiltrate melanomas and astrocytomas in humans express CD94/NKG2A (11, 12), and antibody blockade of CD94/NKG2A receptors on melanoma-specific CD8+ T cells has been shown to restore anti-tumor CTL function in vitro (13). Because autoreactive T cells typically express low-affinity TCRs, TCR signaling would be expected to be at a disadvantage to negative signaling by iNKRs. Moreover, negative signaling tends to dominate positive signaling when iNKRs and activatory NK receptors (aNKRs) co-engage MHC class I ligands on antigen-presenting cells (APCs), possibly due to the higher binding affinity of iNKRs (14). Thus, there is considerable interest in understanding the mechanisms that control iNKR expression on CD8+ T cells. In vitro studies indicate that TCR activation and/or treatment with particular cytokines upregulate expression of certain iNKRs on antigen-specific CD8+ T cells (15). Little is known about factors that control expression of iNKRs on CD8+ T cells in vivo.
Upregulating iNKR ligand expression
An alternative way to control the activity of iNKR+ CD8+ T cells is to manipulate expression of iNKR ligands. There is precedent for this. Human cytomegalovirus (HCMV) and HIV encode proteins that selectively downregulate surface expression of MHC class I molecules that present viral peptides to TCRs but retain expression of those that engage iNKRs (16, 17). Similarly, distressed cells (e.g., infection, neoplasia) upregulate MHC class I–related molecules, such as MICA/B (humans) and Rae1 (mice), that serve as ligands for the activatory NKG2D receptors expressed by NK and CD8+ T cells (18, 19). We previously speculated that the cytokine microenvironment created during viral infection might affect MHC class I expression levels on APCs and alter the balance between TCR and iNKR signaling (7). Specifically, we hypothesized that IFN-γ, a dominant cytokine generated during innate and adaptive phases of immunity to many viral infections, would upregulate both TCR and iNKR ligands. If iNKR-transduced negative signaling overrode TCR positive signaling, IFN-γ could impair antiviral CD8+ T cell function. This possibility is supported by evidence that IFN-γ can facilitate viral evasion of NK killing. The HCMV UL40 glycoprotein, which contains a sequence identical to the HLA-E–binding peptide in the HLA-C leader sequence, triggers CD94/NKG2A receptors on NK only in conjunction with IFN-γ–induced upregulation of HLA-E on APCs (20).
Malmberg et al. (4) provide data supporting this hypothesis in a clinically important tumor model. While assessing the ability of short-term tumor cell lines derived from ovarian carcinoma patients to be lysed by CD8+ CTL, these investigators employed the common practice of pretreating target cells with IFN-γ to enhance MHC class I levels, with the intention of boosting CTL recognition. Unexpectedly, the opposite occurred. Because the allogeneic and peptide-specific CTL lines used in this study uniformly expressed CD94/NKG2A, the authors tested and found that blocking this receptor with a CD94 mAb restored lysis of the IFN-γ–treated tumor target cells. Importantly, the same result was seen for a tumor-associated T cell line against autologous ovarian tumor target cells. We have similarly found that IFN-γ upregulates Qa-1 expression on target cells and enhances their resistance to ex vivo lysis by CD94/NKG2A+ polyoma virus-specific CD8+ T cells (N. Andrews et al., unpublished observations) An initially confounding result in the Malmberg et al. study was that IFN-γ treatment of long-term lines derived from a variety of tumors, including an ovarian carcinoma, improved CTL recognition, despite evidence that these cells expressed HLA-E and IFN-γ upregulated HLA-E expression. The answer lay in whether the tumor cells co-expressed another nonclassical MHC class Ib molecule, HLA-G. Of MHC class I molecules containing leader sequences that bind HLA-E, the leader sequence of HLA-G contains the peptide with highest HLA-E binding affinity (14). Only the short-term ovarian carcinoma cell lines expressed HLA-E and HLA-G, and IFN-γ induced expression of both molecules. By boosting the MHC class I antigen processing machinery, IFN-γ should also promote generation of the HLA-G leader sequence peptide and its assembly with HLA-E. Loss of HLA-G expression by tumor cells with in vitro passage is also consistent with the hypothesis that neoplastic cells maintain expression of this MHC class Ib molecule in vivo to thwart anti-tumor CD94/NKG2A+ CTL and NKs.
Unlike HLA-E (and Qa-1), which has a broad tissue distribution, HLA-G expression is largely restricted to placental extravillous cytotrophoblasts. Because of its highly restricted tissue distribution and evidence that HLA-G expression on cytotrophoblasts increases with invasion of the maternal decidua, HLA-G has been postulated to play a role in blocking maternal immune responses against the semiallogeneic fetus (21). The new mechanistic twist is that HLA-G mediates immunosuppression at the maternal-fetal interface by generating a ligand for the inhibitory CD94/NKG2A receptors on T cells and NKs. By ectopically expressing HLA-G, neoplastic cells appropriate this placental-based immunosuppressive mechanism to escape destruction by lymphocytes.
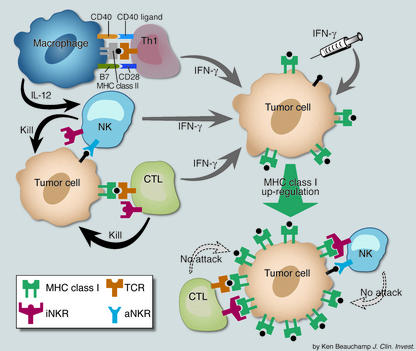
The central question is whether IFN-γ makes use of iNKRs to negatively modulate virus- and tumor-specific CD8+ T cell responses in vivo. IFN-γ may be produced early during the course of primary viral infection by activated NKs (perhaps by IL-12 released by infected or toll-like receptor-activated dendritic cells and macrophages), followed by virus-specific Th1 cells or CD8+ T cells. By upregulating ligands for iNKRs, IFN-γ may provide negative feedback regulation for antiviral CD8+ effector T cells. For tumors, IFN-γ may be secreted early in an anti-tumor immune response by NKs responding to low MHC class I expression on neoplastic cells, by Th1 cells recognizing tumor epitopes displayed by infiltrating MHC class II+ macrophages, and by anti-tumor CD8+ T cells with sufficient avidity to be activated by low numbers of MHC:peptide ligands (Figure 1). IFN-γ from each of these endogenous sources may conspire to nullify the effector activity of NK cells and anti-tumor iNKR+ CTL. Thus, the findings of Malmberg et al. (4) strike a cautionary note for using IFN-γ for tumor immunotherapy. Future studies to define factors that regulate expression and activation of iNKRs on CD8+ T cells may lead to novel strategies to reissue the killing license to anti-tumor CTLs.
Figure 1.
Model for IFN-γ–mediated inhibition of NKs and anti-tumor CTLs. Tumor-infiltrating macrophages present MHC class II:tumor peptide ligands to antigen-specific Th1 cells and induce them to produce IFN-γ. In addition, by upregulating CD40 ligand, these activated Th1 cells ligate CD40 receptors on the macrophages and induce them to secrete IL-12; IL-12 then triggers IFN-γ production by NKs. Low MHC class I–expressing tumor cells also activate high-avidity tumor-specific CTLs and NKs (unengaged iNKRs + activated aNKRs) to produce IFN-γ. By upregulating classical and nonclassical MHC class I surface expression by tumor cells, IFN-γ, either endogenously produced or injected, promotes activation of iNKRs on NKs and anti-tumor CTLs and turns off their cytotoxic effector function.
Footnotes
See the related article beginning on page 1515.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: T cell receptor (TCR); inhibitory NK cell receptor (iNKR) ; human leukocyte antigen (HLA); activatory NK receptors (aNKRs); antigen-presenting cell (APC).
References
- 1.McMahon CW, Raulet DH. Expression and function of NK cell receptors in CD8+T cells. Curr Opin Immunol. 2001;13:465–470. doi: 10.1016/s0952-7915(00)00242-9. [DOI] [PubMed] [Google Scholar]
- 2.Moser JM, Byers AM, Lukacher AE. NK cell receptors in antiviral immunity. Curr Opin Immunol. 2002;14:509–516. doi: 10.1016/s0952-7915(02)00357-6. [DOI] [PubMed] [Google Scholar]
- 3.Ugolini S, et al. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+T cells. Nat Immunol. 2001;2:430–435. doi: 10.1038/87740. [DOI] [PubMed] [Google Scholar]
- 4.Malmberg K-J, et al. IFN-γ protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest. 2002;110:1515–1523. doi:10.1172/JCI200215564. doi: 10.1172/JCI15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braud VM, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 6.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1b. J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8+T cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 8.McMahon CW, et al. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8+T cells. J Immunol. 2002;169:1444–1452. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 9.Miller JD, et al. CD94/NKG2 expression does not inhibit cytotoxic function of lymphocytic choriomeningitis virus-specific CD8+ T cells. J Immunol. 2002;169:693–701. doi: 10.4049/jimmunol.169.2.693. [DOI] [PubMed] [Google Scholar]
- 10.Ugolini S, Vivier E. Regulation of T cell function by NK cell receptors for classical MHC class I molecules. Curr Opin Immunol. 2000;12:295–300. doi: 10.1016/s0952-7915(00)00090-x. [DOI] [PubMed] [Google Scholar]
- 11.Vetter CS, et al. Expression of CD94/NKG2 subtypes on tumor-infiltrating lymphocytes in primary and metastatic melanoma. J Invest Dermatol. 2000;114:941–947. doi: 10.1046/j.1523-1747.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 12.Perrin G, et al. Sister cytotoxic CD8+T cell clones differing in natural killer inhibitory receptor expression in human astrocytoma. Immunol Lett. 2002;81:125–132. doi: 10.1016/s0165-2478(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 13.Speiser DE, et al. In vivo expression of natural killer cell inhibitory receptors by human melanoma-specific cytolytic T lymphocytes. J Exp Med. 1999;190:775–782. doi: 10.1084/jem.190.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vales-Gomez M, et al. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 1999;18:4250–4260. doi: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mingari MC, Moretta A, Moretta L. Regulation of KIR expression in human T cells: a safety mechanism that may impair protective T-cell responses. Immunol Today. 1998;19:153–157. doi: 10.1016/s0167-5699(97)01236-x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen GB, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 17.Tomasec P, et al. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287:1031–1033. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- 18.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groh V, et al. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 20.Cerboni C, et al. Synergistic effect of IFN-γ and human cytomegalovirus protein UL40 in the HLA-E-dependent protection from NK cell-mediated cytotoxicity. Eur J Immunol. 2001;31:2926–2935. doi: 10.1002/1521-4141(2001010)31:10<2926::aid-immu2926>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Rouas-Freiss N, Marchal-Bras G, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA. 1997;94:11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]