Abstract
Acute liver failure caused by viral hepatitis or toxic damage involves both apoptotic and necrotic pathways. IGF binding protein-1 (IGFBP-1), a hepatocyte-derived secreted protein, is required for normal liver regeneration. To determine whether IGFBP-1 could prevent liver injury that entails direct stimulation of hepatocyte apoptosis, IGFBP-1–/– mice, IGFBP-1+/+ mice, and mice pretreated with Ab’s against IGFBP-1 were treated with a normally sublethal dose of Fas agonist. IGFBP-1 deficiency was associated with massive hepatocyte apoptosis and caspase activation within 3 hours of Fas agonist treatment, which could be corrected by pretreatment with IGFBP-1. IGFBP-1–deficient livers had enhanced signaling via the integrin receptor at early times (0.5 to 1 hour) after Fas agonist treatment accompanied by elevated activated matrix metalloproteinase-9 (MMP-9), a known target of fibronectin signaling and activator of TGF-β. Within 3 hours of Fas agonist treatment, elevated expression of active TGF-β1, a hepatocyte apoptogen, was observed in IGFBP-1–deficient livers that correlated with the appearance of the apoptotic process. Both MMP-9 and TGF-β1 expression were suppressed by IGFBP-1 treatment, supporting their role in the apoptotic process. IGFBP-1–/– mice also displayed increased injury in a toxic hepatic injury model caused by CCl4. These findings indicate that IGFBP-1 functions as a critical hepatic survival factor in the liver by reducing the level of proapoptotic signals.
Introduction
Massive hepatocyte apoptosis mediated by Fas or TNF-α pathway activation is observed in liver injury mediated by hepatitis viruses and hepatotoxins (1). Identification of hepatic survival factors is critical for therapeutic intervention in liver failure. We have determined that IGF binding protein-1 (IGFBP-1) is required for normal liver regeneration after partial hepatectomy (2) and sought to determine whether IGFBP-1 can also protect against liver injury. IGFBP-1 is a member of a group of structurally related soluble proteins that specifically bind and modulate the actions of IGF-1 and IGF-2 or act independently of IGFs via interactions with integrin receptors (3). Among the IGFBPs, IGFBP-1 is unique in that its expression is dramatically altered by changes in the metabolic state and increases in hepatocyte proliferation (4, 5), but its functional role has remained elusive. Liver is the primary source of serum IGFBP-1 (6), and its production is localized to hepatocytes (7). Fasting serum IGFBP-1 levels are elevated in patients with cirrhosis and in normal adults following ethanol ingestion (3), and greatly elevated levels of IGFBP-1 are found in hepatic malignancies (8).
IGFBP-1 functions independently of the IGFs via its internal Arg-Gly-Asp (RGD) consensus sequence for cell attachment by specifically inhibiting fibronectin binding to the α5β1 integrin in trophoblasts, resulting in net suppression of trophoblast invasion (9, 10). In human breast cancer cells, interaction of IGFBP-1 with α5β1 integrin induces focal adhesion kinase (FAK) dephosphorylation in an IGF-independent fashion, subsequently leading to cell detachment and death by apoptosis (11).
The majority of the data suggest that IGFBPs are potent inducers of the apoptotic cell death program, in some cases acting via IGF-independent pathways (11–17). However, it has been established that IGFBP-1 functions as a proregeneration factor in the liver (2, 5). IGFBP-1 gene upregulation during liver regeneration is mediated in part by IL-6 (2, 18), which functions as a critical antiapoptotic factor in the liver by its ability to establish and maintain an adequate level of FLICE inhibitory proteins (FLIP) and downstream antiapoptotic factors (19). IGFBP-1 appears to act independently of IL-6 in stimulating liver regeneration. IGFBP-1 acts as a proregeneration factor primarily by upregulating the level of C/EBPβ, a member of the CCAAT enhancer binding protein family of basic leucine zipper transcription factors (20, 21) that is also required for liver regeneration. C/EBPβ deficiency in the liver confers resistance to Fas-mediated apoptosis in the hepatocytes, as shown by reduced activation of caspase-3 and increased expression of antiapoptotic protein Bcl-xL in Fas-treated C/EBPβ–/– livers (22).
We wondered if IGFBP-1 deficiency would result in an apoptotic defect that was similar to that observed with C/EBPβ deficiency. On the contrary, we found that abnormalities in C/EBPβ expression did not play a prominent role in Fas-mediated hepatic injury sustained in IGFBP-1–/– livers. Instead, after Fas stimulation, IGFBP-1 deficiency was associated with rapid onset of massive hepatocyte apoptosis that could be corrected by pretreatment with IGFBP-1. Fibronectin signaling was elevated early in IGFBP-1–deficient livers and was associated with proteolytic activation of matrix metalloproteinase-9 (MMP-9), enhanced activation of the proapoptotic TGF-β1, caspase-3 and caspase-8 activation, and ultimately breakdown of fibronectin. Treatment of IGFBP-1–/– livers with IGFBP-1 corrected these abnormalities and the associated morbidity and hepatic defects, establishing IGFBP-1 as a critical hepatic survival factor.
Methods
Animal studies and generation of mutant mice.
Generation of IGFBP-1–/– animals was described previously (2). Studies were performed on IGFBP-1–/– and IGFBP-1+/+ mice 12–16 weeks of age in a B6.129 hybrid background. Littermates and backcrossing were used to provide a uniform background. IGFBP-1–/– phenotype was confirmed by tail DNA biopsies followed by PCR as described (2).
The Fas injury model and immunohistochemistry.
IGFBP-1–/– and IGFBP-1+/+ mice, 12–16 weeks old, were generated by heterozygous crosses and verified by tail DNA biopsies followed by PCR analyses. Mice were injected intraperitoneally with the Fas agonist mAb Jo-2 (PharMingen, San Diego, California, USA) at a dosage of 0.15 μg per gram body weight. For IGFBP-1–treated mice, animals were injected intraperitoneally with IGFBP-1 at 0.3 μg/g body weight at time 0 or 30 minutes before Fas challenge. IGFBP-1+/+ animals in a B6.129 hybrid background were treated with 0.3 μg/g body weight of anti–IGFBP-1 Ab 30 minutes before Fas challenge. The anterior two-thirds of the liver was processed for protein analyses and the posterior one-third of the liver was fixed in 10% neutral buffered formalin (Formalde-Fresh; Fisher Scientific Co., Fairlawn, New Jersey, USA). The formalin-fixed livers were then paraffin embedded and the liver sections were analyzed by hematoxylin and eosin staining and immunohistochemistry using the following Ab’s: anti–caspase-8 (Novacastra Laboratories Ltd., Burlingame, California, USA), anti–caspase-3 (R&D Systems Inc., Minneapolis, Minnesota, USA), anti–TGF-β1 (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), anti-fibronectin (reactive with extracellular and matrix fibronectin) (Santa Cruz Biotechnology Inc.), and anti–MMP-9 (Calbiochem-Novabiochem Corp., San Diego, California, USA).
Western analyses and Ab’s.
Whole-cell extracts were prepared as previously described and subjected to Western analyses (5). Primary Ab’s used were from Santa Cruz Biotechnology Inc. and Calbiochem-Novabiochem Corp. Secondary Ab’s were from Zymed Laboratories Inc. (South San Francisco, California, USA). Images were scanned densitometrically to quantitate protein levels using Image Quant software (Molecular Dynamics, Sunnyvale, California, USA) and NIH Image 1.62. Statistical analyses were performed with StatWorks (Apple Computer Inc., Cupertino, California, USA) and the Student t test.
IGFBP-1 Ab’s.
IGFBP-1 Ab generated by our lab (23) and anti–IGFBP-1 Ab’s from Santa Cruz Biotechnology Inc. (sc-6000 and sc-6072) were tested. All three Ab’s showed the same result 7 hours after Fas ligand treatment; the Ab generated by Mohn et al. was used in the studies shown. The Ab’s from Santa Cruz Biotechnology Inc. were dialyzed extensively against 1× PBS using the Microdialyzer System 500 (Pierce Biotechnology Inc., Rockford, Illinois, USA) and concentrated in an Amicon Centricon centrifugal filter unit (Millipore Corp., Bedford, Massachusetts, USA).
We used the following Ab’s for Western analyses: Bcl-xL (sc-8392 from Santa Cruz Biotechnology Inc. and 2762 from Cell Signaling Technology Inc., Beverly, Massachusetts, USA); caspase-3 (AF835 from R&D Systems Inc., sc-7148 from Santa Cruz Biotechnology Inc., and 9661 from Cell Signaling Technology Inc.); Bcl-2 (sc-7382 from Santa Cruz Biotechnology Inc. and Ab-2 from Calbiochem-Novabiochem Corp.); C/EBPβ (sc-150 from Santa Cruz Biotechnology Inc.); FAK (sc-932 from Santa Cruz Biotechnology Inc.); integrin β1 (sc-8979 from Santa Cruz Biotechnology Inc.); integrin α5 (sc-10729 from Santa Cruz Biotechnology Inc.); AKT 1/2 (sc-8312 from Santa Cruz Biotechnology Inc.); p130cas (sc-9052 from Santa Cruz Biotechnology Inc.); fibronectin (sc-8422 from Santa Cruz Biotechnology Inc. and Ab-3 from Calbiochem-Novabiochem Corp.); and phosphorylated FAK (pFAK) (Tyr 925, sc-11766 from Santa Cruz Biotechnology Inc.). Anti-FAK detects the total amount of FAK as well as the cleavage product, FAK-related non-kinase (FRNK). Anti-pFAK detects only the phosphorylated form of FAK.
Acute CCl4 injury and immunohistochemistry.
Acute CCl4 injury, BrdU immunohistochemistry, and TUNEL staining were performed as described previously (24, 25). IGFBP-1+/+ and IGFBP-1–/– mice were treated with CCl4 and injected with BrdU 1 hour before sacrifice and liver harvest. Livers were harvested at the indicated times, fixed, sectioned, and stained with anti-BrdU mAb. BrdU-positive hepatocytes for each sample were quantitated by counting positively stained cells in three to four high-power, randomly selected fields. The mean for each timepoint was expressed as a percentage of the mean number of BrdU-labeled cells at the peak time of BrdU incorporation in IGFBP-1+/+ mice (48 hours).
Quantification of damaged areas following CCl4 injection was performed on TUNEL-stained liver sections videomicrographed at original magnification ×40. NIH image 1.61 software was used to map out dark staining (damaged areas) in each videomicrograph field, which was then calculated as a percentage of the total area of the field. Three fields were counted for each animal. All results are based on analysis of at least four IGFBP-1+/+ mice and four IGFBP-1–/– mice.
Results
Increased hepatocellular apoptosis 3 hours after Fas agonist injection in IGFBP-1–/– mice.
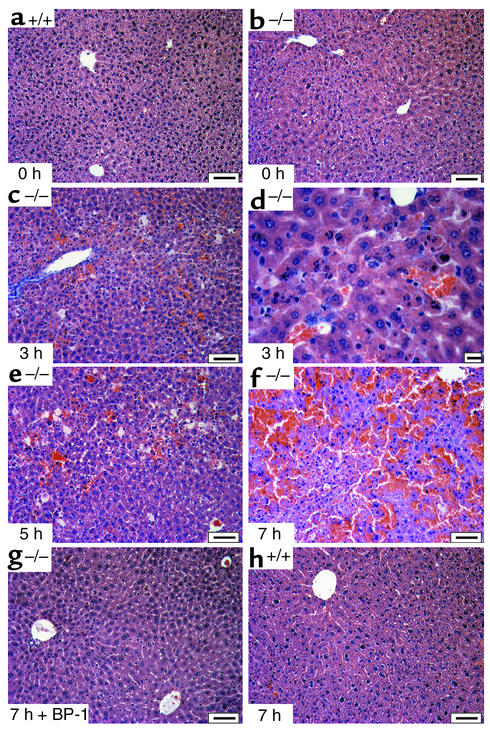
To investigate whether IGFBP-1 could function as an antiapoptotic factor protecting the hepatocytes from apoptosis, IGFBP-1+/+ and IGFBP-1–/– mice were injected intraperitoneally with a low dose (0.15 μg/g body weight) of the Fas agonist Jo-2 mAb, which has been shown to directly stimulate hepatocyte apoptosis only in livers sensitive to Fas agonist; higher doses of 0.25 μg/g resulted in significant mortality in wild-type strains as well as in IGFBP-1–deficient strains (1, 19). Within 5 hours of intraperitoneal injection with the anti-Fas mAb, IGFBP-1–/– mice (n = 11) displayed signs of clinical compromise, including tachypnea, shallow breathing, prostration, and progressive deep hypothermia, consistent with massive liver failure. No significant signs of clinical compromise were observed in the wild-type littermates (n = 11, data not shown). By 3 hours after Fas agonist injection, prominent histologic features of apoptosis were present in IGFBP-1–/– mice, with evidence of hemorrhage (Figure 1c) and hepatocyte apoptosis (Figure 1d) characterized by condensation of chromatin at the nuclear membrane and fragmentation of the cell into subcellular bodies. At the 5-hour timepoint, the normal lobular microarchitecture of the IGFBP-1–/– liver was maintained, but enhanced panlobular hepatocyte apoptosis and sinusoidal congestion were observed (Figure 1e). At 7 hours after anti-Fas injection, histologic examination of IGFBP-1–/– livers revealed multiple areas of focal hemorrhage and necrosis and destruction of the parenchymal architecture of the liver (Figure 1f). However, IGFBP-1–/– livers (n = 8) pretreated with an intraperitoneal dose of 0.3 μg/g weight of IGFBP-1 prior to a lethal challenge of Fas agonist were protected against lethality and Fas-mediated apoptotic injury. At 7 hours after anti-Fas injection, no hemorrhage and parenchymal collapse was observed in the IGFBP-1–/– livers pretreated with IGFBP-1 (Figure 1g). Similarly, the normal lobular microarchitecture of the liver was maintained in the IGFBP-1+/+ livers at 7 hours after anti-Fas injection (Figure 1h).
Figure 1.
Increased acute Fas-mediated injury of IGFBP-1–/– livers following 0.15 μg/g body weight of the Fas agonist was prevented by treatment with IGFBP-1. Hematoxylin and eosin staining of (a) IGFBP-1+/+ liver at time 0, (b) IGFBP-1–/– liver at time 0 and at (c) 3 hours, (d) 3 hours (higher magnification), (e) 5 hours, (f) 7 hours after Fas challenge, (g) 7 hours after IGFBP-1 treatment and Fas challenge, and (h) IGFBP-1+/+ liver at 7 hours after Fas challenge. Scale bars: 50 μm except in d, which is 10 μm. BP-1, IGFBP-1.
Enhanced caspase-8 and caspase-3 activation in IGFBP-1–/– livers after Fas challenge.
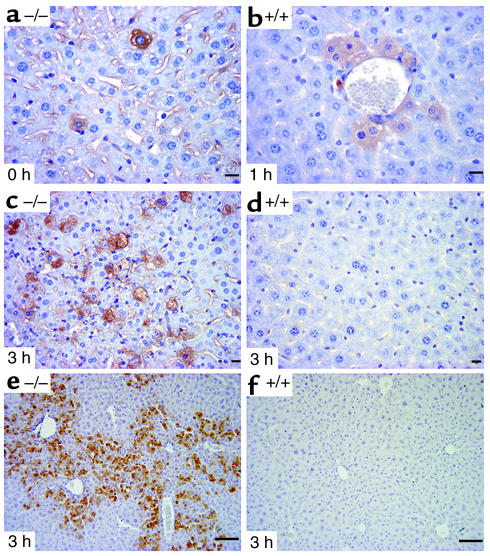
Caspase-8 is an initiator caspase, and processing of procaspase-8 is the first detectable event of Fas-mediated apoptosis (26, 27). To investigate the release of active caspase-8 subunits p18 and p10 into the cytosol following Fas agonist injection, we performed immunohistologic analyses using an Ab that recognizes only the active 10-kDa caspase-8 cleavage product. In IGFBP-1–/– livers, a low level of active caspase-8 subunit was detectable in the hepatocytes of the quiescent liver (Figure 2a), suggesting that IGFBP-1–/– hepatocytes had a preexisting defect in apoptotic pathways. In IGFBP-1+/+ livers, active caspase-8 subunit was detectable 1 hour after Fas injection (Figure 2b) but not in the quiescent liver (data not shown). At 3 hours after anti-Fas injection, an enhanced processing of procaspase-8 into the active 10-kDa caspase-8 subunit was observed in the IGFBP-1–/– livers (Figure 2c). No positive staining was found in the IGFBP-1+/+ livers 3 hours after Fas challenge (Figure 2d).
Figure 2.
Increased caspase-8 and caspase-3 activation in IGFBP-1–/– livers after Fas mAb injection. (a–d) Immunohistologic staining of IGFBP-1–/– liver sections at time 0 (a) and 3 hours (c), and IGFBP-1+/+ liver sections at 1 hour (b) and 3 hours (d) after Fas challenge using anti–caspase-8 p10 Ab. (e and f) Immunohistologic staining of IGFBP-1–/– liver section at 3 hours (e) and IGFBP-1+/+ liver section at 3 hours (f) using anti–caspase-3 p17 Ab. Scale bars: 10 μm (a–d) and 50 μm (e and f).
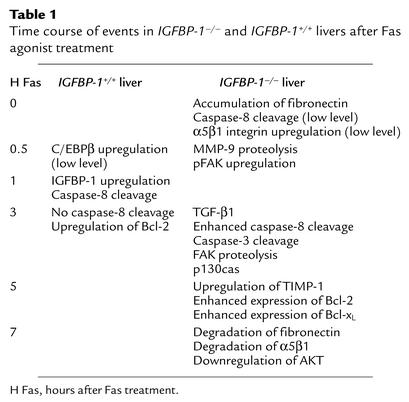
Table 1.
Time course of events in IGFBP-1–/– and IGFBP-1+/+ livers after Fas agonist treatment
Since active caspase-8 subunits may cause cleavage of caspase-3 (19), we used an Ab that recognizes only the active caspase-3 p17 subunit in immunohistochemical analyses. Caspase-3 cleavage was detectable in IGFBP-1–/– liver 3 hours after anti-Fas injection (Figure 2e) but not in the wild-type littermates (Figure 2f).
IGFBP-1 deficiency results in apoptotic pathway abnormalities distinct from C/EBPβ and IL-6 deficiency that are not related to a developmental defect in IGFBP-1–/– livers.
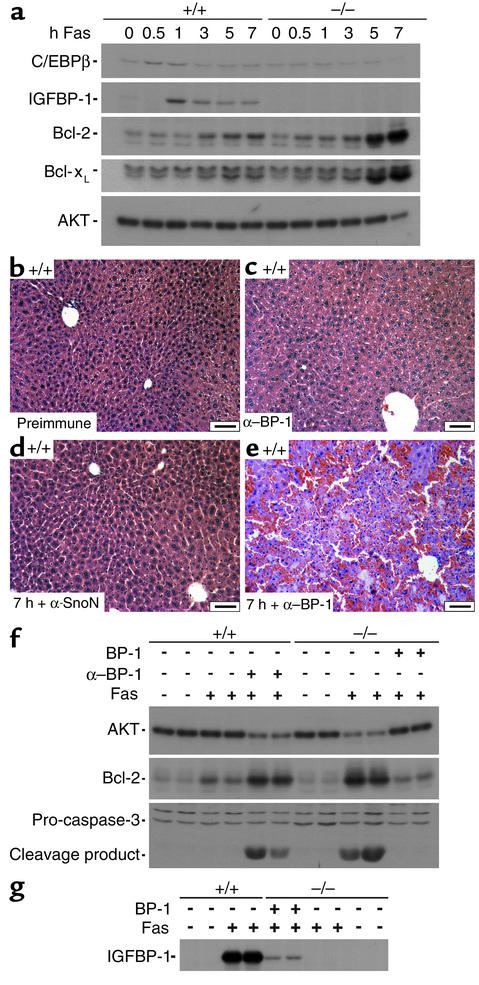
In IGFBP-1–/– livers, we found reduced expression of C/EBPβ in the adult primary hepatocytes and reduced induction of C/EBPβ expression after hepatectomy (2). C/EBPβ prevents caspase-8 activation in Fas-treated hepatic stellate cells (28), and its deficiency in the liver confers resistance to Fas-mediated apoptosis in the hepatocytes, as shown by reduced activation of caspase-3 and increased expression of the antiapoptotic protein Bcl-xL in Fas-treated C/EBPβ–/– livers (22). In contrast to posthepatectomized livers, in which C/EBPβ activation is robust (29, 30), kinetic studies revealed a less than 1.5-fold increase in C/EBPβ expression at 30 minutes and 1 hour after anti-Fas injection (Figure 3a), suggesting that under these conditions C/EBPβ has a minimal role in the apoptotic response. On the other hand, a fivefold increase in IGFBP-1 expression was detectable in the IGFBP-1+/+ livers 1 hour after Fas agonist injection (Figure 3a). Although the precise mechanistic basis for IGFBP-1 activation following treatment with Fas agonist is not known, its induction during the early time period suggested that IGFBP-1 may be protective against Fas-mediated apoptosis.
Figure 3.
Low induction of C/EBPβ and enhanced expression of the major antiapoptotic proteins in IGFBP-1–/– livers after Fas agonist treatment. (a) Western blot demonstrating kinetics of C/EBPβ, IGFBP-1, Bcl-2, Bcl-xL, and AKT induction in Fas agonist–treated IGFBP-1–/– and IGFBP-1+/+ livers at the indicated timepoints. (b–e) Hematoxylin and eosin stain of (b) IGFBP-1+/+ liver 7 hours after treatment with preimmune serum, (c) IGFBP-1+/+ liver 7 hours after treatment with anti–IGFBP-1 Ab, (d) IGFBP-1+/+ liver 7 hours after treatment with anti-SnoN Ab and Fas agonist, and (e) IGFBP-1+/+ liver 7 hours after treatment with anti–IGFBP-1 Ab and Fas agonist. Scale bars: 50 μm. (f) Western blot analyses comparing induction of AKT, Bcl-2, and caspase-3 processing in IGFBP-1+/+ livers, IGFBP-1+/+ and IGFBP-1–/– livers pretreated with anti–IGFBP-1 Ab, and IGFBP-1–pretreated IGFBP-1–/– livers after Fas challenge at the indicated timepoints. (g) Levels of IGFBP-1 detected in whole-cell liver extracts in the indicated animals. α-SnoN, anti-SnoN.
In IL-6–deficient livers (19), the primary defect appears to be a decrease in the level of the antiapoptotic regulators FLIP, Bcl-2, and Bcl-xL. However, no abnormalities in the basal expression of these proteins were observed in IGFBP-1–deficient livers (FLIP not shown) (Figure 3a). After the major apoptotic response had already occurred (5–7 hours after anti-Fas injection), a more than tenfold increase in Bcl-2 and Bcl-xL expression was noted in IGFBP-1–/– livers relative to IGFBP-1+/+ livers (Figure 3a), an apparent compensatory response that was inadequate in preventing liver failure.
To further rule out the possibility that IGFBP-1–/– mice have an intrinsic developmental defect in the liver that is primarily responsible for the apoptotic response, IGFBP-1 deficiency was created in IGFBP-1+/+ mice with the use of neutralizing Ab’s against IGFBP-1. IGFBP-1+/+ animals were pretreated with 0.3 μg/g intraperitoneal anti–IGFBP-1 Ab 30 minutes prior to a lethal challenge of Fas agonist. Findings in these animals paralleled those in IGFBP-1–/– animals treated with Fas agonist. No sinusoidal congestion and collapse of the lobular architecture of the liver was observed in IGFBP-1+/+ animals 7 hours after injection with preimmune serum (n = 6, Figure 3b), with anti–IGFBP-1 Ab (without Fas ligand) for 7 hours (n = 6, Figure 3c), or with anti-SnoN Ab (control Ab) in combination with the Fas agonist (n = 6, Figure 3d). However, destruction of the parenchymal architecture was evident in IGFBP-1+/+ animals pretreated with anti–IGFBP-1 Ab followed by a single 0.15 μg/g intraperitoneal dose of Fas agonist 7 hours after injection (n = 15, Figure 3e). Similar to IGFBP-1–/– livers, livers of wild-type littermates pretreated with anti–IGFBP-1 Ab demonstrated activation of caspase-3 and late induction of Bcl-2 (Figure 3f). As shown, injected IGFBP-1 was detected in hepatic extracts from IGFBP-1–/– mice, indicating that it reached the hepatic parenchyma (Figure 3g), albeit at lower levels than was measured in corresponding IGFBP-1+/+ livers at the same timepoint.
Increased fibronectin and integrin signaling in IGFBP-1–/– livers.
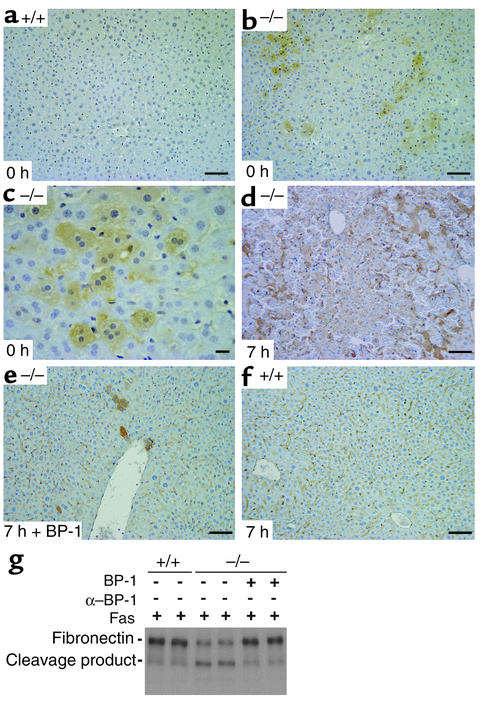
IGFBP-1 may elicit effects via either IGF-dependent or -independent mechanisms. Previously it was shown that the expression of IGF receptors is very low in adult hepatocytes (23, 31). On the other hand, in an IGF-independent mechanism, IGFBP-1 has been shown to prevent fibronectin from binding to α5β1 integrin (10), and fibronectin signaling is highly linked to cell survival pathways (32). We examined the fibronectin signaling axis in IGFBP-1–/– and IGFBP-1+/+ livers following Fas stimulation. Fibronectin is expressed by and associated with multiple cell types in the liver (33). It is known to enhance the apoptotic effect of soluble CD95L (FasL) and the biological efficacy of cytokines such as TGF-β by its ability to retain and increase their local concentrations (34). We therefore determined whether ECM fibronectin was increased in IGFBP-1–/– livers. As shown in Figure 4, a–c, fibronectin was readily detected in quiescent IGFBP-1–/– hepatocytes but not in IGFBP-1+/+ hepatocytes. At 7 hours after Fas challenge, scattered and fragmented fibronectin staining was detected in IGFBP-1–/– livers (Figure 4d), consistent with the hepatocellular injury that had occurred. In contrast, prominent fibronectin staining was seen in sinusoidal areas of IGFBP-1+/+ and IGFBP-1–/– livers pretreated with IGFBP-1 (Figure 4, e and f) and no disorganization was observed.
Figure 4.
Accumulation of fibronectin in the hepatocytes of IGFBP-1–/– quiescent livers and breakdown of fibronectin in Fas-treated IGFBP-1–/– livers. (a–f) Immunohistochemical staining of IGFBP-1+/+ quiescent liver (a), IGFBP-1–/– quiescent liver (b and c), IGFBP-1–/– liver 7 hours after Fas treatment (d), IGFBP-1–pretreated IGFBP-1–/– liver 7 hours after Fas treatment (e), and IGFBP-1+/+ liver 7 hours after Fas treatment (f) sections stained using anti-fibronectin Ab. Scale bars: 50 μm except in c, which is 10 μm. (g) Western blot analysis comparing degradation of fibronectin in IGFBP-1+/+, IGFBP-1–/–, and IGFBP-1–pretreated IGFBP-1–/– livers 7 hours after Fas challenge.
Because the central cell-binding domain of fibronectin (FN120) enhances the expression of matrix metalloproteinases and induces apoptosis (35), we next examined the degradation of fibronectin by Western blot analysis using whole-cell liver homogenates. A prominent 120-kDa cleavage product was observed only in IGFBP-1–/– livers 7 hours after Fas treatment (Figure 4g), compatible with the level of massive apoptosis observed in the livers.
Increased integrin signaling in IGFBP-1–/– livers soon after Fas agonist treatment.
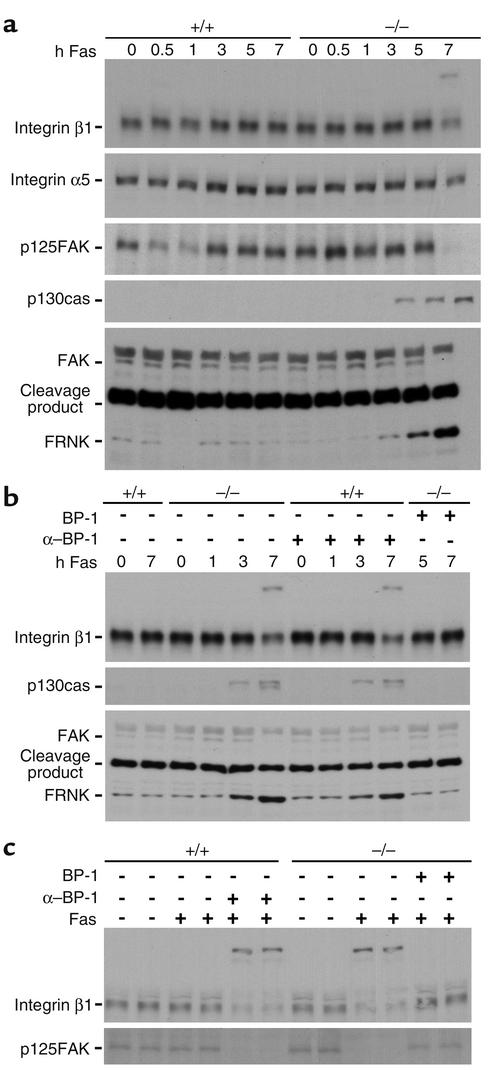
In the liver, fibronectin interacts primarily with α5β1 fibronectin receptors that are expressed on several cell types within the liver and are induced on hepatic stellate cells after liver injury (36). IGFBP-1 interacts with fibronectin receptor α5β1 (10, 37). The expression of integrin subunit α5 and integrin subunit β1 were 1.6-fold and 1.4-fold higher, respectively, in the quiescent IGFBP-1–/– livers than in the wild-type quiescent livers (Figure 5a), not a significant increase. Levels were unchanged after Fas agonist treatment until massive apoptosis had occurred in IGFBP-1–deficient livers, 7 hours after treatment.
Figure 5.
Persistent integrin signaling and progressive FAK degradation in IGFBP-1–/– livers after Fas agonist treatment. (a) Western analyses comparing the expression of integrin β1, integrin α5, phosphorylated p125FAK, and p130cas, and degradation of FAK in Fas agonist–treated IGFBP-1–/– and IGFBP-1+/+ livers at the indicated timepoints. (b and c) Kinetics study showing upregulation of p130cas, FAK proteolysis, and downregulation of phosphorylated p125FAK and integrin β1 in IGFBP-1+/+ livers pretreated with anti–IGFBP-1 Ab and in IGFBP-1–/– livers after Fas challenge. Ab’s against phosphorylated p125FAK and FAK as indicated in Methods.
We hypothesized that IGFBP-1 was exerting its effects on apoptosis in the liver by modulating integrin α5β1 signaling. Depending on the context, integrin signaling can either promote or reduce cellular apoptosis (33–45). Integrin signaling proceeds via the pFAK pathway and subsequently the p130cas pathway. A high basal level of phosphorylated p125FAK was observed in both IGFBP-1+/+ and IGFBP-1–/– livers, which is reflective of basal integrin signaling. However, it was not known whether phosphorylated pFAK levels were generated via α5β1 or other hepatic integrin signals. In contrast to the wild-type livers, where pFAK rapidly decreased after Fas agonist treatment, the expression of phosphorylated p125FAK at 30 minutes was 3.2-fold higher in the IGFBP-1–/– livers and remained elevated 1 hour after Fas ligand treatment, indicating enhanced integrin signaling (Figure 5a). Activation of p130cas in the IGFBP-1–/– livers occurred at 3 hours after anti-Fas mAb challenge (Figure 5a) and was thus dissociated from the level of phosphorylated pFAK. p130cas signaling may occur in response to a variety of signal transduction pathways and is not necessarily linked to integrin signaling (46).
Activation of p125FAK has been functionally linked to the formation of integrin-mediated contact sites between the cell surface and the ECM known as focal adhesions (39). However, cleavage of FAK by caspase-3 generates a truncated isoform of FAK known as FRNK (FAK-related non-kinase) (47), which acts as an inhibitor of p125FAK by transiently blocking the formation of focal adhesions on fibronectin and reducing tyrosine phosphorylation of p125FAK (48). To ascertain whether cleavage of FAK by caspase-3 might play a role in the execution of the suicide program and thereby contribute to the disruption of the cytoarchitecture, leading to eventual collapse of the hepatic lobular architecture, we examined FAK proteolysis. Enhanced expression of FRNK was seen only in the IGFBP-1–/– livers at 5 hours and 7 hours after Fas challenge (Figure 5a), consistent with massive apoptosis observed at those times. Degradation of FAK, activation of p130cas, and downregulation of p125FAK and integrin β1 at 7 hours after Fas challenge were prevented by pretreatment of IGFBP-1–deficient livers with IGFBP-1 (Figure 5, b and c).
Activation of MMP-9 activity in IGFBP-1–/– livers after anti-Fas challenge.
We hypothesized that the elevated integrin signaling observed in the IGFBP-1–deficient livers immediately after Fas agonist treatment plays a role in the ensuing apoptotic response. In the liver, TGF-β is a major hepatocyte apoptogen and can be activated by MMP-9 (49). Fibronectin stimulates MMP-9 secretion via the FAK/Ras signaling pathway (50). Moreover, during monocyte differentiation, fibronectin signaling via integrin α5β1 is required for upregulation of MMP-9 (51).
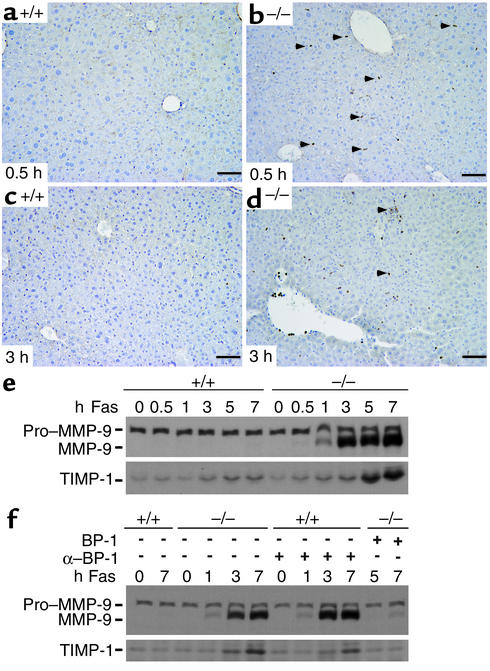
We wondered whether expression of active MMP-9 would be elevated in IGFBP-1–deficient livers. As shown by immunohistologic staining, active MMP-9 was detected in nonparenchymal cells in IGFBP-1–/– livers as early as 30 minutes after Fas challenge (Figure 6b), indicating that MMP-9 expression is an immediate signal in response to Fas agonist. Further upregulation of active MMP-9 was seen in the IGFBP-1–/– livers, but not in IGFBP-1+/+ livers, 3 hours after challenge (Figure 6, c and d). Western analyses using whole-cell liver extracts also revealed progressive conversion of pro–MMP-9 to active MMP-9 in IGFBP-1–/– livers from 30 minutes to 7 hours after Fas challenge (Figure 6e) and in IGFBP-1+/+ livers pretreated with anti–IGFBP-1 Ab (Figure 6f). A greater than tenfold increase in the expression of the tissue inhibitors of metalloproteinase-1 (TIMP-1), an inhibitor of MMP-9 activation (52), occurred in IGFBP-1–/– livers at 5 hours and 7 hours after anti-Fas injection, more than 4 hours after MMP-9 induction and after fulminant apoptotic damage had already occurred (Figure 6e). Pretreatment of IGFBP-1–/– livers with IGFBP-1 prior to a lethal challenge of anti-Fas mAb attenuated the processing of pro–MMP-9 (Figure 6f). No difference in MMP-2 processing was observed between the IGFBP-1+/+ and IGFBP-1–/– livers at different timepoints after anti-Fas mAb challenge (data not shown).
Figure 6.
Activation of MMP-9 and its inhibitor TIMP-1 in IGFBP-1–/– livers after Fas agonist–induced liver injury. (a–d) Immunohistochemical staining of (a) IGFBP-1+/+ liver sections at 0.5 hour and (c) 3 hours, and (b) IGFBP-1–/– liver sections at 0.5 hour and (d) 3 hours after Fas treatment using anti–MMP-9 Ab. Scale bars: 50 μm. Arrowheads indicate positive MMP-9 staining. (e) Western analyses showing MMP-9 activation after a lethal injection of Fas in IGFBP-1–/– livers and enhanced upregulation of TIMP-1 in IGFBP-1–/– livers at 5 hours and 7 hours after Fas challenge. (f) Upregulation of TIMP-1 and activation of MMP-9 in IGFBP-1+/+ livers pretreated with anti–IGFBP-1 Ab and in IGFBP-1–/– livers at the indicated timepoints after Fas challenge.
Activation of TGF-β1 in IGFBP-1–/– livers after anti-Fas challenge.
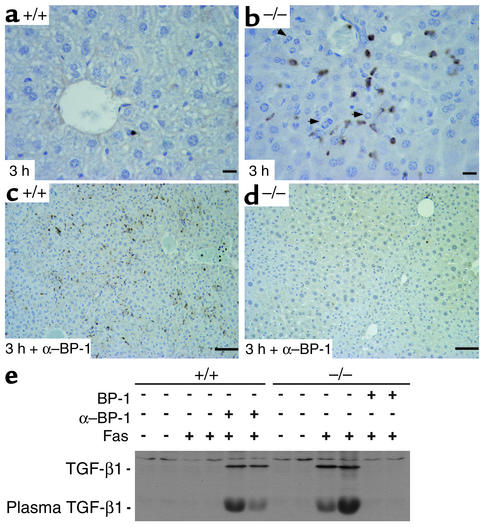
MMP-9 is involved in the proteolytic activation of TGF-β1, a known hepatocyte apoptogen (49). TGF-β1 is involved in the sequential activation of caspase-8 and caspase-3 (53), effects that were found in IGFBP-1–/– livers (See Figure 2). We determined whether TGF-β1 was upregulated in IGFBP-1–deficient livers after anti-Fas mAb challenge. Positive TGF-β1 staining was readily detected within nonparenchymal cells in IGFBP-1–/– livers (Figure 7b) and in wild-type livers treated with anti–IGFBP-1 (Figure 7c) after Fas challenge, and was prevented by IGFBP-1 pretreatment (Figure 7d). These nonparenchymal cells are presumed to be hepatic stellate cells that produce TGF-β1 under pathophysiologic conditions. IGFBP-1 pretreatment blocked the accumulation of active TGF-β1 (Figure 7e).
Figure 7.
Activation of TGF-β1 in IGFBP-1–/– livers after Fas agonist treatment, inhibited by IGFBP-1 treatment. (a–d) Immunohistochemical staining of liver sections using anti–TGF-β1 Ab: IGFBP-1+/+ (a), IGFBP-1–/– (b), IGFBP-1+/+ pretreated with anti–IGFBP-1 Ab (c), and IGFBP-1–/– pretreated with IGFBP-1 (d) 3 hours after Fas treatment. Scale bars: 10 μm (a and b) and 50 μm (c and d). Arrowheads indicate apoptotic hepatocytes. (e) Western analysis showing TGF-β1 activation in IGFBP-1+/+ livers pretreated with anti–IGFBP-1 Ab and in IGFBP-1–/– livers 7 hours after Fas challenge, but not in IGFBP-1–/– livers pretreated with IGFBP-1 7 hours after Fas challenge.
Increased hepatocellular injury and apoptosis after acute CCl4 treatment in IGFBP-1–/– mice.
Like Fas agonist, the hepatotoxin CCl4 also induces acute liver injury. CCl4 causes necrosis and apoptosis in liver cells by altering permeability of cellular, lysosomal, and mitochondrial membranes (19, 54, 55). CCl4 liver injury is associated with high levels of TNF-α, which is felt to enhance injury and promote hepatocyte apoptotic death (56). To determine the role of IGFBP-1 in modulating CCl4-induced acute liver injury, IGFBP-1–/– mice and their wild-type (IGFBP-1+/+) counterparts were injected intraperitoneally with a single 2 μl/g dose of a 50% solution of CCl4 in mineral oil. Morbidity after CCl4 injection was minimal and similar in IGFBP-1+/+ and IGFBP-1–/– mice, and included mild ataxia and mild lethargy for approximately 24 hours; there was no associated mortality.
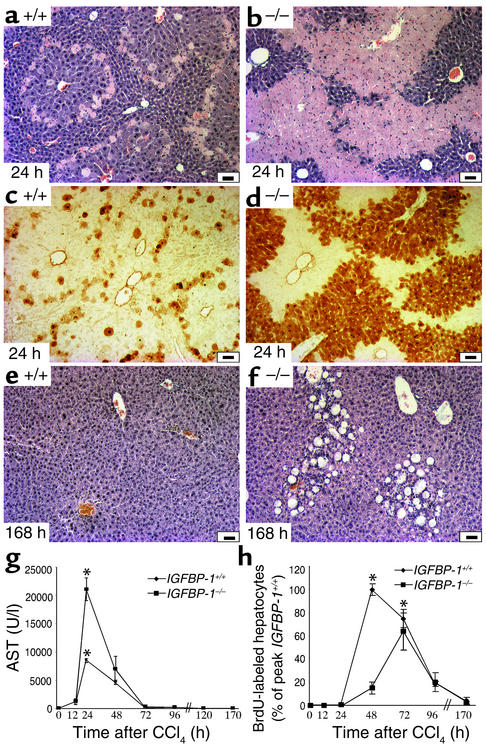
IGFBP-1–/– livers developed more rapid and severe hepatocellular injury following acute CCl4 exposure than did IGFBP-1+/+ livers (Figure 8). At 24 hours after CCl4 treatment, IGFBP-1+/+ livers displayed a localized and mild centrizonal steatosis (Figure 8a). IGFBP-1–/– livers (Figure 8b) showed a diffuse pattern of injury characterized by severe bridging central injury. The severe panlobular steatosis and centrizonal injury noted in IGFBP-1–/– liver parenchyma was associated with congestion, mild inflammatory infiltrate, and increased apoptotic/necrotic hepatocyte death. TUNEL staining preferentially labels DNA strand breaks generated during apoptosis, which allows discrimination of apoptosis from necrosis (57). However, in the CCl4 model, apoptotic cells are frequently surrounded by a mass of necrotic tissue, making them difficult to differentiate. Therefore, TUNEL staining was used to determine total liver cell damage in the CCl4 model (25). Quantification of the damaged area based on TUNEL analyses indicated that the mean area of injury at 24 hours in the IGFBP-1–/– livers was 54.4% ± 6.4% versus 32% ± 5.2% in IGFBP-1+/+ livers (P < 0.05) (Figure 8, c and d). Liver injury persisted at 168 hours in IGFBP-1–/– livers but not IGFBP-1+/+ livers (Figure 8, e and f). Increased liver damage was further substantiated by a 3.5-fold increase in aspartate aminotransferase levels (P < 0.05) (Figure 8g) at 24 hours after CCl4 administration. Levels for total bilirubin, albumin, alkaline phosphatase, creatinine, amylase, glucose, cholesterol, and triglycerides were similar between the IGFBP-1+/+ and IGFBP-1–/– animals (data not shown). Taken together, these data suggest that the presence of IGFBP-1 may confer increased protection from liver damage after CCl4 treatment. Moreover, as in the partial hepatectomy model, in which DNA synthesis is delayed and reduced in IGFBP-1–/– livers, DNA synthesis was delayed and reduced in IGFBP-1–/– livers after CCl4 treatment despite the fact that the amount of injury was less in the IGFBP-1+/+ livers.
Figure 8.
Increased liver injury after acute CCl4 treatment in IGFBP-1–/– livers. (a and b) Hematoxylin and eosin stain of (a) IGFBP-1+/+ liver and (b) IGFBP-1–/– liver 24 hours after CCl4 injection. Scale bars: 50 μm. (c and d) Histologic TUNEL staining of IGFBP-1+/+ (c) and IGFBP-1–/– (d) liver sections 24 hours after CCl4 injection indicates more necrotic damage (dark brown staining areas) in IGFBP-1–/– livers. (e and f) Hematoxylin and eosin stain of (e) IGFBP-1+/+ liver and (f) IGFBP-1–/– liver 168 hours after CCl4 administration. Scale bars: 50 μm. (g) Aspartate aminotransferase (AST) levels in IGFBP-1+/+ and IGFBP-1–/– livers after CCl4 injection at indicated timepoints. IGFBP-1+/+, n = 4 per timepoint. IGFBP-1–/–, n = 6–9 per timepoint. *P < 0.05 vs. IGFBP-1+/+. AST, aspartate aminotransferase, normal = 72–288 U/l. (h) Delayed DNA synthetic response in IGFBP-1–/– hepatocytes after CCl4 injury. BrdU incorporation profile for IGFBP-1+/+ and IGFBP-1–/– mice at various times after CCl4 treatment. *P < 0.05 vs. IGFBP-1+/+. BrdU-positive hepatocytes for each sample were quantitated by counting positively stained cells in three to four high-power, randomly selected fields. The mean for each timepoint was expressed as a percentage of the mean number of BrdU-labeled cells at the peak time of BrdU incorporation in IGFBP-1+/+ mice (48 hours). SDs are shown for each timepoint. Statistical significance was calculated versus wild-type littermates.
Discussion
Apoptosis mediated by Fas agonist (Jo-2 mAb) is restricted to hepatocytes and is an excellent model system for the study of fulminant hepatitis (1). The majority of the data suggest that IGFBPs are potent inducers of the apoptotic cell death program, in some cases acting via IGF-independent effects (11–17). However, our data suggest that IGFBP-1 may function as a critical survival factor in the liver by suppressing the level and activation of specific proapoptotic factors via its regulation of integrin-mediated signaling. Moreover, this hepatoprotective effect was not limited to Fas-mediated acute liver injury, but was also observed in acute toxic damage mediated by CCl4. Although not formally ruled out, IGFBP-1 is unlikely to be acting via modulation of IGF-1 signaling. IGFs have not been shown to have a regulatory role in hepatocytes, which have virtually undetectable IGF-I receptors (3, 6, 23, 31, 58).
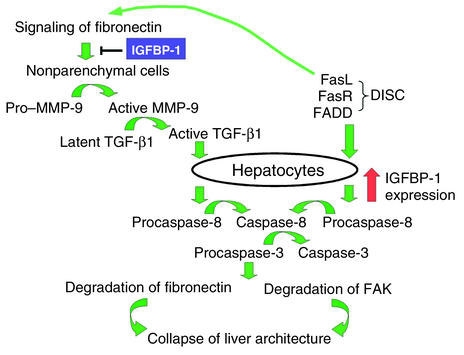
After IGFBP-1–/– mice were treated with anti-Fas mAb, the mice rapidly developed acute fulminant hepatitis associated with hepatocyte apoptosis, hypothermia, sinusoidal congestion, and destruction of hepatic lobular architecture. Apoptosis in IGFBP-1–deficient livers was associated with elevated phosphorylated pFAK at 30 minutes to 1 hour, conversion of pro–MMP-9 to its mature form by 30 minutes, enhanced caspase-8 activation, and procaspase-3 cleavage concomitant with activation of TGF-β1 at 3 hours, simultaneous with the histologic appearance of apoptotic hepatocytes (Figure 9). We hypothesize that the full apoptotic response in IGFBP-1–deficient livers required the combination of TGF-β signaling and Fas pathway activation. Engagement of Fas by anti-Fas mAb treatment leads to recruitment of Fas-associated death domain protein (FADD) and procaspase-8 to the plasma membrane, thereby leading to the formation of the death-inducing signaling complex (DISC) and subsequent self-proteolysis of procaspase-8 (59). This DISC combined with the release of TGF-β, and the ensuing TGF-β–mediated apoptotic response, generated fulminant apoptosis in IGFBP-1–deficient livers. In wild-type livers there was no TGF-β release, and the interaction of Fas with its receptor was insufficient to generate the full apoptotic response. Late changes in the liver were compatible with collapsed hepatic architecture and included increased FAK proteolysis and degradation of fibronectin at 5–7 hours.
Figure 9.
Proposed model for IGFBP-1 protection against Fas-induced lethal hepatitis. In the absence of IGFBP-1, fibronectin signaling in combination with Fas agonist enhances the proapoptotic signaling cascade, leading to processing of pro–MMP-9 to its mature form and subsequent proteolytic activation of TGF-β1, which positively reinforces the processing of procaspase-8 and procaspase-3 to their active forms. Progressive cleavage of fibronectin, proteolysis of FAK by caspase-3, and degradation of α5β1 integrin potentiate focal adhesion disorganization and cell detachment, thereby disrupting the integrity of the hepatic lobular architecture. In normal livers, IGFBP-1 is upregulated after treatment with Fas agonist. IGFBP-1 treatment of IGFBP-1–deficient livers attenuates these abnormalities, thereby preventing the destruction of the parenchymal architecture. FasR, Fas receptor.
Similar changes were seen in IGFBP-1+/+ livers treated with anti–IGFBP-1 Ab in concert with Fas agonist, indicating that no preexisting developmental defect in IGFBP-1–/– livers was responsible for Fas sensitivity. The mechanistic specificity of the apoptotic program was demonstrated by the fact that IGFBP-1 treatment prevented these defects. A number of other Ab’s including the α5β1 integrin–neutralizing Ab, MMP inhibitors, and TGF-β–neutralizing Ab did not alter Fas-mediated apoptosis in preliminary studies (data not shown), further demonstrating the specificity of the anti-IGFBP Ab’s. However, we do not know the ability of these Ab’s to neutralize their target in vivo. Further studies with RGD-blocking peptides or animals treated with IGFBP-1 that has been mutated at the RGD sequence would test the hypothesis that the interaction of IGFBP-1 with integrins is vital for the hepatoprotective effect.
It is not clear why the Fas signal resulted in induction of IGFBP-1 in IGFBP-1+/+ livers. This induction was coincident with the downregulation of pFAK in IGFBP-1+/+ livers. Because pFAK is associated with multiple integrin signaling pathways, it is not yet possible to directly associate IGFBP-1 with downregulation of α5β1 fibronectin receptor signals after Fas agonist treatment. Downregulation of phosphorylated pFAK was not observed in IGFBP-1–deficient livers, suggesting that IGFBP-1 directly or indirectly mediated blockade of integrin signaling after Fas ligation. In IGFBP-1–/– quiescent livers, basal abnormalities such as low levels of procaspase-8 processing and accumulation of fibronectin in the hepatocytes were seen. However, preexisting abnormalities in IGFBP-1–/– livers did not contribute substantially to the apoptotic effect, because we were able to replicate the defect in IGFBP-1+/+ livers treated with IGFBP-1 Ab’s and Fas ligand. Late changes included progressive degradation of fibronectin, proteolysis of FAK by caspase-3, activation of p130cas, and degradation of α5β1 integrin. Breakdown of fibronectin further potentiated focal adhesion disorganization and cell detachment, thereby disrupting the integrity of the hepatic lobular architecture. Compensatory changes such as upregulation of TIMP-1 and Bcl-2 were too late to prevent massive apoptosis that had already ensued. IGFBP-1 may also play a protective role in the late stages of apoptosis by preventing proteolytic cleavage of pFAK to prevent the disassembly of focal adhesions and preserve the integrity of the hepatic cellular architecture (46, 60). Pretreatment of IGFBP-1–/– livers with IGFBP-1 prior to Fas challenge greatly diminished all of these late changes, indicating that they were part of the apoptotic cascade induced by IGFBP-1 deficiency.
Fibronectin signaling has been shown to be both pro- and antiapoptotic, depending on the local environment. For example, in some cells, integrin engagement has been shown to inhibit apoptosis in the basal state, but to stimulate apoptosis in the presence of Fas ligand or TNF-α (45). In our studies, the proapoptotic effect was accompanied by the rapid upregulation of MMP-9 and subsequent TGF-β release. TGF-β is proposed as a key liver apoptogen that normally controls liver size and is elevated in certain viral liver diseases and cirrhosis (53). It is a well-studied hepatic apoptogen in vitro. However, its regulation in vivo in the liver during massive apoptosis has not been extensively explored.
Although MMP-9 has been shown to cleave TGF-β in vitro and to activate TGF-β signaling in angiogenesis models (49), this report represents the first demonstration of a potential in vivo link between MMP-9 activation, TGF-β upregulation, and apoptosis in the liver. MMP-9 expression is upregulated in monocytic cells by fibronectin signaling, and MMP-9 activation rapidly proceeds through membrane contact (51). Gelatinases (MMP-2 and MMP-9) may be expressed in stellate and Kupffer cells in the liver and potentially in endothelial cells (52). In our study, MMP-9 rapidly appeared in nonparenchymal cells, followed by the appearance of active TGF-β, presumably within stellate cells. Although TGF-β has long been known as a hepatic apoptogen, its rapid induction after Fas ligation has not been reported previously, perhaps because IGFBP-1 expression normally prevents the appearance of TGF-β. TGF-β causes apoptosis in hepatocytes through similar pathways to those activated by Fas ligation, that is, through generation of active caspase-8, cytochrome c release by mitochondria, and activation of various execution caspases, including caspase-3 and caspase-7 (53). These findings are consistent with the changes observed in IGFBP-1–deficient livers subjected to Fas ligation.
We have clearly demonstrated the role of IGFBP-1 as a hepatic survival factor in a model of fulminant hepatic apoptosis induced by Fas ligation, a model that is commonly compared with acute viral hepatitis. We have also shown that IGFBP-1–deficient mice are more sensitive than the wild-type to acute liver injury caused by a hepatic toxin. These findings may have implications for therapeutic intervention in the treatment of acute viral hepatitis and liver failure.
Acknowledgments
We thank Gary Swain at the Digestive and Liver Center at the University of Pennsylvania School of Medicine for help with hematoxylin and eosin staining, immunohistochemistry, and imaging. This work was supported in part by Digestive and Liver Center grant P30 DK-50306 (for technical support) and NIHgrants DK-44237 and DK-58315 (to R. Taub).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: IGF binding protein-1 (IGFBP-1); Arg-Gly-Asp (RGD); focal adhesion kinase (FAK); CCAAT enhancer binding protein β (C/EBPβ); matrix metalloproteinase-9 (MMP-9); FAK-related non-kinase (FRNK); death-inducing signaling complex (DISC).
References
- 1.Ogasawara J, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 2.Leu, J.I., Crissey, M.S., Criag, L.E., and Taub, R. 2003. Impaired hepatocyte DNA synthetic response posthepatectomy in insulin-like growth factor binding protein-1 deficient mice with defects in C/EBPB and MAPK/ERK regulation. Mol. Cell. Biol. In press. [DOI] [PMC free article] [PubMed]
- 3.Lee PDK, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc. Soc. Exp. Biol. Med. 1997;216:319–357. doi: 10.3181/00379727-216-44182. [DOI] [PubMed] [Google Scholar]
- 4.Scearce LM, Laz TM, Hazel TG, Lau LF, Taub R. Rapid activation of latent transcription factor complexes reflects initiating signals in liver regeneration. Cell Death Differ. 1996;3:47–55. [PubMed] [Google Scholar]
- 5.Leu JI, Crissey MS, Leu JP, Ciliberto G, Taub R. Interleukin-6-induced STAT3 and AP-1 amplify hepatocyte nuclear factor-1-mediated transactivation of hepatic genes, an adaptive response to liver injury. Mol. Cell. Biol. 2001;21:414–424. doi: 10.1128/MCB.21.2.414-424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taub R. Liver regeneration 4: transcriptional control of liver regeneration. FASEB J. 1996;10:413–427. [PubMed] [Google Scholar]
- 7.Lee J, et al. Structure and localization of the IGFBP-1 gene and its expression during liver regeneration. Hepatology. 1994;19:656–665. doi: 10.1002/hep.1840190317. [DOI] [PubMed] [Google Scholar]
- 8.Kondoh N, et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990–4996. [PubMed] [Google Scholar]
- 9.Gleeson LM, Chakraborty C, Mckinnon T, Lala PK. Insulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through α5β1 integrin via mitogen activated protein kinase pathway. J. Clin. Endocrinol. Metab. 2001;86:2484–2493. doi: 10.1210/jcem.86.6.7532. [DOI] [PubMed] [Google Scholar]
- 10.Irwin JC, Giudice LC. Insulin-like growth factor binding protein-1 binds to placental cytotrophoblast alpha5beta1 integrin and inhibits cytotrophoblast invasion into decidualized endometrial stromal cultures. Growth Horm. IGF Res. 1998;8:21–31. doi: 10.1016/s1096-6374(98)80318-3. [DOI] [PubMed] [Google Scholar]
- 11.Perks CM, Newcomb PV, Norman MR, Holly JM. Effect of insulin-like growth factor binding protein-1 on integrin signaling and the induction of apoptosis in human breast cancer cells. J. Mol. Endocrinol. 1999;22:141–150. doi: 10.1677/jme.0.0220141. [DOI] [PubMed] [Google Scholar]
- 12.Baxter RC. Signaling pathways involved in antiproliferative effects of IGFBP-3: a review. Mol. Pathol. 2001;54:145–148. doi: 10.1136/mp.54.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman RS, et al. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999;13:2604–2616. doi: 10.1101/gad.13.19.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damon SE, Maddison L, Ware JL, Plymate SR. Overexpression of an inhibitory insulin-like growth factor binding protein (IGFBP), IGFBP-4, delays onset of prostate tumor formation. Endocrinology. 1998;139:3456–3464. doi: 10.1210/endo.139.8.6150. [DOI] [PubMed] [Google Scholar]
- 15.Dheen ST, Rajkumar K, Murphy LJ. Islet cell proliferation and apoptosis in insulin-like growth factor binding protein-1 deficient mice. J. Endocrinol. 1997;155:551–558. doi: 10.1677/joe.0.1550551. [DOI] [PubMed] [Google Scholar]
- 16.Sueoka N, et al. Insulin-like growth factor binding protein-6 activates programmed cell death in non-small cell lung cancer cells. Oncogene. 2000;19:4432–4436. doi: 10.1038/sj.onc.1203813. [DOI] [PubMed] [Google Scholar]
- 17.Tonner E, Allan GJ, Flint DJ. Hormonal control of plasmin and tissue-type plasminogen activator activity in rat milk during involution of the mammary gland. J. Endocrinol. 2000;167:265–273. doi: 10.1677/joe.0.1670265. [DOI] [PubMed] [Google Scholar]
- 18.Li W, et al. Global changes in interleukin-6-dependent gene expression patterns in mouse livers after partial hepatectomy. Hepatology. 2001;33:1377–1386. doi: 10.1053/jhep.2001.24431. [DOI] [PubMed] [Google Scholar]
- 19.Kovalovich K, et al. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J. Biol. Chem. 2001;276:26605–26613. doi: 10.1074/jbc.M100740200. [DOI] [PubMed] [Google Scholar]
- 20.Poli V, Mancini FP, Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63:643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- 21.Akira S, et al. A nuclear factor for IL-6 expression (NF-IL-6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee D, Kaestner KH, Kovalovich K, Greenbaum LE. Fas-induced apoptosis in mouse hepatocytes is dependent on C/EBPβ. Hepatology. 2001;33:1166–1172. doi: 10.1053/jhep.2001.24032. [DOI] [PubMed] [Google Scholar]
- 23.Mohn KL, Melby AE, Tewari DS, Laz TM, Taub R. The gene encoding rat insulin-like growth factor binding protein-1 is rapidly and highly induced in regenerating liver. Mol. Cell. Biol. 1991;11:1393–1401. doi: 10.1128/mcb.11.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeAngelis RA, Kovalovich K, Cressman DE, Taub R. Normal liver regeneration in p50/nuclear factor kB1 knockout mice. Hepatology. 2001;33:915–924. doi: 10.1053/jhep.2001.23192. [DOI] [PubMed] [Google Scholar]
- 25.Kovalovich K, et al. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149–159. doi: 10.1002/hep.510310123. [DOI] [PubMed] [Google Scholar]
- 26.Kischkel FC, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medema JP, et al. FLICE is activated by association with the CD95 death inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck M, Poli V, Hunter T, Chojkier M. C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell. 2001;8:807–816. doi: 10.1016/s1097-2765(01)00374-4. [DOI] [PubMed] [Google Scholar]
- 29.Greenbaum LE, Cressman DE, Haber BA, Taub R. Coexistence of C/EBPα, β, growth-induced proteins and DNA synthesis in hepatocytes during liver regeneration. J. Clin. Invest. 1995;96:1351–1365. doi: 10.1172/JCI118170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenbaum LE, et al. CCAAT enhancer binding protein β is required for normal hepatocyte proliferation in mice after partial hepatectomy. J. Clin. Invest. 1998;102:996–1007. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caro JF, et al. Insulin-like growth factor I binding in hepatocytes from human liver, human hepatoma, and normal, regenerating, and fetal rat liver. J. Clin. Invest. 1988;81:976–981. doi: 10.1172/JCI113451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 33.Pujades C, Forsberg E, Enrich C, Johansson S. Changes in cell surface expression of fibronectin and fibronectin receptor during liver regeneration. J. Cell Sci. 1992;102:815–820. doi: 10.1242/jcs.102.4.815. [DOI] [PubMed] [Google Scholar]
- 34.Aoki K, et al. Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat. Immunol. 2001;2:333–337. doi: 10.1038/86336. [DOI] [PubMed] [Google Scholar]
- 35.Kapila YL, Niu J, Johnson PW. The high affinity heparin-binding domain and the V region of fibronectin mediate invasion of human oral squamous cell carcinoma cells in vitro. J. Biol. Chem. 1997;272:18932–18938. doi: 10.1074/jbc.272.30.18932. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, et al. Expression of fibronectin receptor, integrin alpha 5 beta 1 on hepatic stellate cells in rat liver fibrosis. Chin. Med. J. 2000;113:227–236. [PubMed] [Google Scholar]
- 37.Jones JI, Gockerman A, Busby WH, Clemmons DR. IGFBP-1 stimulates cell migration and binds to the a5b1 integrin by means of its Arg-Gly-Asp sequence. Proc. Natl. Acad. Sci. USA. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilic D, et al. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 40.Stupack DG, Puente XS, Boutsaboualoy S, Storgard SM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 2001;155:459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bussell K. A life or death situation? Nat. Rev. Mol. Cell Biol. 2001;2:868–869. doi: 10.1038/35103040. [DOI] [PubMed] [Google Scholar]
- 42.Giancotti FG, Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 43.Plath T, et al. A novel function for the tumor suppressor p16(INK4a): induction of anoikis via upregulation of the alpha(5)beta(1) fibronectin receptor. J. Cell Biol. 2000;150:1467–1478. doi: 10.1083/jcb.150.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol. Biol. Cell. 1995;6:725–740. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential roles for αMβ2 integrin clustering or activation in the control of apoptosis via regulation of Akt and ERK survival mechanisms. J. Cell. Biol. 2000;151:1305–1320. doi: 10.1083/jcb.151.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Neill GM, Fashena SJ, Golemis EA. Integrin signaling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 47.Gervais FG, Thornberry NA, Ruffolo SC, Nicholson DW, Roy S. Caspases cleave focal adhesion kinase during apoptosis to generate a FRNK-like polypeptide. J. Biol. Chem. 1998;273:17102–17108. doi: 10.1074/jbc.273.27.17102. [DOI] [PubMed] [Google Scholar]
- 48.Richardson A, Parsons TA. Mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 49.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 50.Thant AA, et al. Fibronectin activates matrix metalloproteinase-9 secretion via the MEK1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin. Exp. Metastasis. 2000;18:423–428. doi: 10.1023/a:1010921730952. [DOI] [PubMed] [Google Scholar]
- 51.Xie B, Laouar A, Huberman E. Autocrine regulation of macrophage differentiation and 92-kDa gelatinase production by tumor necrosis factor-α via α5β1 integrin in HL-60 cells. J. Biol. Chem. 1998;273:11583–11588. doi: 10.1074/jbc.273.19.11583. [DOI] [PubMed] [Google Scholar]
- 52.Winwood PJ, et al. Kupffer cell-derived 95-kd type IV collagenase/gelatinase B: characterization and expression in cultured cells. Hepatology. 1995;22:304–315. [PubMed] [Google Scholar]
- 53.Cain K, Freathy C. Liver toxicity and apoptosis: role of TGF-β1, cytochrome c and the apoptosome. Toxicol. Lett. 2001;120:307–315. doi: 10.1016/s0378-4274(01)00283-1. [DOI] [PubMed] [Google Scholar]
- 54.Berger ML, Bhatt H, Combes B, Estabrook R. CCl4-induced toxicity in isolated hepatocytes: the importance of direct solvent injury. Hepatology. 1986;6:36–45. doi: 10.1002/hep.1840060108. [DOI] [PubMed] [Google Scholar]
- 55.Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J. Pathol. 1998;153:515–525. doi: 10.1016/S0002-9440(10)65594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czaja MJ, Xu J, Alt E. Prevention of carbon tetrachloride-induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology. 1995;108:1849–1854. doi: 10.1016/0016-5085(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 57.Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 58.Taub, R. 1995. Expression and function of growth induced genes during liver regeneration. In Liver regeneration and carcinogenesis. R. Jirtle, editor. Academic Press. New York, New York, USA. 71–97.
- 59.Scaffidi C, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panetti TS. Tyrosine phosphorylation of paxillin, FAK, and p130cas: effects on cell spreading and migration. Front. Biosci. 2002;7:d143–d150. doi: 10.2741/A771. [DOI] [PubMed] [Google Scholar]