Despite decades of research, corticosteroids and NSAIDs remain the main pharmacological weapons to control inflammation in the clinic. Unfortunately, these drugs have significant side effects, especially when used chronically. Consequently, there is tremendous interest in the development of novel, safer, and more effective anti-inflammatory drugs. Most anti-inflammatory agents directly or indirectly inhibit the formation or the effects of arachidonic acid metabolites collectively known as eicosanoids (prostaglandins, leukotrienes, thromboxanes, endoperoxides, and other mediators) (Table 1). It has long been recognized that inhibition of phospholipase A2–catalyzed (PLA2-catalyzed) arachidonic acid release from cell-membrane glycerophospholipids could potentially block the synthesis of all eicosanoids. The vast PLA2 enzyme family includes cellular isoforms involved in signal transduction, such as three cellular isoforms of PLA2 (cPLA2s), and ten secretory isoforms of PLA2 (sPLA2s) (1). Various sPLA2 isoforms participate in digestive physiology, antimicrobial defense, and inflammation. The cPLA2s, and sPLA2s IIA and V, play key roles in arachidonic-acid release during acute inflammation (1). Two families of endogenous proteins include members whose synthesis and/or secretion are induced by glucocorticoids in the lung that exhibit anti-inflammatory activity in experimental models. These are the lipocortins, or annexins (2), and the secretoglobins, whose prototype is uteroglobin (3). These families include proteins with distinct and pleiotropic biological properties. Lipocortins I and V, as well as rabbit and human uteroglobin, have anti-inflammatory properties that can be explained, at least in part, by their ability to inhibit sPLA2. Human uteroglobin or “Clara Cell 10 kDa protein” is currently in clinical development for the prevention of airway inflammation in neonatal lung disease. The mechanism of sPLA2 inhibition by lipocortins and uteroglobin remains controversial and may depend on the assay system. However, a 9–amino acid sequence that is highly conserved in uteroglobin and the anti-inflammatory lipocortins I and V was identified as early as 1988 (4). Synthetic peptides corresponding to this shared sequence exhibit striking anti-inflammatory activity in vivo and inhibit sPLA2 in vitro. Mutagenesis data show that this sequence is necessary for sPLA2 inhibition by uteroglobin (5). Peptides derived from uteroglobin and lipocortins are collectively known as antiflammins, now recognized as one of the most potent classes of anti-inflammatory agents identified to date (6). The elegant work by Sohn et al. (7) appearing in this issue of the JCI builds on that early discovery and on the observation that some sPLA2s are further activated by post-translational modifications catalyzed by transglutaminases (TGases). Transglutaminases are multifunctional enzymes that form isopeptide bonds between specific lysine and glutamine residues of substrate proteins or crosslink polyamines to glutamine residues (8). Cordella-Miele et al. showed that the TGase-catalyzed formation of an intramolecular isopeptide bond within sPLA2s (9) or the polyamination of sPLA2 (10) enhances the activity of sPLA2s. Basing their work on these findings, Sohn et al. designed a novel series of chimeric peptides that include a fragment of pro-elafin (a TGase substrate in keratinocytes), and the conserved core of antiflammins (the sequence KVLD corresponding to uteroglobin residues 43–46). These new peptides inhibit sPLA2 and TGase activity, and the TGase-catalyzed post-translational activation of sPLA2 (Figure 1). Interestingly, the authors show that even the original antiflammins inhibit TGase, though not as efficiently as the new chimeric peptides. Uteroglobin is a well-known TGase substrate and Lys 43 a likely acyl acceptor (11). The chimeric peptides exhibit dramatic in vivo anti-inflammatory activity in a clinically relevant model of allergic inflammation: ragweed pollen–induced allergic conjunctivitis in guinea pigs. Inhibition of sPLA2 and TGase activity was documented in tissue extracts from treated animals, and in vivo anti-inflammatory activity correlated with in vitro inhibitory potency on sPLA2 and TGase. Chimeric peptide R2 was as potent as topical steroid or antihistamine drops, based on clinical inflammation scores, and was even more effective in reducing eosinophil infiltration. These findings have potentially great therapeutic relevance if one considers the number of patients who are chronically treated with antihistamines or steroids for seasonal allergies.
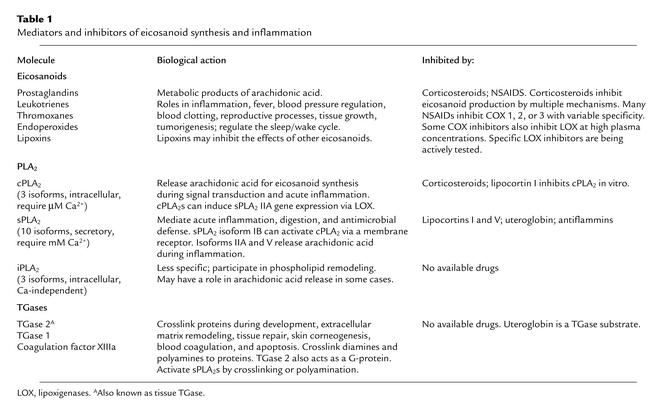
Table 1.
Mediators and inhibitors of eicosanoid synthesis and inflammation
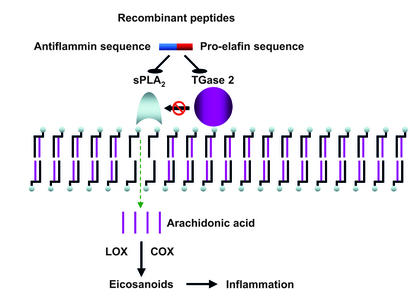
Figure 1.
sPLA2s hydrolyze the ester bond at the sn-2 position of membrane glycerophospholipids, generating free arachidonic acid. This acid is metabolized in a complex series of reactions involving COX or lipoxigenases (LOX), generating pro-inflammatory eicosanoids. TGase-catalyzed post-translational modifications activate sPLA2, potentially increasing eicosanoid production during acute inflammation. The new recombinant peptides contain a pro-elafin sequence that inhibits TGase and an antiflammin sequence that inhibits sPLA2. Thus they prevent TGase-induced sPLA2 activation.
Future directions
The findings of Sohn et al. (7) establish that peptides or recombinant proteins that inhibit TGases and sPLA2 or their peptidomimetic derivatives are highly attractive candidates for clinical development as anti-inflammatory agents. The potential applications of these molecules could go well beyond the realm of seasonal allergies. However, several important questions remain open. First, the precise in vivo mechanism of action of the new chimeric peptides, or for that matter the original antiflammins, remains unclear. Would a pure inhibitor of TGase that does not inhibit sPLA2 be as effective? Are the effects of the new peptides abolished by arachidonic acid, as is the case for antiflammins? Are there additional in vivo mechanisms that were not explored by the relatively simple in vitro assays used in this and other studies? Antiflammins modulate leukocyte adhesion proteins (12), and uteroglobin binds fibronectin (13). Could the new peptides inhibit leukocyte migration via either or both of these mechanisms? Further mechanistic studies are necessary. Nonetheless, it is fair to say that a promising new class of anti-inflammatory agents may soon be added to our therapeutic arsenal.
Footnotes
See the related article beginning on page 121.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: phospholipase A2 (PLA2); cellular isoform of PLA2 (cPLA2); secretory isoform of PLA2 (sPLA2); transglutaminase (TGase).
References
- 1.Murakami M, Kudo I. Phospholipase A2. J. Biochem. (Tokyo). 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. (Lond.). 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee, A.B., and Chilton, B.S. 2000. Uteroglobin/Clara cell family of proteins. New York Academy of Sciences. New York, New York, USA. 358 pp.
- 4.Miele L, Cordella-Miele E, Facchiano A, Mukherjee AB. Novel anti-inflammatory peptides from the region of highest similarity between uteroglobin and lipocortin I. Nature. 1988;335:726–730. doi: 10.1038/335726a0. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury B, et al. Lys 43 and Asp 46 in alpha-helix 3 of uteroglobin are essential for its phospholipase A2 inhibitory activity. Biochem. Biophys. Res. Commun. 2002;295:877–883. doi: 10.1016/s0006-291x(02)00767-2. [DOI] [PubMed] [Google Scholar]
- 6.Miele L. Antiflammins. Bioactive peptides derived from uteroglobin. Ann. N. Y. Acad. Sci. 2000;923:128–140. doi: 10.1111/j.1749-6632.2000.tb05524.x. [DOI] [PubMed] [Google Scholar]
- 7.Sohn J, Kim T-I, Yoon Y-H, Kim J-Y, Kim S-Y. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J. Clin. Invest. 2003;111:121–128. doi:10.1172/JCI200315937. doi: 10.1172/JCI15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem. Sci. 2002;27:534–539. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- 9.Cordella-Miele E, Miele L, Mukherjee AB. A novel transglutaminase-mediated post-translational modification of phospholipase A2 dramatically increases its catalytic activity. J. Biol. Chem. 1990;265:17180–17188. [PubMed] [Google Scholar]
- 10.Cordella-Miele E, Miele L, Beninati S, Mukherjee AB. Transglutaminase-catalyzed incorporation of polyamines into phospholipase A2. J. Biochem. (Tokyo). 1993;113:164–173. doi: 10.1093/oxfordjournals.jbchem.a124021. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee AB, Cordella-Miele E, Kikukawa T, Miele L. Modulation of cellular response to antigens by uteroglobin and transglutaminase. Adv. Exp. Med. Biol. 1988;231:135–152. doi: 10.1007/978-1-4684-9042-8_11. [DOI] [PubMed] [Google Scholar]
- 12.Zouki C, Ouellet S, Filep JG. The anti-inflammatory peptides, antiflammins, regulate the expression of adhesion molecules on human leukocytes and prevent neutrophil adhesion to endothelial cells. FASEB J. 2000;14:572–580. doi: 10.1096/fasebj.14.3.572. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, et al. Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science. 1997;276:1408–1412. doi: 10.1126/science.276.5317.1408. [DOI] [PubMed] [Google Scholar]