Abstract
A pathogenic hallmark of rheumatoid arthritis (RA) is persistent activation of self-reactive CD4+ T cells. The cause of this aberrant activity remains elusive. We report here detection of autoantibodies against B7-H1, a recently described member of the B7 family, in 29% of patients with RA versus 4% of healthy donors. High-level expression of cell surface B7-H1 are found on activated human CD4+, CD8+, and CD45RO+ T cells. Immobilized autoantibodies to B7-H1 are capable of costimulating the proliferation of CD4+ T cells in vitro, and the presence of these autoantibodies correlates with active disease status. Using immobilized B7-H1 mAb’s and programmed death 1Ig, we demonstrate that engagement of B7-H1 on CD4+ T cells costimulates proliferation and secretion of IL-10, and subsequently leads to programmed cell death, accompanied with upregulated expression of TNF-related apoptosis–inducing ligand and activation of caspase-3. Taken together with our previous findings, these data indicate a bidirectional signaling role of B7-H1 in T cell costimulation and apoptosis and implicate B7-H1 autoantibodies as contributing to the progression of RA by inducing aberrant T cell responses.
Introduction
Leukocyte infiltration into the synovium of rheumatoid arthritis (RA) patients results in extensive joint inflammation, characteristic of disease pathogenesis (1). The majority of infiltrating cells are CD4+ T cells, which express activation/memory markers including CD45R, CD44, HLA-DR, and VLA-1 (2, 3), suggesting a persistent state of hyperreactivity. The cause of this aberrant T cell activation has been attributed to the presentation of arthritogenic antigens by HLA-DR (4), existence of superantigen, and homeostatic proliferation (5, 6).
One alternative explanation for persistent T cell activation in systemic autoimmune diseases such as RA may come from the study of T cell costimulation. It is well accepted that, in addition to the engagement of the T cell receptor (TCR) with antigen, costimulation is required for optimal activation of T cells. The best-characterized costimulatory pathways are interactions of CD28 and CTLA-4 on the T cell with B7-1 (CD80) and B7-2 (CD86) on APCs (7, 8). In addition to the natural receptor-ligand interaction, receptor-specific agonistic Ab can also deliver costimulatory signals. In fact, agonistic Ab often results in much stronger signaling than that from natural ligand, possibly due to their high affinity and/or immobilization on cells expressing Fc receptor in vivo (9–12). Agonistic Ab ligation of costimulatory molecules may be particularly relevant in autoimmune diseases in which autoantibodies are commonly detected. In fact, autoantibodies against CTLA-4 recently have been identified in the serum of patients with RA and Behcet disease, although the functional significance of these autoantibodies remains unknown (13).
B7-H1 is a third member of the B7 family that costimulates primary T cells through a putative receptor (14), but may inhibit proliferation of preactivated T cells (15). In vitro stimulation of primary human T cells with B7-H1Ig or B7-H1 transfectants promotes both IL-10 secretion and proliferation, but without the accompanying increased production of IL-2. A putative counter-receptor, PD-1 (programmed death 1), were reported recently (15). Recently, we reported that many human malignancies express B7-H1 and ligation of tumor-associated B7-H1 to a non–PD-1 receptor promotes apoptosis of activated T cells (16), suggesting the expression of B7-H1 may represent a new mechanism for tumor evasion of immunity.
In addition to signals through binding receptor on T cells, select members of the B7 family could also have receptor functions, mediated through a process termed reverse signaling. Specifically, B7-2 demonstrates reverse signaling on B cells, resulting in differentiation and class switching (17). B7-H1 is expressed on activated human and mouse T cells (14, 18). This pattern of B7-H1 expression led us to postulate that engagement of B7-H1 might play a direct role in the regulation of the human T cell response in autoimmune diseases, such as RA, resulting in a persistent state of T cell activation in affected individuals.
Methods
Patients and healthy donors.
Sera samples were obtained from 63 patients with diagnosed RA autoimmune disease and 54 sex- and age-matched healthy donors under the approval of the Internal Review Board of the Mayo Clinic. Diagnosis of RA was defined according to the classification criteria of the American College of Rheumatology. Sera or plasma samples were collected from 63 RA patients (53 women and 10 men, mean age, 58 years; age range, 17–80 years) and 54 healthy donors (42 women and 12 men, mean age, 52; age range 20–69). Human IgG was purified by ImmunoPure (G) IgG purification kits (Piece Chemical Co., Rockford, Illinois, USA).
ELISA.
Purified human B7-H1Ig (mouse IgG2a Fc) (14) or control mIgG2a at 2 μg/ml in PBS was coated overnight on an ELISA plate at 4°C followed by blocking with PBS containing 10% FBS. Sera from healthy donors or RA patients were diluted in PBS at 1:1,000 in triplicate before adding to the plates. After reaction, the wells were washed six times in PBS with 0.1% Tween-20. Bound Ab’s were detected by horseradish peroxidase–conjugated goat anti-human IgG Ab (BioSource International Camarillo, California, USA) at a 1:2,000 dilution for 1.5 hour at room temperature, then reacted with tetramethylbenzidine, and measured using a multimicroplate reader at 450 nm. Nonspecific binding of sera to plates coated with control Ig (mouse IgG2a) was subtracted from each sample.
Generation of mAbs to B7-H1.
BALB/c mice were immunized with purified human B7-H1Ig mixed with complete Freud’s adjuvant (Sigma-Aldrich, St. Louis, Missouri, USA) and boosted three times with B7-H1Ig in incomplete Freud’s adjuvant. Sera from the mice were collected, and their specific binding to B7-H1 was determined by ELISA and by FACS analysis on B7-H1/293 cells (14). The spleen cells from mice with highest titer of antisera were fused with SP2/0 myeloma cells to produce hybridoma cells. After several rounds of selection by ELISA and by FACS, two clones, 2H1 and 5H1, which consistently stain B7-H1/293 cells, were selected. The isotype of 2H1 or 5H1 is IgG1. The culture supernatant of hybridomas was concentrated and purified by a protein G-Sepharose column (Pierce Chemical Co.) and dialyzed in LPS-free PBS. In some experiments, polymyxin B was incorporated in the assays of cell proliferation and cytokine secretion to neutralize residual endotoxin.
T cell activation and FACS analysis.
Freshly isolated human PBMCs (107 cells/ml) were stimulated with 5 μg/ml of phytohemagglutinin (PHA) (Sigma-Aldrich). Cells were harvested and analyzed at 0, 24, and 48 hours upon treatment. For direct immunofluorescence staining, T cells were incubated at 4°C with 1 μg of FITC- or phycoerythrin-conjugated (PE-conjugated) mAb for 30 minutes and analyzed by FACScan flow cytometry (Becton Dickinson Immunocytometry Systems, Mountain View, California, USA) with Cell Quest software (Becton Dickinson Immunocytometry Systems), as described previously (14). The mAb specific for CD4 (RPA-T4), CD8 (RPA-T8), and CD45RO (UCHL1) were purchased from BD-PharMingen (San Diego, California, USA), and rabbit anti-human TNF-related apoptosis–inducing ligand (TRAIL) polyclonal Ab’s were purchased from Alexis Biochemical Corp. (San Diego, California, USA). For indirect immunofluorescence staining, cells were first incubated with B7-H1 mAb (3 μg/sample) at 4°C for 30 minutes. The cells were washed and further incubated with FITC-conjugated (BioSource International) or PE-conjugated (Southern Biotechnology Associates, Birmingham, Alabama, USA) goat anti-mouse IgG F(ab′)2 for 30 minutes at 4°C. The mouse IgG-1 (Sigma-Aldrich) was used as control Ig. In some experiments, cells were treated with human Ig before incubation with FITC- or PE-conjugated mAbs to block interference of Fc receptors.
Costimulation of T cell responses.
Purified human CD4+ T cells at 2 × 105 cells/well in triplicate were cultured in 96-well flat-bottomed plates that were precoated overnight with anti-CD3 mAb (HIT3a; BD-PharMingen) in the presence of B7-H1 mAbs, PD-1Ig, or control Ab (mouse IgG1). To detect cytokines, supernatants were harvested at 24, 48, and 72 hours of the cultures, and the concentrations of IL-10 were determined by sandwich ELISA methods (BD-PharMingen), according to manufacturer’s instructions. Anti-CD28 mAb (CD28.2; BD-PharMingen) was included as positive control. For blocking the effects of B7-H1 mAb, soluble B7-H1Ig or control Ig (mIgG2a) was precultured with coated B7-H1 mAb for 30 minutes before the addition of CD4+ T cells. T cell proliferation was determined by the addition of 1.0 μCi 3H-TdR for the final 16 hours of culture. The incorporation of 3H-TdR was counted by a MicroBeta TriLux liquid scintillation counter (Wallac, Turku, Finland).
Induction and analysis of apoptosis.
Purified human CD4+ T cells (4 × 105/ml) were cultured with B7-H1 mAb’s or a control mAb at 10 μg/ml in the presence of immobilized anti-CD3 mAb (500 ng/ml). At each time point, aliquots (105) of the cells were stained by FITC-conjugated annexin V (AV) (BD PharMingen) at 5 μl/test and propidium iodide (PI) (Sigma-Aldrich) at 5 μg/ml for 1 hour. The samples were analyzed by FACS. For blocking the effect of apoptosis, the neutralizing mAb to IL-10 (R&D Systems Inc., Minneapolis, Minnesota, USA), IL-2 (MQ-17H12; BD-PharMingen), or to Fas ligand (NOK-1; BD-PharMingen) were added at 10 μg/ml at the beginning of culture.
Detection of apoptosis-related genes.
Total RNA was prepared using TRI Reagent (Sigma-Aldrich) from 5 × 106 T cells, which were stimulated by anti-CD3/B7-H1 mAb or anti-CD3/control Ab for 24, 48, 72 hours. RNA (10 μg) was used as a template for 32P cDNA probe synthesis. Human apoptosis1 GEArray (SuperArray Inc. Bethesda, Maryland, USA) is composed of 23 apoptosis-related genes and two housekeeping genes, actin and GAPDH. Analysis of gene expression was carried out by side-by-side hybridization with the cDNA probe according to the user manual. A STORM PhosphoImager system (Molecular Dynamics, Sunnyvale, California, USA) was used to directly quantify the intensity of the signals. The relative abundance of a particular transcript was estimated by comparing its signal intensity to the signal derived from β-actin or GAPDH. The results represent a fold increase of signals from T cells that were stimulated with B7-H1 mAb versus control Ab.
Detection of active caspase-3.
The CaspaTagTM Caspas-3 (DEVD) Activity kit (Intergen Co., Purchase, New York, USA) was used to detect the activated form of caspase-3 in CD4+ T cells. The kit detects active caspases in living cells by using a carboxyfluorescein-labeled caspase inhibitor (FAM-DEVD-FMK). The inhibitor irreversibly binds to active caspases, and the caspase-positive cells are detected by flow cytometry (see the manufacturer’s manual). Briefly, 300 μl of 106 cells/ml was transferred to a fresh tube and incubated with 10 μl of FAM-DEVD-FMK solution diluted 30 times for 1 hour at 37°C under 5% CO2 in the dark. After incubation, the cells were washed twice with 2 ml of 1× wash buffer and then were resuspended in 400 μl of 1× wash buffer before analysis with FACS.
Results
Costimulatory B7-H1 autoantibodies in RA serum.
In an attempt to evaluate the potential role of autoantibodies in prolonged activation of T cells in RA, purified IgG from the sera of RA patients were evaluated for their ability to regulate the proliferation of T cells in vitro. In the presence of suboptimal doses (30–50 ng/ml) of anti-CD3 mAb to mimic TCR signaling, purified IgG from the sera of two RA patients, but not control IgG, significantly enhanced the proliferation of purified CD4+ T cells in vitro in a dose-dependent fashion (Figure 1a). In the absence of anti-CD3 mAb, purified IgG from RA patients had no effect (data not shown). The soluble form of these autoantibodies did not have any activity in the same assay (Figure 1b). The costimulatory activity of the autoantibodies was completely blocked by inclusion of soluble B7-H1Ig fusion protein containing human B7-H1 extracellular portion and mouse IgG2a Fc (14), but not by PD-1Ig or control IgG (mouse IgG2a) (Figure 1c). Our results thus suggest that the costimulation activity for CD4+ T cell was mediated by autoantibodies against B7-H1 in the sera of RA, rather than by the soluble form of B7-H1 per se or autoantibodies to PD-1.
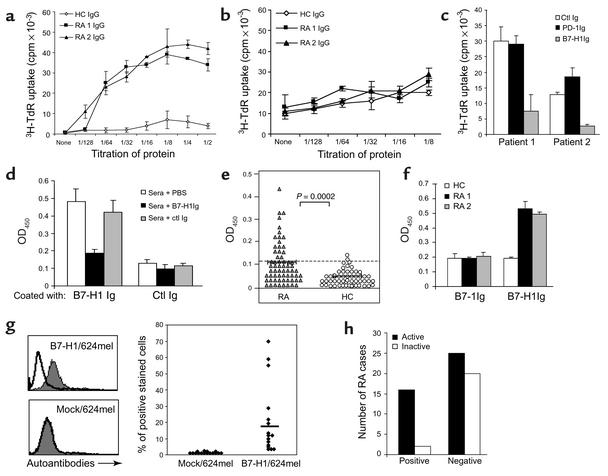
Figure 1.
Detection, costimulatory function, and disease association of B7-H1 autoantibodies in RA patients. CD4+ T cells were cultured with immobilized (a) or soluble (b) sera IgG at the initiated dose of 20 μg/ml in the presence of suboptimal anti-CD3 mAb. The titers of RA 1 and RA 2 patients are 0.32 and 0.25 (OD450), respectively. (c) For blockade, the control (ctl) Ig, PD-1Ig, or B7-H1Ig at 3 μg/ml were added before the addition of CD4+ T cells. The growth of T cells was detected after 72 hours of culture. (d) To examine specificity of RA sera binding to B7-H1, diluted sera were preincubated with PBS, 2 μg/ml of soluble B7-H1Ig or control Ig (mIgG2a). (e) Diluted sera of 63 patients with RA and 54 healthy donors were tested for binding to B7-H1Ig by ELISA. Samples with OD450 values greater than 0.123 were considered positive. P value for differences between cohorts is shown (t test). (f) The sera at 1:1,000 dilution were examined for binding to B7-1Ig–coated vs. B7-H1Ig–coated ELISA plates. (g) Mock/624mel or B7-H1/624mel cells were stained with diluted (1:5) sera from 16 ELISA-positive RA patients. The inset bar shows the average percentage of positive staining. A typical histogram of FACS assay is shown on the left. (h) Sixty-three RA patients were sorted according to the active status of their disease, and the presence of B7-H1 autoantibodies. Statistical analysis of the correlation was performed as P = 0.0179 in a Fisher exact test.
To directly demonstrate the existence of autoantibodies against B7-H1 in the sera of RA patients, sera from 63 patients with RA were examined by a specific sandwich ELISA using plates coated with purified B7-H1Ig. Autoantibodies binding to B7-H1 were detected by anti-human IgG mAb. Our ELISA is highly specific for human B7-H1 because binding of patients’ sera could be selectively blocked by preincubation of sera with soluble B7-H1Ig, but not control mIgG2a (Figure 1d). We used 0.123 OD450 as a cut-off based on the mean (0.057) + 2 SD (0.033) of the values with sera from 54 healthy donors at 1:1,000 dilutions. As shown in Figure 1e, sera from 18 of 63 sera from patients with RA (29%) had elevated autoantibodies to B7-H1, while only 4% of 54 healthy donors were marginally positive for the presence of autoantibodies to B7-H1 (P = 0.0002). Similar to the findings of Matsui et al. (13), we did not detect any autoantibodies to B7-1, even in the sera that were positive for B7-H1 Ab’s (Figure 1f). The presence of B7-H1 autoantibodies was also tested by binding of 624 melanoma line (624mel) that was transfected to express human B7-H1 (B7-H1/624mel) (16). A significant portion of sera from 16 patients, which were positive in ELISA assay, also stained for B7-H1/624mel but not Mock/624mel. The specificity of the binding was also confirmed by complete blockade with B7-H1Ig, which was preincubated with the diluted sera (data not shown). In addition, all the samples that were negative in ELISA for the presence of autoantibodies to B7-H1 did not bind B7-H1/624mel (data not shown). Our results indicate that a significant population of patients suffering from RA has elevated autoantibodies to B7-H1.
Correlation of B7-H1 autoantibodies with RA activity.
As a preliminary functional determinant, we examined the possible relationship between the presence of B7-H1 autoantibodies and disease activity. Active disease was defined as the presence of at least nine tender joints, six swollen joints, and one or both of the following: 1 hour of morning stiffness or elevated Westergren sedimentation rate (19). We found a significant correlation between active disease and the presence of autoantibodies in 63 patients with RA. Eighty-nine percent of RA patients in the B7-H1 autoantibody-positive group had active disease, while only 56% of RA patients in B7-H1 autoantibody-negative group demonstrated disease activity (P = 0.017) (Figure 1h). These results suggest that autoantibodies to B7-H1 may be involved in disease progression by a direct effect on T cells.
Reverse costimulation of CD4+ T cells by B7-H1 mAb.
Autoantibodies from sera are polyvalent with multiple different specificities, limiting the potential for functional analysis. To facilitate further study of the effect of autoantibodies to B7-H1 on T cell response, we generated hybridomas that secrete mAb against human B7-H1. We identified two mAbs, 2H1 and 5H1, which specifically bound to B7-H1 on 293 cells transfected with human B7-H1 plasmid (B7-H1/293), but not mock-transfected 293 cells (Mock/293) by FACS analysis (Figure 2a). In these and later studies, 5H1 and 2H1 showed identical features and were used interchangeably.
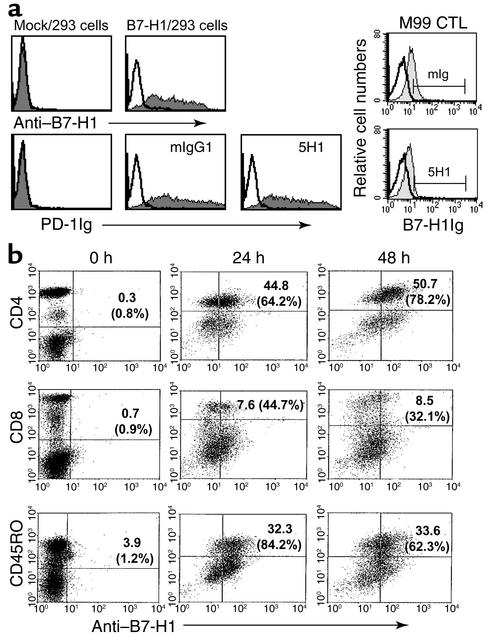
Figure 2.
Preferential expression of B7-H1 mAb on activated CD4+ T cells. (a) On the left, 293 cells were transfected with mock (pcDNA3 vector) or human B7-H1 plasmid (pcDNA3-B7-H1cDNA) for 48 hours. B7-H1/293 cells were pretreated with 20 μg of control Ig (mIgG1) or 5H1 before staining with PD-1Ig (5 μg). On the right, activated M99 CTL cells were pretreated with 10 μg of mIgG1 or 5H1 before staining with B7-H1Ig (10 μg). B7-H1 mAb, PD-1Ig, or B7-H1Ig were used to stain the transfected 293 cells and activated M99 CTL cells. Representative fluorescence histograms of isotype control reagents (open lines) and B7-H1 mAb or fusion proteins (filled lines) are shown. (b) Induction of B7-H1 expression on human T cell subsets. Human PBMCs were activated with PHA for indicated times and subjected to FACS analysis with B7-H1 mAb and mAb to CD4, CD8, or CD45RO. The numbers indicate the percentage of B7-H1 and CD4, CD8, or CD45RO double-positive cells in total populations, and the percentage in parentheses indicates the percentage of B7-H1–positive cells in each CD4+, CD8+, or CD45RO+ subsets.
To determine whether or not our mAb can block interaction between B7-H1 and PD-1, we examine the ability of 5H1 mAb to block the binding of PD-1Ig to B7-H1/293 cells. As shown in Figure 2a, PD-1Ig bound B7-H1/293 cells but not mock/293 cells. Inclusion of 5H1 mAb up to 20 μg/ml during staining did not interfere with the binding of PD-1Ig. Inclusion of 5H1 mAb, however, could inhibit the binding of B7-H1Ig to M99 T cell clone from 31% to 8% (Figure 2a), suggesting that 5H1 could partially block the binding of B7-H1 to a non–PD-1 receptor (16). Similar blocking function was also found using 2H1 mAb (data not shown).
FACS analysis using B7-H1 mAb showed that B7-H1 is not detectable on freshly isolated PBMC subsets with CD4, CD8, or CD45RO markers. However, stimulation by phytohemagglutinin (PHA), a T cell mitogen, rapidly upregulated expression of B7-H1 on 64.2% of CD4+ cells at 24 hours and 78.2% at 48 hours. Meanwhile, only 44.7% and 32.1% of CD8+ T cells expressed B7-H1 at 24 hours and 48 hours after PHA stimulation, respectively. High levels of B7-H1 (84.2% at 24 hours and 62.3% at 48 hours) were also detected on CD45RO+ memory T cells (Figure 2b). We have also found that stimulation of CD4+ T cells with immobilized CD3 mAb in optimal doses rapidly upregulate the expression of B7-H1 within 24 hours, while suboptimal doses of CD3 mAb requires more than 48 hours to induce B7-H1 expression (data not shown). We conclude that B7-H1 is inducible on human T cells and is preferentially expressed on activated CD4+T cells and CD45RO+ memory T cells.
To evaluate the function of CD4+ T cell–associated B7-H1, we stimulated purified human CD4+ T cells with suboptimal concentrations (30 ng/ml) of anti-CD3 mAb in combination with B7-H1 mAb. While anti-CD3 mAb alone induced an absent or weak T cell response, significant increases in T cell proliferation were observed by inclusion of B7-H1 mAb. This effect, however, was less potent than that of anti-CD28 mAb (Figure 3a). Costimulatory activity was also observed using immobilized human PD-1Ig fusion protein, suggesting an agonistic effect of B7-H1 mAb. The effect of the B7-H1 mAbs was dose dependent in a range of 2.5 to 10 μg/ml (Figure 3b). Immobilization of B7-H1 mAb was critical for the effect since B7-H1 mAb in soluble form in doses up to 20 μg/ml were ineffective (Figure 3c). TCR signaling was also required for proliferation, because B7-H1 mAb did not stimulate T cell proliferation in the absence of anti-CD3 mAb (Figure 3a). Inclusion of soluble B7-H1Ig, which competitively inhibits the interaction between T cell–associated B7-H1 and B7-H1 mAb, significantly reduced the costimulatory effect of B7-H1 mAb on T cells. In contrast, soluble B7-1Ig or control Ig had no inhibitory effect (Figure 3d), confirming the specificity of the response.
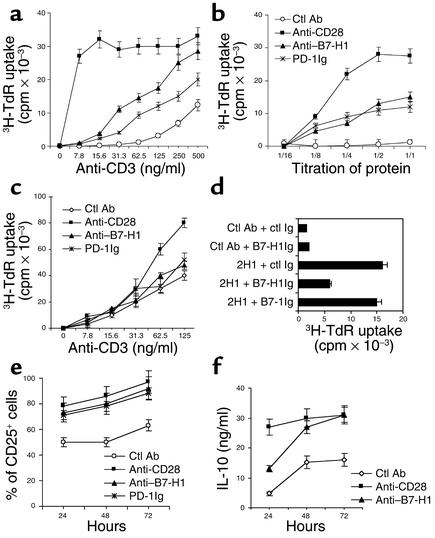
Figure 3.
B7-H1 mAb costimulates human CD4+ T cell proliferation. (a) Purified human CD4+ T cells were cultured with immobilized 10 μg/ml of control (ctl) Ab, B7-H1 mAb (2H1), PD-1Ig, and 2 μg/ml of CD28 mAb in the presence of precoated different dose of CD3 mAb. Cultures were pulsed with 3H-TdR for a final 16 hours, and the cells were harvested at 72 hours. (b) Dose-dependent costimulation of immobilized anti–B7-H1 mAb in the presence of suboptimal dose (30 ng/ml) of CD3 mAb. Titration of mAb or fusion protein was started at 20 μg/ml of control Ab, B7-H1 mAb, PD-1Ig, and 4 μg/ml of CD28 mAb. (c) Human CD4+ T cells were costimulated with 20 μg/ml of soluble control (ctl) Ab, B7-H1 mAb (2H1), PD-1Ig, and 2 μg/ml of CD28 mAb as the same condition in a. (d) Blocking of B7-H1 mAb–mediated costimulation by soluble B7-H1Ig. Soluble control Ig, B7-1Ig, or B7-H1Ig (10 μg/ml) was preincubated with immobilized control (ctl) Ab or B7-H1 mAb (2H1) for 30 minutes before the addition of T cells. B7-H1 costimulation was assayed in the presence of suboptimal dose of CD3 mAb for 72 hours of culture. (e) FACS analysis of IL-2 receptor (CD25) expression in CD4+ T cells after B7-H1 mAb costimulation. (f) IL-10 secretion from CD4+ T cells in the presence of immobilized CD3 mAb (500 ng/ml), and B7-H1 mAb (10 μg/ml), or CD28 mAb (2 μg/ml).
The costimulatory functions of B7-H1 mAb are very similar to the autoantibodies isolated from RA patients, which induced both phenotypic T cell changes and distinct patterns of cytokine secretion. Specifically, B7-H1 mAb induced high-levels of CD25 expression on CD4+ T cells, an effect similar to that using anti-CD3/CD28 mAb (Figure 3e). Additionally, anti-CD3/B7-H1 mAb costimulation led to increased secretion of IL-10 (Figure 3f). A small increase of IFN-γ secretion was also observed in culture supernatant, while IL-2 and IL-4 were not detected (data not shown). Taken together, our results suggest a reverse-signaling function of B7-H1 for CD4+ T cell costimulation.
Promoting apoptosis of activated CD4+ T cells by B7-H1 mAb.
We have shown that one role of B7-H1 mAb is to promote proliferation of CD4+ T cells. This suggests that B7-H1 autoantibodies in RA patients may contribute to the persistent activation of newly recruited T cells when they encounter self antigens. In RA patients, however, many CD4+ T cells are already mature/activated. The effect of B7-H1 triggering by Ab’s on activated T cells may be different from those observed on primary T cells. To examine the effect of B7-H1 mAb on activated CD4+ T cells, we employed an in vitro culture system in which optimal doses of anti-CD3 mAb can drive T cell proliferation without additional costimulation. In this setting, B7-H1 mAb significantly increased apoptosis of CD4+ T cells, as determined by double staining of PI and AV (Figure 4a). The cell death induced by B7-H1 mAb was completely abrogated by preincubation of the mAb with B7-H1Ig, but not with control Ig (Figure 4b). Similarly, immobilized PD-1Ig also increased apoptosis of activated T cells. Neither soluble nor immobilized mAb to B7-H1 or PD-1Ig alone had an effect (data not shown). In a different setting, CD4+ T cells were preactivated by optimal doses of anti-CD3 mAb for 48 hours, in which all CD4+ T cells express high levels of B7-H1, and further stimulated with B7-H1 mAb. Similarly, preactivated T cells also had increased apoptosis after exposure to a coligation of immobilized anti-CD3/B7-H1 mAb, but not to ligation of B7-H1 mAb alone (data not shown). Our results suggest that B7-H1 mAb in the presence of strong TCR signaling promotes apoptosis of activated CD4+ T cells.
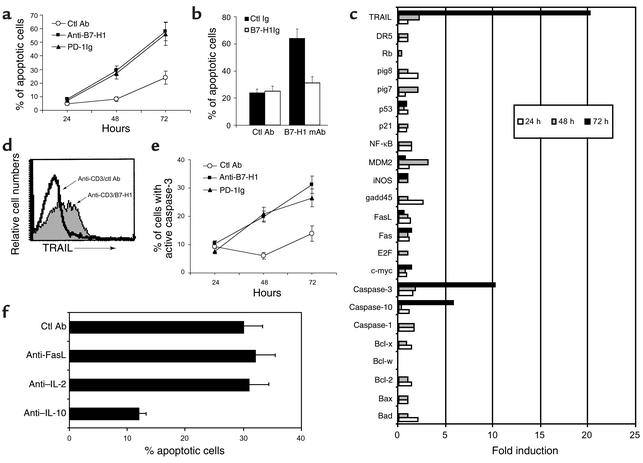
Figure 4.
B7-H1 mAb promotes programmed cell death of activated CD4+ T cells. (a) Human CD4+ T cells were cultured with 10 μg/ml of immobilized control (ctl) Ab, B7-H1 mAb, PD-1Ig in the presence of precoated optimal dose (500 ng/ml) of CD3 mAb. The cells were analyzed by FACS to determine apoptotic cells (positive in AV and negative in PI staining). (b) Blocking of B7-H1 mAb-induced apoptosis by soluble B7-H1Ig. Control Ig or B7-H1Ig at 10 μg/ml was preincubated with immobilized control Ab or B7-H1 mAb for 30 minutes before the addition of T cells. Percentages of apoptotic CD4+ T cells were shown at 72 hours of culture. (c) Expression of apoptotic genes by B7-H1 mAb costimulation. Purified human CD4+ T cells (5 × 106) were cultured with immobilized B7-H1 mAb or control mAb at 10 μg/ml in the presence of optimal dose of CD3 mAb. The mRNA levels of each gene from B7-H1 mAb-stimulated T cells were presented as the fold induction, relevant to that from control mAb-treated T cells. (d) FACS analysis of expression of TRAIL protein on CD4+ T cells 3 days after anti-CD3/B7-H1 mAb costimulation. (e) Purified human CD4+ T cells were stimulated in the presence of indicated mAb or fusion protein in immobilized form as indicated in the legend of a and the activated form of caspase-3 at the indicated time point was stained by FAM-DEVD-FMK and subjected to FACS analysis. (f) Blocking of B7-H1 mAb-induced apoptosis of activated CD4+ T cells by anti–IL-10 neutralizing mAb. Purified human CD4+ T cells were stimulated with immobilized CD3 mAb and B7-H1 mAb for 72 hours, and the apoptotic cells were analyzed by AV and PI staining. Control Ab and mAbs to Fas ligand, IL-2, or IL-10 at 10 μg/ml was included at the beginning of the culture.
To define the mechanism of the apoptotic effect, we used DNA arrays for expression analysis of apoptosis-related genes stimulated by B7-H1 mAb. Up to 72 hours after ligation with anti-CD3/B7-H1 mAb, mRNA from CD4+ T cells was extracted and hybridized to a DNA array membrane. In three separate experiments, transcription of caspase-10 and caspase-3 genes was reproducibly increased. TRAIL was also upregulated (Figure 4c). Enhanced gene expression was also confirmed by protein analysis. Specifically, elevated TRAIL was found in anti-CD3/B7-H1 mAb–stimulated T cells by staining with anti-TRAIL Ab in FACS analysis (Figure 4d). Significant increases of active caspase-3 were also detected at 48 and 72 hours after stimulation (Figure 4e). Neither B7-H1 mAb nor anti-CD3 (at suboptimal doses) alone stimulated these changes. Anti-CD3 at high dose (1 μg/ml) could induce the activation of caspase 3 but not the expression of TRAIL in activated CD4+ T cells (data not shown). These observations emphasize dependence of a TCR signal in the effect of B7-H1 mAb. Our results suggest that B7-H1 mAb upregulates caspases and TRAIL on T cells, which may facilitate activation-induced death of CD4+ T cells.
IL-10 is a potent immunosuppressive cytokine that stimulates Th2 CD4+ T cell responses and enhances the apoptosis of activated human T cells (20–23). B7-H1 mAb stimulated secretion of IL-10 from activated CD4+ T cells (Figure 3f), providing presumptive evidence that IL-10 might play a role in the increased apoptosis induced by B7-H1 mAb. To test this hypothesis, we examined whether neutralization of IL-10 in anti-CD3/B7-H1 mAb inhibited apoptosis. As shown in Figure 4f, inclusion of anti–IL-10 mAb significantly reduced the amount of apoptosis induced by anti-CD3/B7-H1 mAb at 72 hours. In contrast, neutralizing Ab’s against Fas ligand and IL-2 had no effect. Our results suggest that IL-10 facilitates, at least in part, the induction of apoptosis by B7-H1 mAb ligation.
Discussion
Autoantibodies to B7-H1 are found in the sera of a significant portion of the RA patients, and the existence of the autoantibodies is correlated with active status of the disease. This finding suggests a role of B7-H1 autoantibodies in the progression of RA. Further studies indicate that human CD4+ T cells preferentially express B7-H1 and engagement of T cell–associated B7-H1 by Ab costimulate activation, proliferation, cytokine secretion, and programmed cell death. Our results thus support a role of T cell–associated B7-H1 in reverse signaling of CD4+ T cells, and increased B7-H1 autoantibodies may play a role in aberrant activation of T cells in RA and possibly other systemic autoimmune diseases.
Correlation between the existence of elevated autoantibodies in RA patients and active disease provides an important clue to consider regarding the potential role of B7-H1 autoantibodies in the progression of diseases. It is unknown, however, whether or not B7-H1 autoantibodies are also quantitatively correlated with active diseases due to the semiquantitative nature of ELISA and subjective diagnostic standard for active diseases. Our in vitro studies, however, suggest an important contribution of B7-H1 autoantibodies in aberrant T cell responses, which is often observed in RA patients, by costimulating the resting T cells and inducing apoptosis of preactivated T cells. It is attempting to speculate that CD4+ T cells in RA patients will have a higher turnover rate and shorter life of T cells in the presence of B7-H1 autoantibodies. One should keep in mind that our results could represent a gross underestimate of the importance of B7-H1 autoantibodies in the pathogenesis of RA. Specifically, nearly all of the therapeutic agents (e.g., methotrexate) employed have regulatory effects on the immune system and T cell biology, making correlation of clinical markers of disease and B7-H1 expression very difficult (24). Importantly, definition of the effects of individual agents on B7-H1 expression may facilitate correlation of autoantibody detection with both diagnosis and disease activity.
Although our results support that engagement of T cell–associated B7-H1 transmits functional signaling, the nature of this signaling is unknown. The cytoplasmic domain of B7-H1 does not contain obvious structural elements that are directly relevant to signaling for cell activation or death (25, 26). Therefore, adapter proteins might play a role in transmitting B7-H1 signal to T cells. It is possible that multiple signaling pathways might be involved, since B7-H1 signaling leads to diverse outcomes including costimulation of T cell growth, secretion of IL-10, upregulation of caspases, and TRAIL leading to increased apoptosis of activated T cells. In addition to autoantibodies, binding of B7-H1 by PD-1 receptor may also play a role in the B7-H1 signaling. PD-1 is a CTLA-4–like molecule expressed on activated T cells and has been implicated as a receptor for B7-H1 (15). PD-1–deficient mice spontaneously develop systemic lupus-like autoimmune diseases characterized by immune complex deposits in the renal parenchyma of aged B6 mice (27). The in vitro effects of B7-H1 autoantibody ligation were analogous to those observed with immobilized PD-1I g (Figure 3), suggesting a possible role of PD-1 as a ligand to trigger T cell–associated B7-H1 in aberrant CD4+ T cell activation in RA patients. Further studies are needed to clarify these issues.
Our results reveal that activated T cells could simultaneously express B7-H1, PD-1, and the putative second B7-H1 receptor. Therefore, B7-H1 Ab’s can potentially affect the interactions among these molecules. While our results strongly support that B7-H1 autoantibodies directly deliver an activation signal to T cells by cross-linking B7-H1, it is also possible that B7-H1 Ab’s may block or “sequestrate” B7-H1 from interacting with PD-1, leading to a decreased negative signaling for T cell response. Several lines of evidence, however, do not support this hypothesis in our system. We demonstrate that only immobilized B7-H1 Ab’s could induce reverse costimulation while soluble Ab’s did not (Figure 3c). This result emphasizes the importance of cross-linking rather than neutralizing Ab’s in the activation of T cells. Furthermore, our 5H1 mAb could not block the interaction between B7-H1 and PD-1, whereas it can partially inhibit binding of B7-H1 to a non–PD-1 receptor on M99 T cells (Figure 2a). This result suggests that blocking or “sequestration” of B7-H1 by Ab’s in our experimental condition would still allow B7-H1 to interact with PD-1. Consistent with these findings, reverse costimulation by B7-H1 autoantibodies could not be blocked by PD-1Ig (Figure 1b), a result similar to our previous finding that PD-1Ig also did not block the effect of anti-CD3 mAb in the stimulation of T cell proliferation and cytokine production (16). Taken together, our results suggest that PD-1 is not involved in the suppression of T cell responses in our systems.
The prevalence of autoantibodies to B7-H1 in the sera of RA patients raised the question as to why tolerance to B7-H1 is broken. Actually, we did not detect any B7-H1 expression on the freshly isolated T cells from ten RA patients (unpublished observation). This may be caused by transient nature of B7-H1 expression and by the anti-inflammation treatment of these patients. Previously, we have reported that the mRNA of B7-H1 can be detected in various organs, including heart, lung, liver, placenta, and spleen in healthy individuals. However, immunohistochemistry analysis using B7-H1 mAb demonstrates no evidence of protein expression, except macrophage-derived cells, including Kupffer cells in liver and monocytes in blood (16). Expression of B7-H1, however, can be induced in cells such as activated T cells (14, 18). It is thus possible that in autoimmune diseases, inflammatory tissues and cells may lead to ectopic expression of B7-H1, which might serve as an antigen source for elicitation of autoantibodies. Recently we have found similar increases of B7-H1 autoantibodies in patients with systemic lupus erythematosus and autoimmune inner ear disease (unpublished data). Defining the roles of B7-H1 and gaining a better understanding of the molecular pathways elicited after B7-H1 ligation will facilitate the development of novel therapies for the treatment of autoimmune disease.
Acknowledgments
This study was supported by the Mayo Foundation. We thank Jorg J. Goronzy and K. Tamada for helpful discussion, Stephen S. Cha for statistical analysis, Jane Jaquith and Lucinda Hinkley for their work as study coordinators, and Kathy Jensen and Julie Lau for editing the manuscript and graphs.
Footnotes
Haidong Dong and Scott E. Strome contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: rheumatoid arthritis (RA); T cell receptor (TCR); programmed death 1 (PD-1); phycoerythrin (PE); TNF-related apoptosis–inducing ligand (TRAIL); annexin V (AV); propidium iodide (PI); phytohemagglutinin (PHA).
References
- 1.Palmer DG. The anatomy of the rheumatoid lesion. Br. Med. Bull. 1995;51:286–295. doi: 10.1093/oxfordjournals.bmb.a072961. [DOI] [PubMed] [Google Scholar]
- 2.Salmon M, Gaston JS. The role of T-lymphocytes in rheumatoid arthritis. Br. Med. Bull. 1995;51:332–345. doi: 10.1093/oxfordjournals.bmb.a072964. [DOI] [PubMed] [Google Scholar]
- 3.Hemler ME, Glass D, Coblyn JS, Jacobson JG. Very late activation antigens on rheumatoid synovial fluid T lymphocytes. Association with stages of T cell activation. J. Clin. Invest. 1986;78:696–702. doi: 10.1172/JCI112629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 5.Torres BA, Johnson HM. Modulation of disease by superantigens. Curr. Opin. Immunol. 1998;10:465–470. doi: 10.1016/s0952-7915(98)80122-2. [DOI] [PubMed] [Google Scholar]
- 6.Koetz K, et al. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 8.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr. Opin. Immunol. 1997;9:369–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 9.Ye Z, et al. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat. Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 10.Chen L. Antibody gene therapy: old wine in a new bottle. Nat. Med. 2002;8:333–334. doi: 10.1038/nm0402-333. [DOI] [PubMed] [Google Scholar]
- 11.Hazenbos WLW, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 12.Ioan-Facsinay A, et al. FcγRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T, et al. Autoantibodies to T cell costimulatory molecules in systemic autoimmune diseases. J. Immunol. 1999;162:4328–4335. [PubMed] [Google Scholar]
- 14.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 17.Jeannin P, et al. CD86 (B7-2) on human B cells. A functional role in proliferation and selective differentiation into IgE- and IgG4-producing cells. J. Bio. Chem. 1997;272:15613–15619. doi: 10.1074/jbc.272.25.15613. [DOI] [PubMed] [Google Scholar]
- 18.Tamura H, et al. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809–1816. doi: 10.1182/blood.v97.6.1809. [DOI] [PubMed] [Google Scholar]
- 19.Felson DT, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–735. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 20.Georgescu L, Vakkalanka RK, Elkon KB, Crow MK. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J. Clin. Invest. 1997;100:2622–2633. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito I, et al. Fas ligand-mediated exocrinopathy resembling Sjogren’s syndrome in mice transgenic for IL-10. J. Immunol. 1999;162:2488–2494. [PubMed] [Google Scholar]
- 22.Mignon-Godefroy K, Rott O, Brazillet MP, Charreire J. Curative and protective effects of IL-10 in experimental autoimmune thyroiditis (EAT). Evidence for IL-10-enhanced cell death in EAT. J. Immunol. 1995;154:6634–6643. [PubMed] [Google Scholar]
- 23.Clerici M, et al. Type 1/type 2 cytokine modulation of T-cell programmed cell death as a model for human immunodeficiency virus pathogenesis. Proc. Natl. Acad. Sci. USA. 1994;91:11811–11815. doi: 10.1073/pnas.91.25.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genestier L, et al. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J. Clin. Invest. 1998;102:322–328. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 26.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat. Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]