Abstract
Dimeric Fc receptor (FcR) nonbinding anti-CD3 antibodies have been developed to minimize toxicities associated with classical anti-CD3 monoclonal antibodies (e.g., OKT3). Studies with murine analogs of non-FcR–binding antibodies have shown reduced mitogenicity compared to OKT3. In a trial of an FcR nonbinding humanized anti-CD3 mAb hOKT3γ1(Ala-Ala) for treatment of patients with type 1 diabetes, we found significant increases in IL-10 and IL-5 in the serum of 63% and 72% of patients, respectively, and TNF-α and IL-6 levels that were lower than those previously reported following OKT3 therapy. The activation signal delivered by hOKT3γ1(Ala-Ala) was associated with calcium signaling and cytokine production by previously activated human cells in vitro. However, the production of IL-10, compared to IFN-γ on a molar basis, was greater after culture with hOKT3γ1(Ala-Ala) than with OKT3. Flow cytometric studies confirmed that OKT3 induced IFN-γ and IL-10 production, but hOKT3γ1(Ala-Ala) induced only detectable IL-10 production in CD45RO+ cells. Moreover, in vivo, we found IL-10+CD4+ T cells after drug treatment. These cells were heterogeneous but generally CD45RO+, CTLA-4–, and expressed CCR4. A subgroup of these cells expressed TGF-β. Thus, the non-FcR binding anti-CD3 mAb, hOKT3γ1(Ala-Ala) delivers an activation signal to T cells that is quantitatively and qualitatively different from OKT3. It leads to the generation of T cells that might inhibit the autoimmune response and may be involved in the beneficial effect on β cell destruction in Type 1 diabetes.
Introduction
Most immune suppressive agents prevent T cell responses by depletion or inactivation of T cells. For example, glucocorticoids and the calcineurin in-hibitors, such as cyclosporine A and FK-506, block cytokine gene transcription, preventing the production of T cell growth factors, whereas other agents, such as Campath 1H, cause prolonged depletion of T cells (1–4). While these approaches are very effective in the short term, these effects are not antigen specific and may not persist after the drugs are discontinued. Hence, true immunologic tolerance, in which an immune response does not occur after an immunologic agent is withdrawn, is rarely achieved. In experimental systems, immunologic tolerance requires additional approaches, such as modification of effector cell pool size, T cell stimulatory signals, and importantly, immune regulation (5–8).
The mAb against the CD3ε molecule, in contrast, has induced tolerance to autoimmunity in murine models of type 1 diabetes mellitus. Treatment with anti-CD3 mAb reversed diabetes in the NOD mouse and prevented recurrent immune responses toward transplanted syngeneic islets (9–11). This was achieved without the need for continuous immune suppression and persisted at a time when T cells were quantitatively normal. The mechanism underlying this tolerance is not known but has been postulated to involve induction of regulatory cells (12). In the NOD mouse, insulitis cleared after mAb treatment, and then recurred, but did not cause tissue damage, indicating an evolution into a nonpathogenic infiltrate. Mice treated with anti-CD3 mAb were resistant to the transfer of disease with diabetogenic splenocytes (11).
Despite these achievements in mice, tolerance with anti-CD3 mAb has not occurred in humans treated with OKT3 together with other immune suppressive agents (13–16). Furthermore, OKT3 has toxicities that render it unsuitable for use in treatment of many patient groups, including those with type 1 diabetes mellitus. Two side effects of importance are the T cell activation that occur in vivo, leading to the cytokine release syndrome, and the development of human anti-murine Ab’s (17–20). The cytokine release syndrome involves cross-linking of the mAb through binding to the Fc receptor (FcR), activation of T cells in vivo, and release of IL-6 and TNF-α. In an effort to eliminate the toxicity and the development of human anti-mouse Ab’s that occur with OKT3, Xu, Zivin, and Bluestone “humanized” the Ig molecule and substituted alanines for leucines at positions 234 and 235 to render the IgG1 molecule FcR nonbinding (21, 22). Initial studies with this drug showed that the mutation resulted in a 3 log-fold reduction in proliferation in vitro compared with OKT3, with decreased production of TNF-α and IL-6. The expression of early activation markers, including Leu23 and IL-2R following culture with drug, were reduced compared with culture with OKT3, while the binding and modulation properties of the anti-CD3 mAb and its ability to block cytotoxic T lymphocyte (CTL) activity were similar to OKT3 (21). In a clinical study, the FcR nonbinding anti-CD3 mAb, hOKT3γ1(Ala-Ala) was shown to reverse renal and renal/pancreas allograft rejection in five of seven patients without clinical signs of T cell activation in vivo, consistent with the notion that the anti-CD3 mAb was “non-activating” (22–24). However, all of these patients were given other immune suppressive agents at the time of drug administration; therefore, the effects of the anti-CD3 mAb on T cell activation could not be determined.
Further studies with FcR nonbinding anti-CD3 mAb’s have suggested that this form of anti-CD3 mAb can inactivate selective subsets of T cells (25, 26). Studies by Smith and Tang et al. indicated that FcR nonbinding anti-CD3 mAb induced anergy of previously activated Th1 cells, but Th2 cells and naive T cells were not inhibited. This effect was fundamentally different from the FcR binding anti-CD3 mAb that induced cytokine release and proliferation of T cells. The mechanism that accounted for the induction of anergy was not clear. It had been thought that the FcR nonbinding anti-CD3 mAb was nonactivating, since calcium signaling and cell proliferation was not observed in vitro. Nonetheless, other studies suggested that a signal is delivered to T cells, is quantitatively different from activating anti-CD3, and resulted in a qualitatively different outcome. Moreover, the relationship between the induction of anergy on cell subsets in vitro and immune regulatory cells in vivo has not been established.
Patients with new onset type 1 diabetes mellitus were administered hOKT3γ1(Ala-Ala) in a Phase I/II trial (27). This was the first reported experience with this anti-CD3 mAb given without other immune suppressive agents, which may have affected the activation of T cells or the ability of the anti-CD3 mAb to induce signals that may lead to toleragenic activity. We observed mild symptoms of T cell activation and an unusual rash suggesting that there had been activation of T cells in vivo. This prompted us to examine the effect of the drug on T cell activation in vivo and in vitro to determine whether the drug may induce T cell signaling and how this signaling may affect immune responses. Our data suggest that treatment with hOKT3γ1(Ala-Ala) delivers a signal to T cells causing release of cytokines that may have a role in immune regulation in type 1 diabetes.
Methods
Treatment of patients with hOKT3 γ1(Ala-Ala).
A total of 20 patients received hOKT3γ1(Ala-Ala) in a randomized control trial of the drug for treatment of new onset type 1 diabetes mellitus. Data on the first 12 subjects have been published (27). The patients all had type 1 diabetes for ≤ 6 weeks before the drug was administered. There were 14 men and 6 women with an average age of 14 ± 1.2 years. The drug dosing was modified after treatment of the first 12 patients to increase the coating and modulation of the CD3 molecule in vivo. The drug was given intravenously at doses as follows: day 1, 1.42 μg/kg; day 2, 5.67 μg/kg; day 3, 11.3 μg/kg; day 4, 22.6 μg/kg; days 5–14, 45.4 μg/kg for patients 1–12. For patients 13–20, the doses were: day 1, 450 μg/m2; day 2, 919 μg/m2, days 3–12, 1,818 μg/m2. Patients were questioned about side effects daily. All patients gave informed consent or assent, if under age 18. This protocol was approved by the Institutional Review Boards at Columbia University, the University of Utah, the University of California, San Francisco, and the National Institute of Diabetes and Digestive and Kidney Diseases.
Measurement of cytokines in the serum of drug-treated patients.
Serum samples were collected before, 1, 2, and 4 hours after the drug was administered on days 1, 2, 5, and 6, for patients 1–12 and days 1, 2, 3, and 4 for patients 13–20. These days corresponded to the first and second initial doses of the drug and the first and second full doses of the drug during the two dosing protocols. Cytokines were measured by ELISA. IL-10, IL-6, TNF-α, IFN-γ, and IL-2 were from Biosource Europe SA (Niuelles, Belgium) and IL-5 was from Immunotech (Marseilles, France). Because of the volume of serum required, measurements could be completed for IL-5 in 18 patients; IFN-γ, IL-6, TNF-α, and IL-2 in 19 patients; and IL-10 in 20 patients. The lower limits of detection of these assays were 20 pg/ml for IL-2, 8.0 pg/ml for IL-5, 5.0 pg/ml for IL-6, 10.0 pg/ml for IL-10, 20 pg/ml for IFN-γ, and 1 pg/ml for TNF-α. There were detectable levels of cytokines in the serum of patients before treatment as follows: IL-2, – 0/18; IL-5, 10 of 18 patients (mean = 22.6 pg/ml, range = 9–70 pg/ml); IL-6, 3 of 18 patients (mean = 20.3 pg/ml, range = 6–26 pg/ml); IL-10, 3 of 18 patients (mean = 37 pg/ml, range = 21–57 pg/ml); IFN-γ, 1 of 18 patients (30 pg/ml); TNF-α, 14 of 18 patients (mean = 36 pg/ml, range = 6–189 pg/ml).
Analysis of cell surface markers by flow cytometry.
PBMCs were isolated from the blood of patients undergoing treatment with hOKT3γ1(Ala-Ala) by Ficoll-Hypaque gradient centrifugation. The cells were stained with mAb’s to CD4, CD8, CD25, CD69, CD62L, CTLA-4, CD45, CD45RO (PharMingen, San Diego, California, USA), and TGF-β (R&D Systems Inc., Minneapolis, Minnesota, USA) in PBS with 1% BSA and 0.1% NaN3, and analyzed on a FACScan or FACScalibur cytometer. Data from a total of 10,000 lymphocytes were collected, and were analyzed with CellQuest software.
Calcium flux studies.
The mobilization of intracellular calcium was studied in previously activated T cells using described techniques (28). To prepare these cells, PBMCs from normal donors were stimulated with phytohemagglutinin (PHA) (2 μg/ml; Sigma-Aldrich, St. Louis, Missouri, USA) in RPMI with 10% FCS, N-[2-hydroxyethyl]piperzine-N-[2-ethanesulfonic acid] (HEPES), glutamine, 2-mercaptoethanol (2ME), and antibiotics for 3 days and then harvested, washed, and placed in culture with rIL-2 (25 U/ml) for 8–10 days. Dead cells and debris were then removed by Ficoll-Hypaque gradient centrifugation, and the cells were suspended in HBSS and loaded with indo-1 (indo-1/acetoxymethlester) and the detergent Pleuronic (Molecular Probes Inc., Eugene, Oregon, USA) in HBSS for 45 min at 37°C. The cells were washed and kept at 4°C until analysis. They were then warmed to 37°C and analyzed on a Mo-Flow cytometer, first in HBSS alone, and then with the addition of anti-CD3 mAb’s, followed by rabbit anti-mouse Ig (RAM) Ab (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA). The fluorescence at 390/20 and 530/20 nm for bound and free probe, respectively, were collected. To calculate the percentage of responding cells, the background staining (with HBSS alone) was subtracted from the staining in the presence of anti-CD3 mAb and RAM Ab.
Stimulation of T cells in vitro.
PBMCs were isolated from normal donors (n = 10) and patients with type 1 diabetes of duration 1–24 years (n = 10) and placed in culture in wells coated with goat anti-mouse Ab (1 μg/ml) at 2 × 106 cells/ml in RPMI with 10% FCS, HEPES, 2ME, penicillin/streptomycin, and glutamine with or without OKT3 (1 μg/ml), or hOKT3γ1(Ala-Ala) (1 μg/ml) with or without soluble anti-CD28 mAb (1 μg/ml). After 72 hours, the culture supernatants were harvested. The concentrations of cytokines in the supernatant fluids were measured with cytometric bead array (CBA) beads by flow cytometry (Becton Dickinson, San Jose, California, USA). The lower limits of detection of cytokines in this assay were IFN-γ, 20 pg/ml; IL-10, 3 pg/ml; and IL-5, 3 pg/ml.
Separation of CD45RO+/– and CD45RO– T cells.
To study the effects of anti-CD3 mAb’s on T cell subsets, T cells were purified from PBMCs by staining with paramagnetic beads (Miltenyi Biotec, Auburn, California, USA) and separation with magnetized columns. The purified T cells were then further separated into CD45RO+ and CD45RO– cells by staining with anti-CD45 paramagnetic beads and a magnetized column (Miltenyi Biotec). The cells were stimulated for 6 hours with anti-CD3 and anti-CD28 mAb’s in the presence of monensin and stained for surface CD3 and CD45RO and intracellular cytokines as described above and below.
Intracellular cytokine staining.
Freshly isolated PBMCs were cultured for 6 hours with OKT3, 1 μg/ml, or hOKT3γ1(Ala-Ala), 1 μg/ml, in the presence of monensin. They were then washed and stained with mAb to cell surface markers CD45RO and CD3. The cells were washed and resuspended in Cytofix/Cytoperm (PharMingen) solution for 20 min at 4°C. Cells were washed twice with Perm/Wash solution and then resuspended in Perm/Wash and stained for intracellular cytokines at 4°C for 45 min. Cells were washed twice with Perm/Wash and analyzed on a FACSCalibur instrument. Electronic gates were placed around the CD3+CD45RO+ or CD3+CD45RO– cells to identify cytokine production within these populations. The percentage of positive cells staining for a cytokine was calculated after subtracting the background staining with an isotype control mAb and dividing by the total number of cells of an identified phenotype.
PBMCs from drug-treated patients were also stained for intracellular cytokines, IFN-γ, and IL-10, and cell surface markers. Frozen PBMCs isolated before or at specified time points after drug treatment were thawed and then cultured for 6 hours in monensin with or without anti-CD28 mAb. The cells were then stained with Ab’s against CD3, CD4, or CD8, CD25, CD45RO, CD62L, TGF-β, permeabilized, stained for intracellular IL-10, IFN-γ (PharMingen), or CTLA-4 and then analyzed on a flow cytometer. To confirm the specificity of staining, IL-10 (0.5 μg/106 cells) was added to replicate tubes during staining for intracellular IL-10. Data were collected on approximately 150,000 lymphocytes. Electronic gates were placed around cells with positive staining (i.e., above background staining with isotype control mAb’s) for CD4, IL-10, or CD45RO.
Data analysis.
Data are presented as mean ± SEM data from replicate experiments or the indicated number of subjects. Continuous and nominal variables were compared with a Mann-Whitney or Fisher exact test, respectively. Cytokine data were compared after log transformation.
Results
Release of cytokines during treatment with hOKT3γ1(Ala-Ala).
Twenty patients with recent onset type 1 diabetes received a 12- (protocol 1) or 14-day (protocol 2) course of the anti-CD3 mAb hOKT3γ1(Ala-Ala). A description of the treatment group and the effects of the drug on clinical disease in the first 12 patients have been published (27). The clinical symptoms that occurred during administration of hOKT3γ1(Ala-Ala) consisted of fever in 62%, rash in 81%, and less commonly, myalgia in 14%, arthralgia in 14%, and headache in 33% of the patients. In comparison with the clinical experience with OKT3 in transplant recipients, the symptoms seen with hOKT3γ1(Ala-Ala) were milder and of brief duration, occurring on the first day the maximal dose was given.
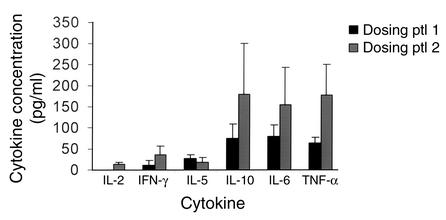
We measured the levels of cytokines in the serum of patients before and after administration of the first two escalating and the first two maximal doses of the drug (Figure 1). The peak levels of cytokines occurred either following the second of the escalating doses of drug or following the first full dose of drug and were, on average, higher with the second dosing protocol. Drug treatment caused release of detectable levels of TNF-γ and IL-6 in 20 of 20 and 16 of 19 (9 of 11 in protocol 1 and 7 of 8 in protocol 2) subjects, respectively. Release of IFN-γ in 5 of 20 (1 of 12 in protocol 1 and 4 of 8 in protocol 2) and IL-2 in 3 of 20 (0 of 12 in protocol 1 and 3 of 8 in protocol 2) were infrequent and seen more often with the second dosing protocol. However, IL-5 and IL-10 levels increased in 16 of 19 (9 of 11 subjects in protocol 1, 7 of 8 subjects in protocol 2), and 13 of 20 (7 of 12 subjects in protocol 1 and 6 of 8 subjects in protocol 2), respectively (P < 0.01 and < 0.04 IL-10 versus IFN-γ or IL-2; P < 0.01 IL-5 versus IFN-γ or IL-2), and were higher than levels of IL-2 and IFN-γ.
Figure 1.
Cytokine levels following administration of hOKT3γ1(Ala-Ala). The levels of cytokines in serum were measured as described in Methods. The average (± SEM) of the highest level of cytokines following drug administration on days 1, 2, 5, and 6 (first 12 patients) or days 1, 2, 3, and 4 (patients 9–20) for each patient in the two dosing protocols (see Methods) are shown. ptl, protocol.
Expression of activation markers on T cells during treatment with hOKT3γ1(Ala-Ala).
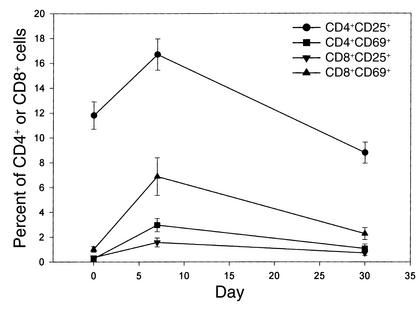
These symptoms and release of cytokines suggested T cell activation in vivo. To determine which cells were affected and whether there was phenotypic evidence for T cell activation, we studied the expression of the activation markers, CD25 and CD69, on peripheral blood T cells (Figure 2). Before treatment, CD25 was expressed on 11.8% ± 1.1% of CD4+ T cells and 0.36% ± 0.1% of CD8+ T cells. CD69 was expressed on 0.26% ± 0.07% of CD4+ and 1.01% ± 0.22% of CD8+ T cells. The expression of CD25 on CD4+ T cells was lower on average than that seen with cells from seven normal control subjects studied in the same assay (19.7% ± 3.42%, P = 0.02), consistent with previous reports of reduced numbers of CD25+CD4+ T cells in patients with new onset type 1 diabetes mellitus (29). During mAb treatment the expression of CD25 increased on the CD4+ cells to 16.7% ± 0.26% (P < 0.002) and on a smaller percentage of the CD8+ cells to 1.57% ± 0.36% (P = 0.0001). The expression of CD69 increased on the CD8+ cells (to 6.88% ± 1.52%, P < 0.0005) and on a smaller percentage of the CD4+ cells (2.97% ±0.53%, P < 0.0001). These activation markers were transiently expressed — by day 30 the percentage of CD4+CD25+ and CD8+CD69+ cells was at or below pretreatment levels (Figure 2).
Figure 2.
Expression of CD25 and CD69 on peripheral T cells in patients receiving hOKT3γ1(Ala-Ala). The percentage of CD4+ and CD8+ cells expressing CD25 and CD69 are shown before, during, and after treatment with hOKT3γ1(Ala-Ala). There was a significant increase in the percentage of CD4+CD25+ (P = 0.0015), CD4+CD69+ (P < 0.0001), CD8+CD25+ (P = 0.0001), and CD8+CD69+ (P < 0.0005) T cells at day 7 (for the first 12 patients) or day 8 (for patients 9–20).
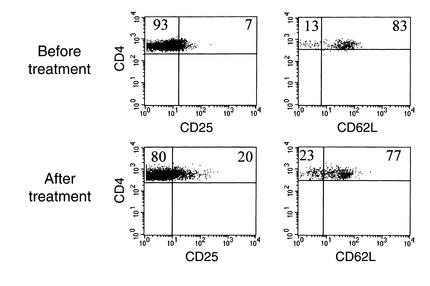
The increase in the proportion of CD4+CD25+ cells may have been due to an expansion of a CD4+CD25+ subpopulation that has been shown to have “regulatory” function, rather than acquisition of CD25 on a previously CD25– population. The former, CD4+CD25+ regulatory cells, can be identified by their expression of high levels of CD62L (L-selectin) (30). However, an increase in this population was not the explanation for the increase in the CD4+CD25+ population, because the expression of CD62L was reduced on the CD25+CD4+ T cells in four of five patients in whom this analysis was done (Figure 3).
Figure 3.
Downmodulation of CD62L on CD4+/–CD25+/– T cells during treatment with hOKT3γ1(Ala-Ala). PBMCs were isolated from patients before and after treatment with hOKT3γ1(Ala-Ala). The cells were stained with mAb’s to CD4, CD25, and CD62L, or isotype controls. Electronic gates were placed around CD4+ lymphocytes, and the percentage of the CD4+ cells staining with CD25 (left panels) or CD62L (right panels) are shown. These data are from a single patient, representative of four of five patients in whom this analysis was performed. The expression of CD62L was reduced on the CD4+CD25+ population of cells.
FcR nonbinding anti-CD3 mAb causes calcium flux and cytokine production in previously activated T cells.
Our observations suggesting that hOKT3γ1(Ala-Ala) induced T cell activation in vivo were unexpected, since in previous clinical studies there were not symptoms of cytokine release. However, in the other trials, the drug had been administered in the presence of other immune-suppressive agents that may have blocked signs and symptoms of T cell activation. Furthermore, our finding of IL-10 and IL-5, but not IFN-γ and IL-2, suggested a pattern of activation different from conventional immunostimulants.
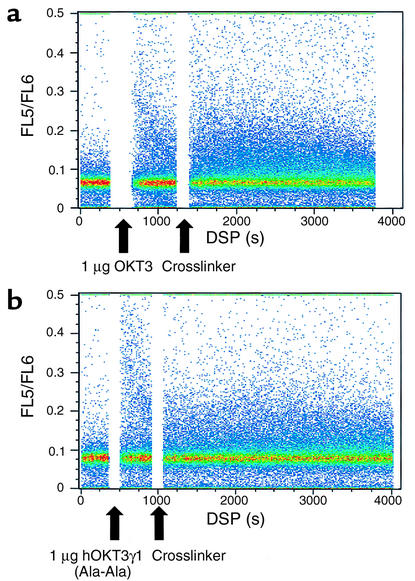
To test whether hOKT3γ1(Ala-Ala) induces a calcium flux, an indication of activation, we compared calcium flux during culture with hOKT3γ1(Ala-Ala) to culture with OKT3 in previously activated T cells (Figure 4). T cells, previously activated with PHA and IL-2, were loaded with the calcium dye indo-1, and calcium release was studied following addition of drug or OKT3 with a cross-linking anti-rabbit Ab by flow cytometry. In four separate experiments, the addition of OKT3, 1 or 10 μg/ml, with rabbit anti-mouse Ab to cross-link the mAb induced calcium flux in an average of 4.4% and 7.7% of the cells. Cells cultured with 10 μg/ml or 1 μg/ml of hOKT3γ1(Ala-Ala) showed calcium flux in 4.8% and 11.1% of the cells, respectively, in the same experiments.
Figure 4.
Density plot of calcium flux in recently activated T cells cultured with OKT3 or hOKT3γ1(Ala-Ala). An aliquot of cells that had been cultured with PHA followed by IL-2 were loaded with indo-1 and placed in culture (at 37°C) in HBSS alone, followed by OKT3 (top) or hOKT3γ1(Ala-Ala) (bottom) (1 μg/ml) with the addition of RAM at the indicated times (in seconds). The ratio of FL5/FL6, indicates intracellular calcium levels in individual cells, where an increased ratio reflects increased intracellular calcium. The addition of either hOKT3γ1(Ala-Ala) or OKT3 followed by cross-linking Ab led to increase in intracellular calcium.
Whereas OKT3 induces both IL-10 and IFN-γ production by CD45RO+/– T cells, hOKT3γ1(Ala-Ala) induces IL-10 production.
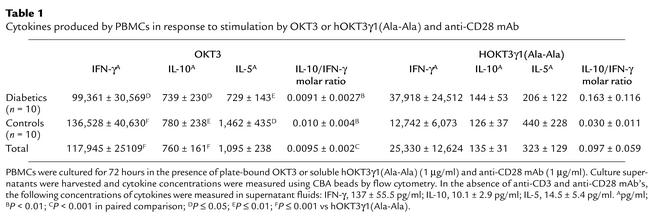
Thus, both forms of anti-CD3 mAb induced calcium flux in T cells. To determine whether this signal caused similar cytokine production, we cul-tured PBMCs from normal individuals and patients with type 1 diabetes, in the presence of OKT3 or hOKT3γ1(Ala-Ala) and anti-CD28 mAb, and measured cytokines released in the supernatants (Table 1). Compared with nondiabetic control subjects, diabetic subjects produced higher levels of IL-5 in response to either anti-CD3 mAb with anti-CD28 mAb, but the differences were not statistically different; the production of other cytokines was similar in diabetic and control subjects (P = 0.2 for both Ab’s). The amount of all cytokines produced in the presence of drug was significantly less than that produced in the presence of OKT3. Despite the reduced overall levels of cytokine production, when compared on a molar basis, there was greater relative production of IL-10 compared with IFN-γ when cells were cultured with the drug and anti-CD28 mAb compared with OKT3 and anti-CD28 mAb (P < 0.001). In other studies (not shown), and similar to previously reported studies, culture of the cells with the hOKT3γ1(Ala-Ala) did not induce cellular proliferation (31, 32).
Table 1.
Cytokines produced by PBMCs in response to stimulation by OKT3 or hOKT3γ1(Ala-Ala) and anti-CD28 mAb
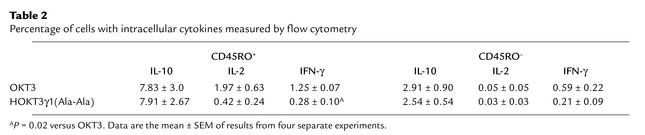
The levels of cytokines in the supernatants reflect both the number of activated cells as well as the amount of cytokine produced by individual cells. Furthermore, previous studies had indicated that the activation state of the cell may affect the response to anti-CD3 mAb. Therefore, to differentiate cytokine production in individual naive and memory T cells, we studied the cytokine production by CD45RO+ and CD45RO– T cells freshly isolated from peripheral blood to OKT3 or hOKT3γ1(Ala-Ala) by flow cytometry (Table 2). Culture of T cells with OKT3 induced IFN-γ in a significantly greater proportion of CD45RO+ T cells than hOKT3γ1(Ala-Ala) (P = 0.02). An equivalent proportion of CD45RO+ cells stained with mAb to IL-10 following culture with the two anti-CD3 mAb’s. There was also a tendency for increased frequency of IL-2–producing cells following incubation with OKT3 as compared with hOKT3γ1(Ala-Ala), but the difference between the two culture conditions was not statistically significant (P = 0.08). Less than 1% of CD45RO– cells produced IFN-γ or IL-2 with either mAb. A smaller proportion of CD45RO– cells produced IL-10 compared with CD45RO+ cells, but the proportion of CD45RO– cells responding to the two mAb’s was similar.
Table 2.
Percentage of cells with intracellular cytokines measured by flow cytometry
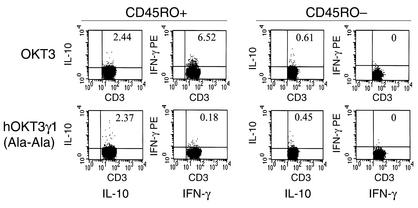
The expression of IFN-γ and IL-10 with OKT3 and the expression of IL-10 without IFN-γ and with hOKT3γ1(Ala-Ala) was confirmed in additional studies with populations of purified CD45RO+ and CD45RO– cells stimulated with anti-CD3 and anti-CD28 mAb’s (Figure 5). In these studies, IFN-γ and IL-10 was induced in CD45RO+ cells cultured with OKT3 and anti-CD28 mAb, whereas only IL-10 was expressed in these cells cultured with hOKT3γ1(Ala-Ala) and anti-CD28 mAb. Less than 1% of CD45RO– cells expressed cytokine with either anti-CD3 mAb.
Figure 5.
Induction of cytokines by anti-CD3 mAb’s. CD45RO+- and CD45RO–-enriched T cells were separated with paramagnetic beads and cultured with either OKT3 (10 μg/ml) or hOKT3γ1(Ala-Ala) (10 μg/ml) with anti-CD28 (1 μg/ml) in wells coated with RAM antibody (10 μg/ml) for 6 hours in the presence of monensin. The cells were then stained with mAb’s to CD45RO and CD3, fixed and permeabilized, and stained for intracellular cytokines as described in Methods, then analyzed by flow cytometry. For analysis, electronic gates were placed around the CD3+CD45RO+ or CD3–CD45RO– subsets.The percentage of gated cells staining for each cytokine are shown in the upper-right corner of each dot plot.
Treatment with hOKT3γ1(Ala-Ala) results in induction of IL-10+CD4+ T cells in vivo.
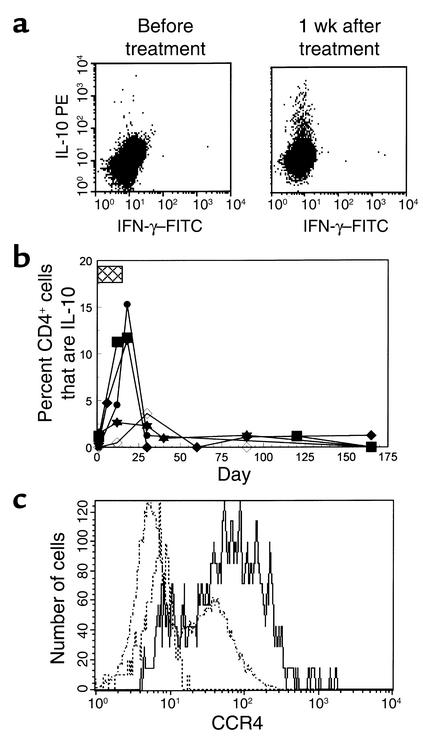
The chronic effects of drug treatment on T cell function may be different from the acute activation events that were characterized by the release of cytokines after the initial doses of the drug. Furthermore, the cytokine release that occurred following the initial doses of the drug may affect the functional properties of cells that differentiate in the presence of the cytokines. For example, IL-4 or IFN-γ affects the differentiation of undifferentiated T cells into Th1 or Th2 cells (33). Likewise, human type 1 regulatory cells (Tr1) were initially identified as cells that had been activated in the presence of IL-10 and rendered anergic (34). We therefore studied PBMCs from six patients who had been treated with hOKT3γ1(Ala-Ala) for evidence of cells of a differentiated phenotype that may persist after the initial doses of drug. Cells were harvested from drug-treated patients before and after treatment with the mAb and cultured for six hours in vitro in the presence of monensin and anti-CD28 mAb, but without additional anti-CD3 Ab. The cells were then stained for surface and intracellular markers and analyzed by flow cytometry. We identified a subpopulation of IL-10+CD3+ cells in five of six drug-treated patients at the conclusion of drug treatment (Figure 6a). We confirmed the specificity of staining for IL-10 by demonstrating the ability of unlabeled IL-10, added during staining, to block binding of the anti–IL-10 mAb (data not shown). The IL-10+ T cells were seen in the PBMCs even without the addition of anti-CD28 mAb to the cultures, suggesting that the production of IL-10 by these cells had occurred in vivo without the need for costimulatory signals. There was no detectable IFN-γ production by these cells.
Figure 6.
Expression of IL-10 in CD3+/– T cells after treatment with hOKT3γ1(Ala-Ala). (a) PBMCs were isolated from a patient before and 1 week after the last dose of drug. The cells were cultured for 6 hours in the presence of monensin and CD28 and then stained for CD3 on the cell surfaces and intracellular IFN-γ (x axis) and IL-10 (y axis) after permeabilization. Further studies identified the IL-10+ cells as being CD4+ (see text). (b) The presence of IL-10+CD4+ T cells was studied before, during, and after treatment with the anti-CD3 mAb. The box indicates the time that the anti-CD3 mAb was given. The data represent the percentage of CD4+ T cells that are IL-10+ over time in the five of six patients in whom these cells were identified. (c) The IL-10+CD4+ T cells express CCR4 on their surfaces. The histogram shows surface staining with CCR4 on IL-10+CD4+ (solid line), IL-10–CD4+ (dashed line), or background staining on CD4+ cells with an isotype control Ab (dotted line).
The IL-10+CD3+ cells were CD4+. On day 12 of treatment, when 10.4% ± 2.5% of the CD4+ cells were IL-10+, < 0.3% of the CD8+ cells were IL-10+. The IL-10+CD4+ cells were seen after the conclusion of drug treatment. One week after the last dose of drug, at time at which the level of coating and modulation of the CD3 molecule was 25.5% ± 6.8% and 30.9% ± 7.3%, the IL-10+CD4+ cells were still found in three of three patients. The frequency of the cells diminished with time, but in three of the four individuals in whom the cells were detectable, there were still IL-10+CD4+ T cells present 1 month after the start of treatment (Figure 6b). By 3 months after treatment, the IL-10+CD4+ cells were not detectable over background.
The IL-10+CD4+ subpopulation was heterogeneous. The majority (65.9% ± 9.9%, range 42–89%) of the IL-10+CD4+ cells were CD45RO+ compared with 39.3% ± 7.8% (range 20–58%)of the IL-10–CD4+ cells (P < 0.001), and 7.9% ± 2.1% of the CD45RO+CD4+ cells were IL-10+ compared with 1.1% ± 0.4% of the CD45RO-CD4+ cells (P < 0.05). In addition, the IL-10+CD4+ cells expressed CCR4 on their cell surfaces (68.3% ± 12.0%, range 44.5–83%), unlike the IL-10–CD4+ cells (14.0% ± 9.3%, range 3.5–32.6%, P = 0.05) (Figure 6c). The majority of the IL-10+ cells were CCR5– (21.8% ± 7.5%, range 8.6–34.5%), which was not significantly different from the IL-10–CD4+ cells (4.5% ± 3.7%, range 0–11.9%). The expression of CD62L was minimally lower on IL-10+ (35.8 ± 6.6 compared with IL-10– (39.6 ± 7.1) (P = 0.1), consistent with activation in at least some of the cells. We found that 93.5% of the IL-10+CD4+ cells were CD25–, which was similar to the IL-10–CD4+ cells (93.1%). Their expression of CTLA-4 on IL-10+ or IL-10– T cells was similar to background levels. However, there was a higher proportion of IL-10+CD4+ T cells with surface expression of TGF-β (12.6% ± 4.8%) compared with the IL-10–CD4+ cells (0.28% ± 0.18%) (P = 0.057).
Discussion
These studies demonstrate that hOKT3γ1(Ala-Ala) has activation properties that differ substantially from the parent compound OKT3 or even other humanized FcR nonbinding anti-CD3 mAb’s and suggest a mechanism whereby the drug may modulate autoimmune responses involved in type 1 diabetes. The differences in the activation properties of these anti-CD3 mAb’s have important implications for use in clinical medicine.
First, although hOKT3γ1(Ala-Ala) induces calcium-like flux OKT3 in short-term studies in vitro, the cytokines produced in response to the two mAb’s are different. Overall, the quantity of cytokines produced in response to hOKT3γ1(Ala-Ala) is less than OKT3, and hOKT3γ1(Ala-Ala) preferentially induces IL-10, whereas cells stimulated with OKT3 produce more IFN-γ on a molar basis. Earlier studies in murine systems indicated that FcR nonbinding anti-CD3 caused anergy, rather than activation, of previously activated Th1 cells. Increases in intracellular calcium were not observed when murine T cell clones were cultured with a FcR nonbinding anti-CD3 mAb (25). It should be noted, however, that NFAT was clearly shown to translocate in these cells consistent with at least a small Ca++ flux (26). Like other anti-CD3 mAb’s, there is specificity of the activation effects of hOKT3γ1(Ala-Ala) for previously activated (CD45RO+) T cells. Indeed, nonFcR binding anti-CD3 mAb’s have been shown to induce apoptosis of activated human T cells (34–37).
Partial T cell receptor (TCR) agonists induce different signaling pathways compared with those activated by OKT3. These signaling responses at the molecular level may account for differences in cell activation. Studies with the murine analogue of hOKT3γ1(Ala-Ala) and murine T cell clones showed that the FcR nonbinding anti-CD3 mAb induced similar phosphorylation of CD3ε and p21δ, but less phosphorylation of p23δ and ZAP-70 kinase and minimal phospholipase Cγ1 phosphorylation compared with FcR binding anti-CD3 (25). In contrast to TCR agonists, which cause association of active lck and active ZAP-70 with p120-GTPase-activating protein (p120-GAP), partial agonists of the TCR did not activate lck or ZAP-70 but induced association of p120-GAP with inactive ZAP-70 (38). Studies with another humanized FcR nonbinding anti-CD3 mAb, HuM291, have described greater sustainable phosphorylation of extracellular signal-regulated kinase-2 compared with OKT3 (34, 35, 39). Hu291, however, induced IFN-γ, TNF-α, and IL-6 after the first dose of drug and induced rapid apoptosis of T cells. Therefore, the constant region of the anti-CD3 Ig reflected in the comparison of OKT3 with hOKT3γ1(Ala-Ala), as well as the TCR affinity for the ε chain reflected by the comparison of hOKT3γ1(Ala-Ala) and the reported effects of Hu291, influence the activation properties of TCR agonists.
Second, in vivo, hOKT3γ1(Ala-Ala) induces IL-10 and IL-5 production, whereas OKT3 and other non-FcR binding anti-CD3 mAb’s have been shown to be potent stimulators of IFN-γ release. The early induction of IL-10 without IFN-γ in vivo may account for the immune modulatory properties of hOKT3γ1(Ala-Ala) by affecting the autoimmune response toward a nonpathogenic phenotype. Several reports in different animal models of autoimmune diseases such as type 1 diabetes have implicated IFN-γ as an important mediator in the autoimmune attack on islet cells, either through its direct cytotoxic effects on β cells or indirectly through the induction of immune effectors (40–44). In contrast, production of IL-10 and IL-5 promote development of “protective” Th2 cells in mice (45–47). Altering the phenotype of cells expressing the TCR from pathogenic T cells toward a Th2 phenotype can render those cells nonpathogenic in rodent models of type 1 diabetes and may account for the protective effects of IL-10 in the NOD mouse (41). The mechanisms, however, involved in the effects of immune deviation are not clear, since the outcomes of experiments depend on the experimental system studied (48). Indeed, some investigators have postulated that the protection from autoimmune diabetes with Th1 to Th2 cytokine shifts in the NOD mouse is the outcome rather than the cause of resistance elicited by immunostimulation (49). Our findings, both in terms of the acute activation effects as well as the chronic effects of the drug involving induction of IL-10+CD4+CCR4+ T cells are consistent with activation and/or deviation of immune responses toward a Th2 phenotype (50). The fact that the activation properties of hOKT3γ1(Ala-Ala), characterized in vitro, were reflected by our findings in vivo, suggest that the immune deviation induced by the mAb is the cause rather than the outcome of the effect of the drug on type 1 diabetes.
Results from studies in the NOD mouse have suggested that anti-CD3 mAb induces a population of regulatory cells that inhibit diabetogenesis and induce tolerance to the disease (12). The effects of the mAb in mice was long lasting, even after the drug administration was discontinued. They could not be explained simply by the elimination of pathogenic cells. Diabetogenic cells were still present in the mice at a time after treatment, but were blocked from causing disease. Cyclosporin A prevented the induction of tolerance suggesting that cytokine production may be important in the mechanism of anti-CD3 mAb. Our studies in patients with type 1 diabetes are also consistent with establishment of regulatory mechanisms following non-FcR binding anti-CD3 mAb treatment. The clinical effects of the drug treatment persisted for beyond 1 year after drug treatment, at a time when the numbers of circulating T cells were normal and mAb could not be detected on their surfaces or in the peripheral blood.
After the initial effects in inducing cytokine release, the drug induces cells that may account for the effects of the drug through immune regulation. We identified a population of IL-10+CD4+ T cells in the peripheral blood at the conclusion and after treatment in five of six drug-treated patients in whom these studies were done. While this finding suggests that these cells are induced in the majority of drug-treated patients, a larger study will be needed to determine the actual prevalence of these cells. The IL-10+CD4+ T cells diminished with time, but were still detectable over background levels 1 month after drug treatment in three-quarters of the patients. In this regard, IL-10 itself or the CD4+ cells that produce IL-10 may affect the autoimmune effectors directly by the cytokines they produce or as regulatory T cells that can suppress antigen-specific responses (51, 52). Activation of human T cells in the presence of IL-10 has been found to induce anergy or nonresponsiveness of T cells that cannot be reversed by culture with anti-CD3 with anti-CD28 mAb’s (51). In the NOD mouse, systemic administration of an IL-10/Fc fusion protein prevented the occurrence of diabetes with lasting protection after treatment was discontinued, and T cell clones transduced with the IL-10 gene prevented diabetes in the NOD mouse (53, 54) The phenotype of the IL-10+CD4+ T cells is similar to other reports of regulatory cells. Sebastiani et al. identified a subpopulation of IL-10+CD4+ regulatory T cells that block, in an IL-10–dependent manner, the differentiation and maturation of dendritic cells, thereby impairing their capacity to activate Tc1 and Th1 effector cells (55). However, the cells reported by Sebastiani et al. expressed both CCR4 and CCR5, whereas a report by Iellem et al. identified CCR4 and CCR8 expression on CD4+CD25+ regulatory T cells (56). In preliminary studies, we have been unable to induce further cytokine production in CD3+ T cells in drug-treated patients by culture with cross-linked anti-CD3 mAb and anti-CD28, suggesting that these cells are anergic (unpublished observations). Dieckmann et al. have shown that anergized CD4+CD25– T cells can then suppress proliferation of syngeneic CD4+ T cells via an IL-10–dependent mechanism that is independent of cell contact (57).
Interestingly, we found that the surface expression of TGF-β was greater on the IL-10+ cells compared with the IL-10– CD4+ cells. The role of TGF-β in regulatory T cell activity has been controversial — some investigators have found that suppressive activity of CD4+CD25+ T cells is partially inhibited by anti–TFG-β Ab’s, whereas others have reported that this activity is independent of TGF-β (58, 59). Roncarolo et al. have found that the regulatory function of CD25+CD4+ T cells is partially dependent on TGF-β, but these cells are distinct from IL-10+CD4+ Tr1 cells (60). Thus, further studies will be needed to determine the functional role of the IL-10+ cells and the importance of their surface expression of TGF-β.
In summary, the structural alterations in the anti-CD3 molecule OKT3 to produce hOKT3γ1(Ala-Ala) have resulted in an anti-CD3 mAb with activation properties that are distinct from the parent molecule or other anti-CD3 mAb’s and suggest a mechanism for immune modulation by the drug. Acutely, hOKT3γ1(Ala-Ala) induces activation of T cells resulting in production of IL-10 in CD45RO+ T cells. Afterward, the mAb induces a heterogeneous population of CD4+IL-10+ cells. The functional role of the IL-10+ T cells is under study, but these observations suggest a mechanism whereby activation of T cells with FcR nonbinding anti-CD3 mAb may skew immunologic responses and/or induce a population of regulatory cells.
Acknowledgments
This work was supported by R01DK157846, U19AI46132, M01 RR00645, P60 DK20595, a Special Grant (4–1999–711), and a Center Grant (4–1999–841) from the Juvenile Diabetes Research Foundation.
Footnotes
Conflict of interest: Jeffrey A. Bluestone has a financial interest in the monoclonal antibody hOKT3γ1(Ala-Ala) consisting of a patent application and a commercial agreement with Centocor and Johnson and Johnson Pharmaceuticals.
Nonstandard abbreviations used: Fc receptor (FcR); cytotoxic T lymphocyte (CTL); phytohemagglutinin (PHA); N-[2-hydroxy-ethyl]piperzine-N-[2-ethanesulfonic acid] (HEPES); 2-mercaptoethanol (2ME); indo-1/acetoxymethlester (indo-1); rabbit anti-mouse Ig (RAM); cytometric bead array (CBA); human type 1 regulatory cells (Tr1); T cell receptor (TCR).
References
- 1.Staruch MJ, Sigal NH, Dumont FJ. Differential effects of the immunosuppressive macrolides FK-506 and rapamycin on activation-induced T-cell apoptosis. Int. J. Immunopharmacol. 1991;13:677–685. doi: 10.1016/0192-0561(91)90180-f. [DOI] [PubMed] [Google Scholar]
- 2.Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu. Rev. Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 3.Kottaridis PD, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96:2419–2425. [PubMed] [Google Scholar]
- 4.Pangalis GA, et al. Campath-1H (anti-CD52) monoclonal antibody therapy in lymphoproliferative disorders. Med. Oncol. 2001;18:99–107. doi: 10.1385/mo:18:2:99. [DOI] [PubMed] [Google Scholar]
- 5.Li XC, et al. T cell death and transplantation tolerance. Immunity. 2001;14:407–416. doi: 10.1016/s1074-7613(01)00121-2. [DOI] [PubMed] [Google Scholar]
- 6.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 7.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 8.Herbelin A, et al. Mature mainstream TCR alpha beta+CD4+ thymocytes expressing L-selectin mediate “active tolerance” in the nonobese diabetic mouse. J. Immunol. 1998;161:2620–2628. [PubMed] [Google Scholar]
- 9.Chatenoud L, et al. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herold KC, et al. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes. 1992;41:385–391. doi: 10.2337/diab.41.3.385. [DOI] [PubMed] [Google Scholar]
- 11.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J. Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 12.Bach JF, Chatenoud L. Tolerance to islet autoantigens in type 1 diabetes. Annu. Rev. Immunol. 2001;19:131–161. doi: 10.1146/annurev.immunol.19.1.131. [DOI] [PubMed] [Google Scholar]
- 13.Chatenoud L, Bach JF. Anti-CD3 antibodies. Immunol. Ser. 1993;59:175–191. [PubMed] [Google Scholar]
- 14.Chatenoud L. Use of CD3 antibodies in transplantation and autoimmune diseases. Transplant Proc. 1994;26:3191–3193. [PubMed] [Google Scholar]
- 15.Smith SL. Ten years of Orthoclone OKT3 (muromonab-CD3): a review. J. Transpl. Coord. 1996;6:109–119. doi: 10.7182/prtr.1.6.3.8145l3u185493182. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K. Muromonab CD3 (Orthoclone OKT3) J. Toxicol. Sci. 1995;20:483–484. [PubMed] [Google Scholar]
- 17.Chatenoud L, Ferran C, Bach JF. The anti-CD3-induced syndrome: a consequence of massive in vivo cell activation. Curr. Top. Microbiol. Immunol. 1991;174:121–134. doi: 10.1007/978-3-642-50998-8_9. [DOI] [PubMed] [Google Scholar]
- 18.Chatenoud L. Humoral immune response against OKT3. Transplant Proc. 1993;25:68–73. [PubMed] [Google Scholar]
- 19.Chatenoud L. OKT3-induced cytokine-release syndrome: prevention effect of anti-tumor necrosis factor monoclonal antibody. Transplant Proc. 1993;25:47–51. [PubMed] [Google Scholar]
- 20.Woodle ES, et al. T-cell activation and lymphokine production induced by antihuman CD3 monoclonal antibodies. Transplant Proc. 1991;23:81–82. [PubMed] [Google Scholar]
- 21.Alegre ML, et al. Effect of a single amino acid mutation on the activating and immunosuppressive properties of a “humanized” OKT3 monoclonal antibody. J. Immunol. 1992;148:3461–3468. [PubMed] [Google Scholar]
- 22.Woodle ES, et al. Humanized, nonmitogenic OKT3 antibody, huOKT3 gamma(Ala-Ala): initial clinical experience. Transplant Proc. 1998;30:1369–1370. doi: 10.1016/s0041-1345(98)00278-4. [DOI] [PubMed] [Google Scholar]
- 23.Woodle ES, Hussein S, Bluestone JA. In vivo administration of anti-murine CD3 monoclonal antibody induces selective, long-term anergy in CD8+ T cells. Transplantation. 1996;61:798–803. doi: 10.1097/00007890-199603150-00021. [DOI] [PubMed] [Google Scholar]
- 24.Woodle ES, et al. Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3gamma1(Ala-Ala) in the treatment of acute renal allograft rejection. Transplantation. 1999;68:608–616. doi: 10.1097/00007890-199909150-00003. [DOI] [PubMed] [Google Scholar]
- 25.Smith JA, et al. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J. Exp. Med. 1997;185:1413–1422. doi: 10.1084/jem.185.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JA, Tang Q, Bluestone JA. Partial TCR signals delivered by FcR-nonbinding anti-CD3 monoclonal antibodies differentially regulate individual Th subsets. J. Immunol. 1998;160:4841–4849. [PubMed] [Google Scholar]
- 27.Herold KC, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 28.Rabin RL, et al. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J. Immunol. 1999;162:3840–3850. [PubMed] [Google Scholar]
- 29.Kukreja A, et al. Multiple immuno-regulatory defects in type-1 diabetes. J. Clin. Invest. 2002;109:131–140. doi:10.1172/JCI200213605. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shevach EM. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 31.Xu D, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 32.Alegre ML, et al. A non-activating “humanized” anti-CD3 monoclonal antibody retains immunosuppressive properties in vivo. Transplantation. 1994;57:1537–1543. [PubMed] [Google Scholar]
- 33.Toellner KM, et al. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J. Exp. Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpenter PA, et al. Non-FcR-binding, humanized anti-CD3 antibody Hu291 induces apoptosis of human T cells more effectively than OKT3 and is immunosuppressive in vivo. Transplant Proc. 2000;32:1545–1546. doi: 10.1016/s0041-1345(00)01343-9. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter PA, et al. Non-Fc receptor-binding humanized anti-CD3 antibodies induce apoptosis of activated human T cells. J. Immunol. 2000;165:6205–6213. doi: 10.4049/jimmunol.165.11.6205. [DOI] [PubMed] [Google Scholar]
- 36.Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J. Immunol. 1993;150:4338–4345. [PubMed] [Google Scholar]
- 37.Janssen O, Wesselborg S, Kabelitz D. Immunosuppression by OKT3 — induction of programmed cell death (apoptosis) as a possible mechanism of action. Transplantation. 1992;53:233–234. [PubMed] [Google Scholar]
- 38.Chau LA, Bluestone JA, Madrenas J. Dissociation of intracellular signaling pathways in response to partial agonist ligands of the T cell receptor. J. Exp. Med. 1998;187:1699–1709. doi: 10.1084/jem.187.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter PA, et al. A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease. Blood. 2002;99:2712–2719. doi: 10.1182/blood.v99.8.2712. [DOI] [PubMed] [Google Scholar]
- 40.Herold KC, et al. Regulation of cytokine production during development of autoimmune diabetes induced with multiple low doses of streptozotocin. J. Immunol. 1996;156:3521–3527. [PubMed] [Google Scholar]
- 41.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 42.Wang B, et al. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 1997;94:13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zipris D, et al. Cytokine gene expression in islets and thyroids of BB rats. IFN-gamma and IL-12p40 mRNA increase with age in both diabetic and insulin-treated nondiabetic BB rats. J. Immunol. 1996;156:1315–1321. [PubMed] [Google Scholar]
- 44.Rabinovitch A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 1998;14:129–151. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 45.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today. 1991;12:A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 46.de Waal Malefyt R, et al. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore KW, et al. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 48.Pakala SV, Kurrer MO, Katz JD. T helper 2 (Th2) T cells induce acute pancreatitis and diabetes in immune-compromised nonobese diabetic (NOD) mice. J. Exp. Med. 1997;186:299–306. doi: 10.1084/jem.186.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serreze DV, et al. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J. Immunol. 2001;166:1352–1359. doi: 10.4049/jimmunol.166.2.1352. [DOI] [PubMed] [Google Scholar]
- 50.D’Ambrosio D, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J. Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 51.Groux H, et al. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roncarolo MG, et al. Type 1 T regulatory cells. Immunol. Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 53.Moritani M, et al. Prevention of adoptively transferred diabetes in nonobese diabetic mice with IL-10-transduced islet-specific Th1 lymphocytes. A gene therapy model for autoimmune diabetes. J. Clin. Invest. 1996;98:1851–1859. doi: 10.1172/JCI118986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng XX, et al. A noncytolytic IL-10/Fc fusion protein prevents diabetes, blocks autoimmunity, and promotes suppressor phenomena in NOD mice. J. Immunol. 1997;158:4507–4513. [PubMed] [Google Scholar]
- 55.Sebastiani S, et al. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J. Immunol. 2001;166:996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]
- 56.Iellem A. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dieckmann D, et al. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 1-producing, contact-independent type 1-like regulatory T cells. J. Exp. Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Annunziato F, et al. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J. Exp. Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piccirillo CA, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J. Exp. Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]