Abstract
Chlorosomes are the main light harvesting complexes of green photosynthetic bacteria. Recently, a lamellar model was proposed for the arrangement of pigment aggregates in Chlorobium tepidum chlorosomes, which contain bacteriochlorophyll (BChl) c as the main pigment. Here we demonstrate that the lamellar organization is also found in chlorosomes from two brown-colored species (Chl. phaeovibrioides and Chl. phaeobacteroides) containing BChl e as the main pigment. This suggests that the lamellar model is universal among green sulfur bacteria. In contrast to green-colored Chl. tepidum, chlorosomes from the brown-colored species often contain domains of lamellar aggregates that may help them to survive in extremely low light conditions. We suggest that carotenoids are localized between the lamellar planes and drive lamellar assembly by augmenting hydrophobic interactions. A model for chlorosome assembly, which accounts for the role of carotenoids and secondary BChl homologs, is presented.
INTRODUCTION
Chlorosomes are the main light harvesting complexes of green photosynthetic bacteria. A typical chlorosome is an ellipsoidal body (typically 100–200 nm in length, 20–50 nm in width) that is composed mainly of bacteriochlorophylls and carotenoids with minor contributions from quinones, lipids, and proteins (1,2). The major difference from all other light harvesting complexes is that the main chlorosome pigments, bacteriochlorophyll (BChl) c, d, or e, are not associated with proteins and self-assemble into aggregates. The BChl aggregates were originally proposed to assemble into rodlike elements (3,4). An alternative lamellar model was put forward for the pigment arrangement in Chlorobium tepidum chlorosomes (5). Recently, further support for the lamellar model was obtained by a careful fixation of whole cells and electron microscopy (EM) (6). The density patterns and striations observed for most Chl. tepidum chlorosomes in this study were inconsistent with the presence of hexagonally packed, rod-shaped, BChl c aggregates, but could be explained by the lamellar structure of the aggregates. However, it is not obvious whether similar structural features are common to chlorosomes from the other members of Chlorobiaceae, which exhibit different pigment compositions.
Chl. tepidum is a green-colored, green sulfur bacterium containing BChl c as the main pigment, and chlorobactene and OH-chlorobactene as the main carotenoids. For comparison we selected two brown-colored green sulfur bacteria, Chl. phaeovibrioides and Chl. phaeobacteroides, which contain BChl e, isorenieratene, and β-isorenieratene as the main pigments (7). Compared to green-colored bacteria, chlorosomes from brown-colored species contain larger amounts of carotenoids and BChl secondary homologs with esterifying alcohols longer than farnesyl (8,9). Recently, carotenoids were shown to play a pivotal role in nucleating the aggregation of BChl c in vitro (10). Carotenoids were also proposed to play a structural role in the lamellar assembly (5). This suggests that carotenoids may play an important role in chlorosome morphogenesis. The carotenoid content of chlorosomes can be manipulated by growing the bacteria in the presence of 2-hydroxybiphenyl (HBP) which acts as an inhibitor of carotenoid synthesis (11,12). This treatment reduces the carotenoid amount available during chlorosome assembly. Alternatively, carotenoids can be extracted from isolated chlorosomes by hexane in vitro (13).
In this study we use a combination of structural and analytical techniques to explore the roles of carotenoids and secondary BChl homologs in chlorosome structure and self-assembly. The results show that chlorosomes from the two brown-colored species exhibit the same lamellar organization of BChl e pigments as previously observed for BChl c in Chl. tepidum. However, the long-range organization is fundamentally different. The results also unequivocally demonstrate that carotenoids are an integral part of the lamellar structure.
MATERIALS AND METHODS
Organisms and growth conditions
Chl. phaeobacteroides strain CL1401 and Chl. phaeovibrioides strain UdG7006 were grown in standard Pfennig mineral medium (14) in 10-L glass bottles under continuous stirring. For the brackish Chl. phaeovibrioides the medium was supplemented with 2% NaCl. The inoculum was 3% using active cultures of both species. Illumination was continuously provided by four Philips SL25 fluorescent lamps giving an average light intensity of 100 μmol photons/m2/s at the surface of the culture bottles. Cells were harvested at the stationary phase by centrifugation at 16,000× g for 20 min at 4°C. Pellets were suspended in 50 mM Tris-HCl pH 8.0 and stored at −20°C until use. Chl. tepidum was grown as previously described (5). Inhibition of carotenoid biosynthesis was accomplished by supplementing the culture media with HBP at a final concentration of 20 μg/mL (11).
Chlorosome preparation and carotenoid extraction
Chlorosomes from brown-colored species and Chl. tepidum were isolated as previously described (5,11). Carotenoids and quinones were extracted from chlorosomes as described in (13,15) with minor modifications. The chlorosome-containing sucrose gradient band was diluted eightfold with 50 mM Tris-HCl pH 8.0 and centrifuged at 125,000× g for 1 h at 4°C. To remove traces of sucrose, the chlorosome pellet was resuspended in buffer and centrifuged again under the same conditions. The pellet was resuspended in a minimal volume of buffer, quickly frozen in liquid nitrogen while swirling the flask and freeze-dried for 90 min. The dried film of chlorosomes was washed at least three times with hexane. To ensure efficient carotenoid extraction, the film was resuspended in a minimal volume of buffer and the freeze-drying procedure and the hexane wash were repeated at least three times.
HPLC analysis
Photosynthetic pigments were extracted from thawed chlorosomes using acetone:methanol (7:2) (Scharlau, HPLC grade). The extract was stored at −30°C during 24 h and then centrifuged at 13,400× g for 15 min. Before HPLC analyses, 1 mL of clear supernatants were mixed with 1 M ammonium acetate (10% final concentration), which was used as ion pairing agent to improve the resolution of pigment separation (16). Samples were equilibrated for 5 min and then analyzed by reverse-phase HPLC according to (16) with minor modifications as described in (12).
The HPLC detection system was calibrated by injecting pigment standards of known concentration extracted from pure cultures of Chl. phaeobacteroides (BChl e, isorenieratene, and β-isorenieratene) and Chl. limicola (BChl c and chlorobactene). The standards were quantified by measuring the extinction at λmax for each pigment and determining the areas of the corresponding peaks. The molar absorption coefficients used for calibration were (in mM−1cm−1): 65.3 for BChl a at 771 nm (17), 74 for BChl c at 434 nm (18), 41 for BChl e at 654 nm, and 107 for isorenieratene at 450 nm (19). For the quantification of colorless phytoene an absorption coefficient of 68 mM−1cm−1 at 287 nm was used (20). The calibrated areas of peaks at 287 nm (phytoene), 434 nm (BChl c), 453 nm (isorenieratene), 464 nm (chlorobactene), 473 nm (BChl e), and 771 nm (BChl a) were used to estimate the pigment composition of samples.
Structural analysis
Sample preparations and EM were done as previously described (5). X-ray scattering of the native and HBP-chlorosomes presented in Fig. 3 was collected at beamline ID1 of European synchrotron radiation facility with a radiation wavelength of 0.92 Å. The measurements presented in Fig. 4 were done on an in-house rotating Cu-anode source equipped with focusing optics and an image plate detector. The typical optical density of the samples per cm was 1000 at 715 nm. Extensive irradiation did not affect optical properties of chlorosomes (Supplementary Fig. 1).
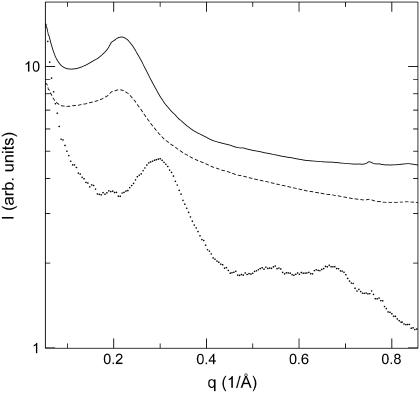
FIGURE 3.
SAXS obtained from solutions of Chl. phaeovibrioides control (CTRL1, solid) and HBP-chlorosomes (dashed). Scattering from Chl. tepidum chlorosomes (dotted) is shown for comparison (data from Psencik et al. (5)).
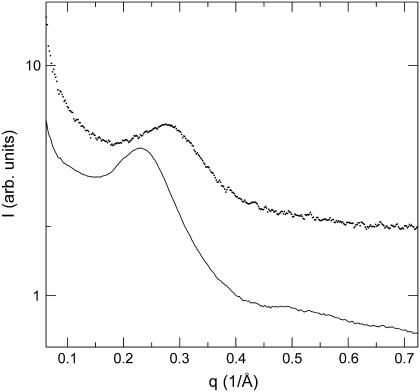
FIGURE 4.
SAXS obtained from solutions of Chl. phaeovibrioides control (CTRL2, solid) and hexane-washed (HEX2, dotted) chlorosomes.
RESULTS
Pigment composition
One of the aims of this study was to establish a possible correlation between pigment composition and chlorosome structure and assembly. In particular the role of carotenoids and secondary BChl homologs was examined. The composition of several different preparations of Chl. phaeovibrioides chlorosomes was analyzed and compared with control chlorosomes from Chl. tepidum and Chl. phaeobacteroides (Table 1). Two different batches of control Chl. phaeovibrioides chlorosomes (CTRL1 and CTRL2) were washed with hexane to remove lipophilic molecules (carotenoids and quinones) resulting in samples HEX1 and HEX2, respectively. These were also compared with chlorosomes from HBP treated bacteria (HBP-chlorosomes), where carotenoid synthesis was inhibited. Absorption spectra of hexane washed and HBP-chlorosomes exhibited the same features as that of control chlorosomes except the changes due to loss of carotenoids and BChl a (Supplementary Fig. 2).
TABLE 1.
Pigment composition of chlorosomes obtained from the HPLC separation and analysis
| Sample | BChl c, e main homologues % | BChl c, e secondary homologues % | Car/ BChl c, e (mol/mol) | BChl a/ BChl c, e (mol/mol) |
|---|---|---|---|---|
| Chl. tepidum | 82.7 | 17.3 | 0.084 | 0.009 |
| Chl. phaeovibrioides CTRL1 | 79.0 | 21.5 | 0.111 | 0.0072 |
| Chl. phaeovibrioides HEX1 | 79.3 | 20.5 | 0.033 | 0.0039 |
| Chl. phaeovibrioides CTRL2 | 57.8 | 42.1 | 0.2 | 0.016 |
| Chl. phaeovibrioides HEX2 | 63.3 | 36.7 | 0.007 | 0.011 |
| Chl. phaeovibrioides HBP | 61.3 | 37.9 | 0.040* | 0.0022 |
| Chl. phaeobacteroides | 53.5 | 46.5 | 0.247 | 0.0054 |
Phytoene was accumulated in HBP-chlorosome, phytoene/BChl e (mol/mol) ratio of 1.9; phytoene was not detected in any other sample.
Concentrations of pigments were determined with an error of ∼1.5%.
Native brown-colored bacteria contained more carotenoids and secondary homologs than Chl. tepidum. However, batch to batch variation in their content was observed (cf. CTRL1 and CTRL2 in Table 1). Hexane washing of control chlorosomes led to the removal of 70−95% of the original carotenoid and 30−80% of the BChl a content, respectively (HEX1 and HEX2 in Table 1). The carotenoid content of HBP-chlorosomes from Chl. phaeovibrioides was significantly reduced with respect to control chlorosomes. However, the effect of the HBP treatment on carotenoid biosynthesis inhibition was less efficient than in the case of Chl. phaeobacteroides (10), which might be due to a different cellular response. HBP treatment resulted in the accumulation of colorless phytoene in chlorosomes (Table 1), beyond which the carotenoid biosynthesis is inhibited by HBP.
Electron cryomicroscopy
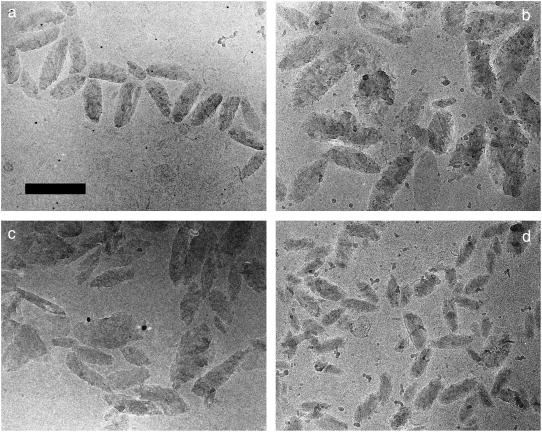
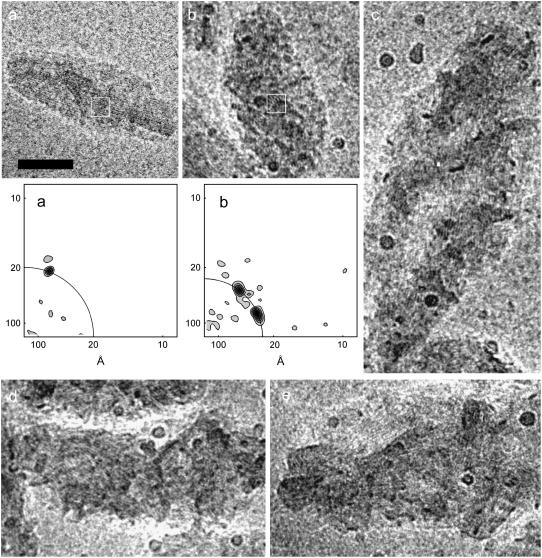
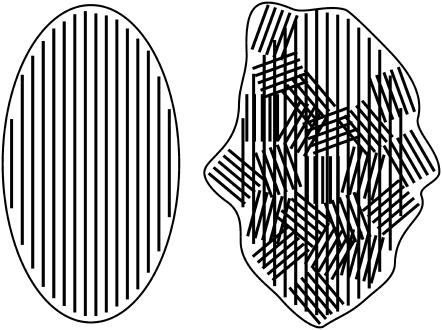
Fig. 1 compares a field view of control chlorosomes from Chl. tepidum and Chl. phaeovibrioides. Whereas chlorosomes from Chl. tepidum had an approximately elliptical shape with a relatively smooth outline, chlorosomes from Chl. phaeovibrioides were irregular and exhibited a rather rough outline. Fig. 2 compares a typical chlorosome from Chl. tepidum with several control chlorosomes from Chl. phaeovibrioides. In all of the chlorosomes striations of parallel dark and light stripes were discernible. However, a closer inspection revealed a different long-range organization of BChl aggregates in the two species: In Chl. tepidum, the striations run close to parallel with the long axis of the chlorosome and span the whole length of the chlorosome (see also Fig. 1 in Psencik et al. (5)). In contrast, the direction of striation in Chl. phaeovibrioides chlorosomes was discontinuous and contained within distinct domains typically 100−200 Å in size. The relative orientation of the domains was more or less random although a preference for having the striation oriented at sharp angles with the long axis of the chlorosome was apparent. The random arrangement of domains correlates with the rough outline of Chl. phaeovibrioides chlorosomes. Such domains were also found occasionally in chlorosomes of Chl. tepidum (not shown).
FIGURE 1.
Comparison of overall chlorosome shapes. Electron micrographs of samples embedded in vitreous ice: (a) Chl. tepidum chlorosomes, (b) Chl. phaeovibrioides control chlorosomes, (c) hexane-washed Chl. phaeovibrioides chlorosomes and (d) HBP-chlorosomes from Chl. phaeovibrioides. Scale bar 200 nm.
FIGURE 2.
EM analysis of representative chlorosomes of (a) Chl. tepidum and (b) Chl. phaeovibrioides embedded in vitreous ice. The lower panels (a) and (b) show power spectra of the boxed areas in the upper panels. The quarter-circles indicate the spacing of the main diffraction maxima, which was 20 and 24 Å, respectively. Additional chlorosomes from Chl. phaeovibrioides are shown in panels (c–e) to illustrate the variability of the domain arrangement and their involvement in the rough surface formation. Scale bar 50 nm.
The spacing between the striae was determined from the computed power spectra of several regions in different chlorosomes (Fig. 2). Multiple areas with pronounced striation were analyzed and the ranges of spacing obtained are summarized in Table 2. Control chlorosomes from Chl. phaeovibrioides exhibited spacing (23−25 Å) larger than that seen for Chl. tepidum (19–22 Å). The presence of domains in Chl. phaeovibrioides chlorosomes often manifested itself in several peaks with similar spacing but with different orientation, i.e., different azimuthal position in the power spectra (Fig. 2). In contrast, a single intense peak was usually observed for Chl. tepidum chlorosomes (Fig. 2 here and Fig. 1 in Psencik et al. (5)).
TABLE 2.
Structural properties of chlorosomes
| Bacterium | BChl | Spacing (EM) (Å) | Domains* | Spacing x-ray‡ (Å) |
|---|---|---|---|---|
| Chl. tepidum | BChl c | 19.5−21.5 | Rarely | 20.9 |
| Chl. phaeovibrioides CTRL1 | BChl e | 23–25 | Almost always | 28.9 |
| Chl. phaeovibrioides HEX1 | BChl e | 21−25 | Almost always | n.d. |
| Chl. phaeovibrioides CTRL2 | BChl e | n.d. | n.d. | 27.3 |
| Chl. phaeovibrioides HEX2 | BChl e | n.d. | n.d. | 22.4 |
| Chl. phaeovibrioides HBP | BChl e | 24–26 | ∼50% | 29.8 |
| Chl. phaeobacteroides | BChl e | 21–33† | Only in “thick” chlorosomes | 29.9 |
Organization of visible striation; the visibility of striation is described in text.
The “thick” chlorosomes exhibited spacing 21−26 Å, whereas “thin” chlorosomes exhibited larger spacing (27−33 Å).
Spacing was determined with an accuracy of ±0.1 Å.
The overall shapes of hexane-washed and HBP-chlorosomes are compared with the control in Fig. 1. The shape of the hexane-washed chlorosomes remained unchanged. The striation and domains were less often observed than in the control chlorosomes, presumably due to a loss of order in the BChl aggregates and consequently lower domain size. The range of spacing between striations was smaller (21–25 Å) than that of the control chlorosomes (Table 2). HBP-chlorosomes were smaller and more ellipsoidal than control chlorosomes (Fig. 1) and resembled Chl. tepidum chlorosomes. The striation and domains were also less frequently encountered and striation was more often parallel to the long axis of the chlorosome. The spacing was slightly larger (24–26 Å) for HBP-chlorosomes than for the control (Table 2).
Preliminary results for Chl. phaeobacteroides indicated the existence of two distinct types of chlorosomes, which were denoted as “thin” and “thick” chlorosomes, respectively. A “thin” chlorosome is ellipsoidal and exhibits striation (spacing 27–33 Å) parallel with the long axis (similar to those of Chl. tepidum, although less ordered). A “thick” chlorosome has a rough surface and contains domains (similar to those from Chl. phaeovibrioides). The striation spacing (21−26 Å) was larger than that seen for Chl. tepidum and approached that of Chl. phaeovibrioides.
X-ray scattering
Solution x-ray scattering from Chl. phaeovibrioides (control and HBP-chlorosomes) and Chl. tepidum chlorosomes is compared in Fig. 3. Both the control and HBP-chlorosomes from Chl. phaeovibrioides exhibited a single diffraction peak between 0.21−0.23 Å−1. This corresponds to lamellar spacing between 27−30 Å. No discernible features were observed at wide angles (q between 0.5−0.8 Å−1). On the other hand, Chl. tepidum chlorosomes yielded the main diffraction peak at q = 0.3 Å−1 (lamellar spacing 20.9 Å) and distinct features were also discernible at wide angles (5) (Table 2). The wide-angle features were assigned to an ordered lattice of BChl molecules within the Chl. tepidum lamellae (5). The absence of these features for Chl. phaeovibrioides indicates a significantly disordered pigment lattice. For chlorosomes from Chl. phaeobacteroides, a spacing of 29.9 Å was obtained (Table 2).
Removal of carotenoids by hexane washing produced a profound change in the position of the main diffraction peak from 0.23 to 0.28 Å−1 (Fig. 4). This corresponds to a substantial decrease (18%) in the lamellar spacing upon removal of carotenoids (Table 2).
DISCUSSION
Lamellar organization of pigment aggregates
Both EM and x-ray scattering show that BChl e molecules in Chl. phaeovibrioides and Chl. phaeobacteroides chlorosomes are organized into lamellae in a fashion similar to that proposed for Chl. tepidum (5). No evidence for a rodlike arrangement of pigments was found. Instead, striation corresponding to lamellae was directly observed in cryo-EM images.
Although the values of spacing between lamellae obtained from EM images are systematically lower than the ensemble average yielded by x-ray (diffraction peak maxima) they still fit within the width of the diffraction peak (Fig. 3). One explanation of the discrepancy is that the EM analyzed areas, which were selected to contain pronounced striation, correspond to well-ordered and tightly packed regions with smaller spacing. The second reason is that the underlying parallel striation (see below) often exhibits larger spacing than that of domains, but is not well represented in the power spectra. Given that cryo-EM and SAXS give the same spacing for chlorosomes which do not possess domains (e.g., Chl. tepidum, Chl. phaeobacteroides “thin” chlorosomes) the second explanation represents the likely reason for the apparent discrepancy. Hence EM does not provide the correct average spacing for chlorosomes with domains. Thus, we use the spacing obtained from x-ray diffraction for quantitative comparisons.
The spacing values obtained for the two brown-colored species were found to be substantially larger (27−30 Å) than that of Chl. tepidum (20.9 Å) chlorosomes. In addition, the diffraction peaks obtained for Chl. phaeovibrioides were wider than that for Chl. tepidum chlorosomes (Fig. 3). This, together with the absence of higher angle diffraction maxima and EM results indicate lower lamellar order in the BChl e containing chlorosomes. The increased spacing and disorder could be a result of the different composition (e.g., carotenoid content or variation in secondary homologs), differences between intrinsic structural properties of BChl c and e and the way chlorosomes are being assembled in different cells (e.g., mechanism of nucleation). As discussed below all these factors may play a role.
Rough surface of chlorosomes is due to lamellar domains
Although the basic lamellar arrangement of pigments in chlorosomes is common to both green and brown-colored species, there is a fundamental difference in their higher order organization. The brown-colored species often contain many lamellar domains with random orientations (Fig. 2). In contrast, the lamellae span the whole length of the chlorosome in the green-colored Chl. tepidum, effectively forming a single domain. The presence of domains correlates with the rough outline of these chlorosomes in EM. This observation is consistent with results from atomic force microscopy (21). These authors reported that chlorosomes of Chloroflexus aurantiacus, Chloronema sp., and Chl. tepidum exhibited a smooth surface, whereas those of Chl. phaeobacteroides and Chl. vibrioforme had rough surfaces. Thus, we conclude that the rough surface represents the outline of the underlying domains.
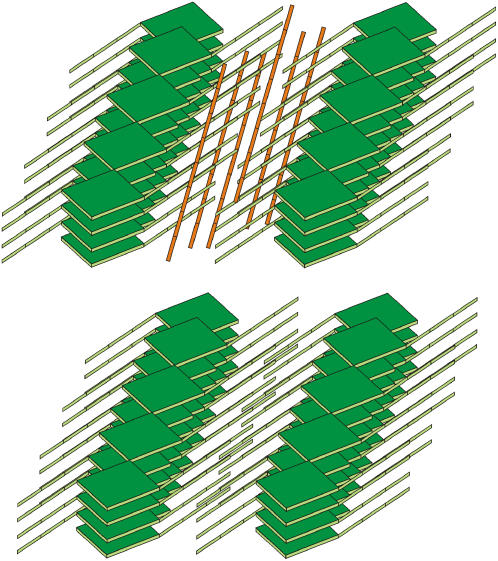
The development of domains is not limited to chlorosomes from brown-colored species but may also happen in green-colored species and their emergence may be related to changes in growing conditions, e.g., self-shading in dense cultures (see below). On the other hand, the “single domain” arrangement is also observed for the “thin” Chl. phaeobacteroides chlorosomes and for a fraction of HBP-chlorosomes from Chl. phaeovibrioides. The results suggest that there are two stages of chlorosome assembly. The first stage is nucleated from the baseplate and results in the single domain smooth chlorosomes. During the second stage, randomly oriented domains nucleate on top of the baseplate-nucleated regular layer. The latter process produces the rough, domain-ridden surface appearance (Fig. 6). We propose that carotenoids and secondary homologs play an important role in the nucleation of domains (see below).
FIGURE 6.
Schematic representation of the two phases of chlorosome assembly: During the first phase, lamellae parallel to the long axis are formed with the help of the ordered baseplate, resulting in a “thin”, single-domain, chlorosome (left). Domains are added onto this regular surface during the second phase for which a larger content of carotenoids and secondary homologs is required and results in a “thick” chlorosome with rough surface (right).
Physiological significance of domains
The brown-colored bacteria are able to survive at extremely low-light conditions (22,23). Under these conditions efficient capture of all photons becomes essential. The presence of domains in chlorosomes may help to increase the light-harvesting efficiency of photons with arbitrary polarization. In all models for the BChl aggregate structure in chlorosomes (including rod and lamellar) the aggregates and their main transition dipole moments are oriented along the long axis of the chlorosome (e.g., single domain chlorosome). Consequently, photons with a polarization component perpendicular to this axis are absorbed with lower efficiency. In the absence of a polarization conversion mechanism, a substantial part of the incident light would not be captured by a given chlorosome. Domains with their lamellae oriented at an angle with the long chlorosome axis provide such a polarization conversion mechanism. The domains provide a transition dipole component in the perpendicular direction while their prevailing sharp angle with the long chlorosome axis still assures reasonable coupling with the rest of the underlying, baseplate-proximal aggregates (Fig. 6).
Localization of carotenoids
Previously it has been suggested that carotenoids occupy the hydrophobic space between the chlorin planes and interact with the esterifying alcohols (5). This model would predict that the lamellar spacing would decrease upon carotenoid content reduction. Indeed, this was observed in this work for chlorosomes from which more than 95% of original carotenoids were removed by hexane. The lattice constant decrease corresponds to roughly an 18% total volume reduction. The corresponding loss of carotenoids (Table 1) and possibly also quinones could account for the volume decrease. The visible absorption spectra of this sample does not indicate any significant departure in short range order from the control (Supplementary Fig. 2) and thus it is fair to assume that hexane treatment affects only the lamellar spacing. Thus, we conclude that carotenoids and possibly quinones occupy the volume between lamellae as illustrated in Fig. 5.
FIGURE 5.
Schematic representation of carotenoid (orange) localization (top) and the effect of their removal on the lamellar lattice of BChl (green) molecules (bottom). Note that the length of the relatively stiff conjugated carotenoids does not favor their orientation along the esterifying alcohol chains.
A comparison of the average lamellar spacing in chlorosomes from Chl. tepidum (∼21 Å), the control Chl. phaeovibrioides (∼28 Å), and Chl. phaeobacteroides (∼30 Å) suggest that the value is proportional to the average length of the esterifying alcohols. In different samples from the same species this effect is often masked by the effect of carotenoids as discussed in the next section.
Role of carotenoids in assembly
Given that carotenoids occupy a substantial percentage of the space between lamellae and that their removal in vitro led to a reduction of lamellar spacing, one would expect the amount of carotenoids to correlate with the spacing value. However, the correlation observed for native bacteria is opposite to that predicted: a larger amount of carotenoids led to a decrease in the spacing (cf. CTRL1 and CTRL2, Tables 1 and 2). Thus, the excluded volume of carotenoids is not the sole determinant of the spacing.
The results of in vitro assembly experiments demonstrated that the hydrophobic effect is the major driving force for BChl aggregate assembly: carotenoids (or lipids) together with esterifying alcohols drive aggregate formation by increasing the hydrophobic interaction between the planes of the relatively polar chlorine rings (10). The strength of the interactions during assembly may determine the spacing. In effect, larger amount of carotenoids (or other hydrophobic substance which can partition into the lamellae) would bolster the interaction between the lamellae and decrease the spacing, as seen for CTRL1 versus CTRL2 (Tables 1 and 2), or for “thin” chlorosomes versus domains. However, as demonstrated by carotenoid extraction with hexane the lamellar arrangement is stable once fully assembled. Thus, the carotenoid-mediated stabilization is essential only during the early stages of assembly. In the fully assembled lamellae the cooperative interactions of the esterifying alcohols are strong enough to drive lattice transformation when carotenoids are removed by hexane (Fig. 5).
Comparison of Chl. phaeovibrioides with Chl. tepidum and HBP-chlorosomes suggests that carotenoids play important role in domain morphogenesis. Under certain stress circumstances, e.g., low light conditions, the bacteria respond by producing additional pigment varieties, in particular secondary homologs and carotenoids (9,24,25). As shown in vitro (10) carotenoids may then facilitate nucleation of the new pigment assembly into small domains on the surface of existing parallel aggregates (Fig. 6). The domains provide additional light harvesting capacity to counter the stress condition.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
We thank Prof. Michael Verkhovsky for helpful discussions.
This study was supported by Academy of Finland grants (206926 and 211986 to R.T.; 208661 to S.B.) Czech Science Foundation and Czech Ministry of Education, Youth, and Sports (contracts 206/05/2739 and MSM0021620835 to J.P.), and the Spanish Ministry of Education and Science (No. BFU2004-04914-C02-02/BMC).
References
- 1.Blankenship, R. E., and K. Matsuura. 2003. Antenna complexes from green photosynthetic bacteria. In Light Harvesting Antennas in Photosynthesis. B. R. Green, and W. W. Parson, editors. Kluwer Academic Publisher, Dordrecht. 195–217.
- 2.Frigaard, N. U., and D. A. Bryant. 2004. Seeing green bacteria in a new light: genomics-enabled studies of the photosynthetic apparatus in green sulfur bacteria and filamentous anoxygenic phototrophic bacteria. Arch. Microbiol. 182:265–276. [DOI] [PubMed] [Google Scholar]
- 3.Staehelin, L. A., J. R. Golecki, R. C. Fuller, and G. Drews. 1978. Visualization of the supramolecular architecture of chlorosomes (Chlorobium type vesicles) in freeze-fractured cells of Chloroflexus aurantiacus. Arch. Microbiol. 119:269–277. [Google Scholar]
- 4.Staehelin, L. A., J. R. Golecki, and G. Drews. 1980. Supramolecular organization of chlorosomes (chlorobium vesicles) and of their membrane attachment sites in Chlorobium limicola. Biochim. Biophys. Acta. 589:30–45. [DOI] [PubMed] [Google Scholar]
- 5.Psencik, J., T. P. Ikonen, P. Laurinmäki, M. C. Merckel, S. J. Butcher, R. E. Serimaa, and R. Tuma. 2004. Lamellar organization of pigments in chlorosomes, the light harvesting complexes of green photosynthetic bacteria. Biophys. J. 87:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohmann-Marriott, M. F., R. E. Blankenship, and R. W. Roberson. 2005. The ultrastructure of Chlorobium tepidum chlorosomes revealed by electron microscopy. Photosynth. Res. 86:145–154. [DOI] [PubMed] [Google Scholar]
- 7.Liaaen-Jensen, S. 1965. Bacterial carotenoids. XVIII. Aryl-carotenes from Phaeobium. Acta Chem. Scand. 19:1025–1030. [DOI] [PubMed] [Google Scholar]
- 8.Francke, C., and J. Amesz. 1997. Isolation and pigment composition of the antenna system of four species of green sulfur bacteria. Photosynth. Res. 52:137–146. [Google Scholar]
- 9.Glaeser, J., L. Bañera, H. Rutters, and J. Overmann. 2002. Novel bacteriochlorophyll e structures and species-specific variability of pigment composition in green sulfur bacteria. Arch. Microbiol. 177:475–485. [DOI] [PubMed] [Google Scholar]
- 10.Klinger, P., J. B. Arellano, F. E. Vacha, J. Hala, and J. Psencik. 2004. Effect of carotenoids and monogalactosyl diglyceride on bacteriochlorophyll c aggregates in aqueous buffer: implications for the self-assembly of chlorosomes. Photochem. Photobiol. 80:572–578. [DOI] [PubMed] [Google Scholar]
- 11.Arellano, J. B., J. Psencik, C. M. Borrego, Y. Z. Ma, R. Guyoneaud, J. Garcia-Gil, and T. Gillbro. 2000. The effect of carotenoid biosynthesis inhibition on the organisation and function of chlorosomes from Chlorobium phaeobacteroides CL1401. Photochem. Photobiol. 71:715–723. [DOI] [PubMed] [Google Scholar]
- 12.Arellano, J. B., C. M. Borrego, A. Martinez-Planells, and L. J. Garcia-Gil. 2001. Effect of carotenoid deficiency on cells and chlorosomes of Chlorobium phaeobacteroides. Arch. Microbiol. 175:226–233. [DOI] [PubMed] [Google Scholar]
- 13.Brune, D. C., T. Nozawa, and R. E. Blankenship. 1987. Antenna organization in green photosynthetic bacteria. 1. Oligomeric bacteriochlorophyll c as a model for the 740 nm absorbing bacteriochlorophyll c in Chloroflexus aurantiacus chlorosomes. Biochemistry. 26:8644–8652. [DOI] [PubMed] [Google Scholar]
- 14.Trüper, H. G., and N. Pfennig. 1992. The family Chlorobiaceae. In The Prokaryotes. A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications. A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer, editors. Springer-Verlag, Berlin. 3583–3592.
- 15.Frigaard, N. U., K. Matsuura, M. Hirota, M. Miller, and R. P. Cox. 1998. Studies of the location and function of isoprenoid quinones in chlorosomes from green sulfur bacteria. Photosynth. Res. 58:81–90. [Google Scholar]
- 16.Borrego, C. M., and L. J. Garcia-Gil. 1994. Separation of bacteriochlorophyll homologues from green photosynthetic sulfur bacteria by reversed-phase HPLC. Photosynth. Res. 41:157–163. [DOI] [PubMed] [Google Scholar]
- 17.Oelze, J. 1985. Analysis of bacteriochlorophylls. Methods Microbiol. 18:257–284. [Google Scholar]
- 18.Sauer, K., J. R. Lindsay-Smith, and A. J. Schultz. 1986. The dimerization of chlorophyll a, chlorophyll b, and bacteriochlorophyll in solution. J. Am. Chem. Soc. 88:2681–2688. [Google Scholar]
- 19.Borrego, C. M., J. B. Arellano, C. A. Abellà, T. Gillbro, and L. J. Garcia-Gil. 1999. The molar extinction coefficient of bacteriochlorophyll e and the pigment stoichiometry in Chlorobium phaeobacteroides. Photosynth. Res. 60:257–264. [Google Scholar]
- 20.Britton, G. 1995. UV/visible spectroscopy. In Carotenoids, Vol. 1B: Spectroscopy. G. Britton, S. Liaaen-Jensen, and H. Pfander, editors. Birkhäuser, Basel. 13–62.
- 21.Martinez-Planells, A., J. B. Arellano, C. M. Borrego, C. Lopez-Iglesias, F. Gich, and J. Garcia-Gil. 2002. Determination of the topography and biometry of chlorosomes by atomic force microscopy. Photosynth. Res. 71:83–90. [DOI] [PubMed] [Google Scholar]
- 22.Repeta, D. J., D. J. Simpson, B. B. Jørgensen, and H. W. Jannasch. 1989. Evidence for anoxygenic photosynthesis from the distribution of bacteriochlorophylls in the black-sea. Nature. 342:69–72. [DOI] [PubMed] [Google Scholar]
- 23.Overmann, J., H. Cypionka, and N. Pfennig. 1992. An extremely low-light-adapted phototrophic sulfur bacterium from the Black Sea. Limnol. Oceanogr. 37:150–155. [Google Scholar]
- 24.Airs, R. L., C. M. Borrego, L. J. Garcia-Gil, and B. J. Keely. 2001. Identification of the bacteriochlorophyll homologues of Chlorobium phaeobacteroides strain UdG6053 grown at low light intensity. Photosynth. Res. 70:221–230. [DOI] [PubMed] [Google Scholar]
- 25.Guyoneaud, R., C. M. Borrego, A. Martinez-Planells, E. T. Buitenhuis, and L. J. Garcia-Gil. 2001. Light responses in the green sulfur bacterium Prosthecochloris aestuarii: Changes in prosthecae length, ultrastructure, and antenna pigment composition. Arch. Microbiol. 176:278–284. [DOI] [PubMed] [Google Scholar]