Abstract
Ca2+ efflux from the sarcoplasmic reticulum decreases when store Ca2+ concentration falls, particularly in skinned fibers and isolated vesicles where luminal Ca2+ can be reduced to very low levels. However ryanodine receptor activity in many single channel studies is higher when the luminal free Ca2+ concentration is reduced. We investigated the hypothesis that prolonged exposure to low luminal Ca2+ causes conformational changes in calsequestrin and deregulation of ryanodine receptors, allowing channel activity to increase. Lowering of luminal Ca2+ from 1 mM to 100 μM for several minutes resulted in conformational changes with dissociation of 65–75% of calsequestrin from the junctional face membrane. The calsequestrin remaining associated no longer regulated channels. In the absence of this regulation, ryanodine receptors were more active when luminal Ca2+ was lowered from 1 mM to 100 μM. In contrast, when ryanodine receptors were calsequestrin regulated, lowering luminal Ca2+ either did not alter or decreased activity. Ryanodine receptors are regulated by calsequestrin under physiological conditions where calsequestrin is polymerized. Since depolymerization occurs slowly, calsequestrin can regulate the ryanodine receptor and prevent excess Ca2+ release when the store is transiently depleted, for example, during high frequency activity or early stages of muscle fatigue.
INTRODUCTION
In this study we address the effect of low luminal Ca2+ on the conformation of the Ca2+-binding protein calsequestrin (CSQ) and on its ability to regulate the ryanodine receptor (RyR) Ca2+ release channel. We also address the way in which this regulation may define the RyRs response to a fall in luminal [Ca2+]. Ca2+ release from the sarcoplasmic reticulum (SR) of skeletal and cardiac muscle fibers is determined by the amount of Ca2+ that is loaded into the store under resting conditions (1–4). Store-dependent Ca2+ release is also found in many other cell types (5–7). The effect of store load on Ca2+ release is particularly apparent in isolated skinned muscle fibers or SR vesicle preparations where store Ca2+ can be reduced to very low concentrations (4–8). In contrast to the consistent results with whole cell and vesicle studies, RyR channels in lipid bilayer systems have been found to respond in a variety of ways to changes in luminal [Ca2+]. For example, changing luminal Ca2+ between 0.5 or 50 μM to ∼1 mM either activates, inhibits, or causes biphasic changes in RyR activity (9–13). These different responses do not appear to be correlated with the use of native or purified RyRs or with the presence of the cytoplasmic channel activators Ca2+ or ATP but may be due to the presence or absence of Ca2+-sensitive proteins on the luminal side of the channel. It is known that there are sensors for [Ca2+] on the RyR protein and/or on luminal proteins that are associated with the RyR (13–15). Little is known about these Ca2+ detectors or the interactions between them and the RyR.
CSQ is the major Ca2+-binding protein in the lumen of the SR where it forms a linear polymer that is seen to be closely associated with the junctional face membrane in ultrastructural images (16). CSQ inhibits the RyR when anchoring proteins triadin and junctin are present (15,17,18). Triadin and junctin are both transmembrane proteins which bind to CSQ and the RyR to form a stable quaternary (RyR/Tri/Jun/CSQ) complex (19). CSQ is a prime candidate for a luminal [Ca2+]-detector because Ca2+ stabilizes CSQ conformation and the CSQ polymer (20). Although CSQ binds to triadin and junctin when [Ca2+]s are lowered (19), reducing luminal [Ca2+] may destabilize the CSQ polymer and alter the way in which it influences RyR activity. Several lines of evidence support this theory. First, we have shown that the RyR/Tri/Jun/CSQ complex facilitates the response of the skeletal RyR to increases in luminal [Ca2+] from 1 to 10 mM (21). Second, a reconstituted RyR/Tri/Jun/CSQ complex is necessary for the cardiac RyR to respond to an increase in luminal Ca2+ from 20 μM to 5 mM (15). Cardiac CSQ exists as a monomer when the [Ca2+] is low (22). Cardiac and skeletal CSQ are the products of different genes and contain sequence differences in the proposed Ca2+-binding sites (23,24). The response of skeletal CSQ to changes in luminal Ca2+ between 100 nM and 1 mM has not been reported previously, to our knowledge.
We have examined the effects of luminal Ca2+ on the conformation of CSQ and the ability of the Ca2+-binding protein to regulate the RyR under conditions where CSQ exists either mostly as a multimer or mostly as a monomer. Luminal [Ca2+] was varied between physiological levels of 1 mM and lower levels (100 μM, 1 μM, or 100 nM). Lowering luminal [Ca2+] resulted in conformational changes in CSQ, with a reduction in the amount of CSQ polymer and an increase in monomer content. In addition, a reduction in luminal [Ca2+] caused a slow dissociation of a fraction of CSQ from the junctional face membrane and a loss of CSQ regulation of RyR activity. When CSQ was either fully dissociated from the RyR (by high ionic strength (25)) or unable to regulate the channel (at low luminal Ca2+—results from this study), the channels were rapidly activated by lowering luminal Ca2+ to nanomolar levels. When RyRs were not subjected to CSQ-dissociation/deregulation procedures, channel activity was either initially unchanged or rapidly reduced by lowering luminal [Ca2+], depending on the degree of cytoplasmic Ca2+ activation. The results suggest that a), CSQ protects the Ca2+ stores from excess Ca2+ depletion by reducing RyR activity when the luminal [Ca2+] is lower than the physiological concentration (∼1 mM), and b), the ability of CSQ to perform this function depends on it maintaining its native polymer conformation. The data also suggest that CSQ is only one of several [Ca2+] detectors on the luminal side of the skeletal muscle RyR. Taken together with our previous studies (26), the effects of CSQ on the RyR suggest that the Ca2+-binding protein would enhance the increase in Ca2+ release from the SR when the store is fully loaded but protects the SR from Ca2+ depletion when the Ca2+ load falls.
METHODS
Materials
The monoclonal VD111D12 anti-CSQ antibody was obtained from Affinity Bio-Reagents (Gymea, Australia). Other chemicals were from Sigma-Aldrich (Castle Hill, Australia).
SR vesicles
Back and leg muscle was removed from New Zealand male white rabbits, and SR vesicles were prepared using standard methods (27) with minor changes (28). Briefly, a crude microsomal preparation was extracted from tissue via differential centrifugation. After sucrose gradient fractionation, RyR-enriched heavy SR vesicles were collected from the 35%/45% interface, snap frozen, and stored in liquid nitrogen or at −70°C.
Junctional face membrane
Junctional face membrane was prepared by solubilization of RyR-enriched heavy SR vesicles by 0.5% Triton X-100, using the methodology published by Costello et al. (29) with minor changes (30). The junctional face membrane preparation contains fragments of the terminal cisternae membrane (not membrane vesicles) (29) and associated SR proteins which face the triad junction between the terminal cisternae and the sarcolemma. These proteins include the RyR, CSQ, triadin, and junctin. Comparisons of native SR vesicles (used in bilayer experiments) and solubilized junctional face membrane (used in this experiment with SDS-PAGE and immunoblot) have shown that the relative amounts of RyR, CSQ, triadin, and junctin are the same in both preparations (26,30).
CSQ expression and purification
CSQ was either purified from rabbit skeletal muscle tissue or recombinant cDNA cloned and expressed in Escherichia coli and subsequently purified by affinity chromatography. To purify CSQ from tissue, junctional face membrane fragments (prepared as above) were exposed to 0.5 M NaCl or 2 mM EGTA. After a second high-speed centrifugation at 48,000 × g for 1 h, the supernatant containing solubilized CSQ was collected and dialyzed overnight against either 1 mM CaCl2, 100 mM KCl, and 10 mM TES (pH 7.4) to remove residual triton X-100 for use in subsequent single channel recording experiments, or 20 mM MOPS, 50/150 mM NaCl, and 200 μM EGTA (pH 7.4) for analytical ultracentrifugation, stopped-flow, spectrophotometry and cross-linking experiments.
Recombinant rabbit skeletal muscle CSQ was cloned, expressed, and purified as previously described (26). In brief, after subcloning of CSQ into a pGEX5x1 vector (containing an N-terminal GST tag), CSQ was expressed in E. coli strain BL21DE3 colonies and purified by glutathione sepharose 4B chromatography. CSQ was then cleaved from the GST-glutathione sepharose 4B complex by incubation with Factor Xa for 16 h at 25°C. Eluted CSQ was dialyzed against the same buffer as muscle CSQ according to different experiments. Purified CSQ was stored at −70°C.
Electrophoresis and immunoblot—determination of CSQ association with junctional face membrane
SDS-PAGE was performed using the Laemmli buffer system (31) with 7.5%, 10%, or 4–15% gradient polyacrylamide gels. Immunoblot was as per Towbin et al. (32). To determine the effect of [Ca2+] on CSQ association with the RyR/Tri/Jun complex, junctional face membrane was resuspended at 3 mg/ml in bilayer-like buffer containing 230 mM CsMS, 20 mM CsCl, 1mM CaCl2, 10 mM TES (pH 7.4) in the presence of protease inhibitors (1 μg/ml aprotinin, 1 μM benzamadine, 1 μg/ml leuopeptin, and 1 μM pepstatin A). The suspension was divided into four 250-μl fractions and incubated at 4°C for 1 h in 1 mM, 100 μM, 1 μM, or 100 nM Ca2+ after the appropriate adjustment of [Ca2+] by the addition of BAPTA: 0.9 mM (for 100 μM Ca2+), 1.4 mM (for 1 μM Ca2+), and 5.4 mM (for 100 nM Ca2+). Fractions were then ultracentrifuged at 100,000 × g for 20 min. After centrifugation, the supernatant was removed and the pellet was resuspended in 250 μl of the bilayer-like buffer. The CSQ content of the resuspended pellets and supernatants were analyzed by electrophoresis and immunoblot. Images of SDS gel electrophoresis and immunoblot were taken by Genesnap 6.0 and quantified by Genetools Analysis Software 3.0 (Synoptics, Cambridge, UK).
Analytical centrifugation
The relative molecular mass of CSQ is indicative of the degree of association of CSQ molecules (i.e., monomer, dimer, or polymer). To determine relative molecular mass at different [Ca2+]s, sedimentation equilibrium experiments were performed in a Beckman optima XL-A analytical ultracentrifuge (Beckman Instruments, Fullerton, CA) using 12-mm double sector cells and an An-60Ti rotor. Purified recombinant CSQ was dialyzed against 20 mM MOPS, 50/150 mM NaCl, and 200 μM EGTA before the experiments. The final absorbance of sample was adjusted to 0.4–0.7 at A279. After adding an appropriate amount of CaCl2 to achieve a final [Ca2+] of 100 nM, 100 μM, or 1 mM, the sample was incubated on ice for 30 min before being loaded into the centrifugation cell. Sedimentation runs were carried out at a rotor speed of 15,000–40,000 × g at 10°C. A higher speed centrifugation was required to reach equilibrium at lower [Ca2+] and was taken into account in the final analysis. The data obtained in these experiments were collected in digital form using the step scan mode set at 1-mm radial increments. Each data point was the average of 10 separate absorbance measurements taken at each radial position. Finally, the molecular mass of sample was obtained using the Ultrascan data analysis program.
Kinetic measurements
Stopped-flow spectrophotometry studies were carried out to examine the kinetics of conformational changes in CSQ using an Applied Photophysics (Leatherhead, UK) SX 18.MV stopped-flow spectrophotometer with an emission monochromator attachment for multiwavelength detection. Purified dialyzed recombinant CSQ in one chamber was initially incubated in a solution containing 1 mM, 100 μM, 100 nM, or 4 nM Ca2+ before mixing. The [Ca2+] was then instantaneously either increased to 1 mM or 100 μM or decreased to 100 μM, 100 nM, or 4 nM by rapid mixing with dialysis buffer in the second chamber containing appropriate amounts of CaCl2 and EGTA. In the regular mixing mode, the contents of the chambers were symmetrically driven into an optical cell by a flush drive ram at 20°C. The fluorescence of the mixed sample in the cell was measured at an emission wavelength of 295 nm for 5, 50, or 200 s. Analysis of the data was carried out using Pro-Kineticist software (Applied Photophysics). Intrinsic fluorescence of tryptophan residues in CSQ was measured. A change in fluorescence indicated a change in the environment of the tryptophan residues. The increase in fluorescence can be explained by a change to a less hydrophilic environment. This is entirely consistent with the shielding of these residues that occurs upon the formation of multimers. Conversely, a decrease in fluorescence would be observed upon dissociation into monomers.
Cross-linking
To visualize the degree of association of CSQ (i.e., dimerization or polymerization) at various [Ca2+]s, 10 μg of purified skeletal muscle or recombinant CSQ (dialyzed as above, at a concentration of >0.5 mg/ml) was incubated at room temperature for 10 min after the [Ca2+] was adjusted to 100 nM, 100 μM, or 1 mM in the presence of 50 mM or 150 mM NaCl and 20 mM MOPS. The samples were further incubated with the cross-linker 20 mM 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and 50 mM N-hydroxysuccinimide (NHS) for 15 min at room temperature. The reaction was stopped by the addition of 1:1 SDS-PAGE sample buffer and loaded on 7.5% or 4–15% gradient gel immediately for electrophoresis and subsequent immunoblotting.
Single channel recording
Lipid bilayers were formed across a 150-μm aperture in a delrin cup, which separated two solutions, denoted cis (cytoplasmic) and trans (luminal). The artificial lipid composition was as follows: phosphatidylethanolamine, phosphatidylserine, phosphatatidylcholine (5:3:2 v/v), as described previously (25). SR vesicles (50 μg) were added to the cis solution so that the cytoplasmic surface of the SR and RyRs faced that solution after incorporation into the lipid bilayer (25). For SR vesicle incorporation, the standard solution compositions were as follows: cis: 230 mM CsMS, 20 mM CsCl, 50 μM or 1 mM CaCl2, and 10 mM TES (pH 7.4); and trans: a), physiological Ca2+ solution: 30 mM CsMS, 20 mM CsCl, 1 mM CaCl2, and 10 mM TES (pH 7.4); b), 100 μM Ca2+ solution, with the same composition as the physiological solution except that the [Ca2+] was 100 μM; or c), 100 nM Ca2+ solution: 30 mM CsMS, 20 mM CsCl, 10 mM TES, 100 μM Ca2+, and 0.218 mM EGTA (pH 7.4). For recording, the following changes to standard solutions were made after channel incorporation. In the cis chamber, 2 mM ATP was added and [Ca2+] was lowered to 50 μM (by the addition of 0.95 mM EGTA or 0.96 mM BAPTA) or 100 nM (by the addition of either 1.71 mM EGTA or 4.25 mM BAPTA). In the trans chamber, Cs+ was raised to 250 mM by the addition of 200 mM CsMS. When using a trans solution with 1 mM Ca2+ for incorporation, trans [Ca2+] was subsequently lowered from 1 mM to 100 μM or 1 μM (by addition of 0.9 mM or 1.3 mM BAPTA or 0.9 mM or 1.07 mM EGTA) or from 1 μM to 100 nM (by addition of 2.95 mM BAPTA or 0.64 mM EGTA). Alternatively, when channels were incorporated at low trans [Ca2+] (100 μM or 100 nM), trans [Ca2+] was then increased to 1 mM by the addition of 0.9 mM or 1.28 mM CaCl2. All electrical potentials are expressed here using standard physiological convention (i.e., cytoplasmic side relative to the luminal side at virtual ground). Single channel recordings were obtained using bilayer potential differences of +40 or −40 mV. Measurements were carried out at 23°C ± 2°C. A fall in luminal pH from 7.4 to 7.0 was measured when EGTA (buffered to a pH of 7.4 by 10 mM TES) was added to the bilayer solutions; however, this was not sufficient to alter RyR activity (33). There was no change in pH when BAPTA, ATP, or CaCl2 were added to the solutions.
Single channel analysis
Single channel parameters were obtained using the Channel2 program (developed by P. W. Gauge and M. Smith, John Curtin School of Medical Research, Canberra, Australia). Channel activity was assessed from a 30-s record and was expressed as relative open probability (Po). In this study, Po was measured in two ways, either by using a threshold discriminator, which is set above the baseline noise at ∼20% of maximal current (this was used where only one channel opened and allows detection of any substates of channel openings >2 pA), or from fractional mean current (I′F), which is the average of all data points obtained during a recording period, divided by the maximum single channel current (used when 2 or 3 channels opened simultaneously). For the Po measurement, records are carefully checked by eye before analysis to make sure that the noise level is less than the level of the threshold discriminator. Po was obtained from total open time divided by total recording time of the channel trace (usually 30 s). The accuracy of the I′F measurement depends on the noise level being equally distributed above and below the baseline so that mean current is close to zero when the channel is inactive. Therefore we corrected fluctuations in baseline levels with the program Baseline (developed by Dr. D. R. Laver, University of Newcastle, Callaghan, Australia). I′F is approximately equal to the Po measured by threshold discrimination when most of channel openings are to the maximum conductance so that relative Po = relative I′F. Therefore, regardless of whether Po was measured directly or as I′F, all normalized data are expressed as relative Po.
CSQ dissociation in bilayer experiments
Trans [Cs+] was increased from 250 mM (control) to 500 mM to dissociate CSQ. This treatment has been shown to dissociate >95% CSQ from the junctional face membrane (25). After an increase in channel activity consistent with CSQ dissociation (25), the trans chamber was perfused with control solution (250 mM Cs+) to both lower ionic strength and remove dissociated CSQ. Replacing the high ionic strength solution with control solution (250 mM Cs+) did not alter channel activity, indicating that the increased activity was caused by an irreversible event, such as CSQ dissociation, which was seen under the same conditions using electrophoresis and immunoblot (25). Therefore, the change in channel activity induced by high luminal ionic strength is believed to be due to CSQ dissociation.
Statistics
Average data are presented as mean ± SE. The significance of differences between control and test values was tested using an analysis of variance (ANOVA, single factor or two-factor without replication. Two-factor was used unless otherwise stated). A Student's t-test for paired data or a sign test (34) was used as appropriate. A P value of <0.05 was considered to be significant.
RESULTS
Effects of low Ca2+ on the CSQ polymer
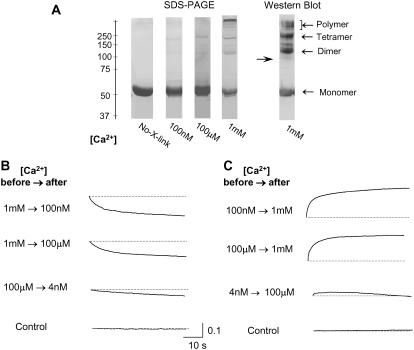
The degree of CSQ polymerization in solutions containing 1 mM, 100 μM, or 100 nM Ca2+ was determined. Purified recombinant CSQ was exposed to each [Ca2+] and then cross-linked with the carboxyl-amine cross-linker EDC and NHS. SDS-PAGE of the cross-linked CSQ showed that at 1 mM Ca2+ much of the CSQ existed as tetramers and higher molecular mass polymers (i.e., CSQ at a molecular mass that is greater than that of a tetramer, i.e., >220 kDa) at 1 mM Ca2+. The resolution at the top of the gel in Fig. 1 allows clear identification of hexamers. Higher molecular mass entities can be seen, but their exact molecular mass not resolved. In contrast to the distribution at 1 mM Ca2+, most of the CSQ was monomeric at 100 μM and 100 nM. Minimal amounts of CSQ existed as dimers or tetramers, and higher molecular mass polymers were not observed at the lower [Ca2+] (Fig. 1 A, lanes 2–4). That the higher molecular mass moieties were CSQ polymers was confirmed by immunoblot using anti-CSQ antibody (Fig. 1 A, lane 5). The existence of CSQ polymers at 1 mM Ca2+ is consistent with ultrastructural observations of muscle fibers fixed under physiological conditions where luminal Ca2+ is 1 mM. These studies show that ribbons of CSQ believed to be CSQ polymers (16) are associated with the junctional face membrane. Thus CSQ in situ under physiological conditions is a polymer. The results in Fig. 1 A show that isolated CSQ retains a largely polymer structure if maintained in 1 mM Ca2+. However, if Ca2+ is lowered to ≤100 μM for prolonged periods, it dissociates into monomers.
FIGURE 1.
Cross-linking and stopped-flow data using purified recombinant CSQ suggest reversible conformational changes including polymerization at 1 mM Ca2+ and depolymerization at 100 μM and 100 nM Ca2+. (A) 7.5% SDS-PAGE after cross-linking revealed that CSQ was mainly a monomer at 100 nM and 100 μM Ca2+ (lanes 2 and 3) but existed as both monomers and multimers at 1 mM Ca2+ (lane 4). Monomeric CSQ not exposed to cross-linker is shown in lane 1 as a control. Immunoblot of the cross-linked CSQ (using anti-CSQ antibody) confirmed that all bands contained CSQ and confirmed the presence of monomers and multimers at 1 mM Ca2+. (B and C) show changes in fluorescence detected in a stopped-flow kinetic study at wavelength 295 nm, temperature 20°C. (B) A decrease in CSQ fluorescence (indicating an increased hydrophilic environment around tryptophan residues) is apparent when Ca2+ is lowered from 1 mM Ca2+ to 100 nM or 100 μM (first and second panels). Only a slight decrease in the fluorescence signal was detected when Ca2+ was dropped from 100 μM to 4 nM (third panel). There was no fluorescence signal in control experiments with buffers only (fourth panel). (C) An increase in fluorescence (indicating a decreased hydrophilic environment around tryptophan residues) occurred when Ca2+ was increased from 100 nM or 100 μM to 1 mM (top two panels). There was only a small increase in fluorescence when Ca2+ was raised from 4 nM to 100 μM (third panel). These results indicate reversible Ca2+-dependent conformational changes occurring in CSQ with changes in [Ca2+], and these conformational changes mostly occur within the physiological range of 100 μM–1 mM. In control experiments, there was no change in fluorescence when buffer [Ca2+] was changed in the absence of CSQ. Each record is the average of at least three experiments. The vertical scale is in arbitrary fluorescence units.
Conformational changes with alterations in [Ca2+] were examined using stopped-flow analysis of the intrinsic fluorescence of tryptophan residues in CSQ (Methods). When the [Ca2+] was decreased from 1 mM to 100 μM or 100 nM, a decrease in fluorescence (indicating an increased hydrophilic environment, consistent with protein unfolding and/or depolymerization) occurred over a 50-s period (Fig. 1 B). When [Ca2+] was raised from 100 nM or 100 μM to 1 mM, an increase in fluorescence over a similar time course indicated a decreased hydrophilic environment and protein folding and/or polymerization (Fig. 1 C). Interestingly, no significant change in fluorescence was seen when Ca2+ was varied between 100 μM and 4 nM (Fig. 1, B and C), indicating that the structural changes detected with the stopped-flow analysis mostly occurred within a physiological range of luminal Ca2+ between 100 μM and 1 mM. These changes in fluorescence occur within the period that changes were observed in single channel activity (below) and are consistent with the cross-linking data, which showed a decreased association between CSQ moieties when [Ca2+] was 100 nM or 100 μM compared with that at 1 mM Ca2+. It is not clear whether the conformational changes detected in stopped-flow reflect changes in the folding and compaction of individual CSQ molecules that precede polymerization/depolymerization or whether it reflects the polymerization process itself. In either case, the stopped-flow data are indicative of effects of Ca2+ on CSQ conformation that are consistent with the final polymerization/depolymerization seen in the cross-linking study. Both the cross-linking and stopped-flow experiments were conducted at physiological ionic strength (150 mM NaCl). Since it has been suggested that monovalent ions disrupt the polymerization of CSQ (22), cross-linking was repeated at low ionic strength (50 mM NaCl). However even under low ionic strength conditions, minimal CSQ dimer and tetramer formation was observed with 1 mM Ca2+ (data not shown).
Changes in CSQ conformation were also examined by measuring particle molecular mass using analytical centrifugation of purified recombinant CSQ exposed to 1 mM, 100 μM, and 100 nM Ca2+ in the presence of either 150 mM or 50 mM NaCl. The apparent molecular mass was more than twofold higher when [Ca2+] was 1 mM than when [Ca2+] was 100 nM (with 150 mM NaCl, Table 1). The molecular mass of CSQ in the presence of 100 nM Ca2+ was consistent with the calculated monomeric molecular mass for CSQ of 48 kDa. The twofold increase in apparent molecular mass is not likely to simply reflect dimer formation, since the cross-linking data show that ∼45% CSQ is a monomer at 1 mM Ca2+. Since 45% monomer plus 55% dimer would give an apparent molecular mass of only 74 kDa, higher molecular mass entities must exist. For example, an apparent molecular mass of ∼100 kDa would be consistent with 45% monomer, 37% dimer, 12% tetramer, and 6% octamer. Therefore, a twofold increase in apparent molecular mass in 1 mM Ca2+ is consistent with the coexistence of monomers, dimers, tetramers, and the higher molecular mass forms seen in the cross-linking study (Fig. 1 A). A similar increase in apparent molecular mass (2.3-fold) was detected with low ionic strength (50 mM Na+) at 1 mM Ca2+ (data not shown). There was a relatively small change in apparent molecular mass between 100 nM and 100 μM Ca2+, suggesting that the protein was mostly monomeric over this concentration range. The results further indicate that dimerization and polymerization occur when Ca2+ is increased from 100 μM and 1 mM, as was also indicated by the stopped-flow and cross-linking data.
TABLE 1.
Sedimentation equilibrium data showing different average size of CSQ at 100 nM, 100 μM, and 1 mM Ca2+ with 150 mM NaCl
| 100 nM Ca2+ | 100 μM Ca2+ | 1 mM Ca2+ | |||
|---|---|---|---|---|---|
| Molecular mass (kDa) (mean ± SE) | Relative molecular mass | Molecular mass (kDa) (mean ± SE) | Relative molecular mass | Molecular mass (kDa) (mean ± SE) | Relative molecular mass |
| 45.33 ± 1.52 | 1 | 65.24 ± 2.7 | 1.44 | 97.33 ± 5.13 | 2.15 |
Effects of low Ca2+ on CSQ association with the RyR/Tri/Jun/CSQ complex
SDS-PAGE and immunoblot analysis were used to determine whether skeletal muscle CSQ did dissociate from the RyR/Tri/Jun/CSQ complex under the same conditions in which conformational changes were seen in CSQ when Ca2+ was lowered. Solubilized junctional face membrane (obtained from skeletal muscle SR preparations) used in this experiment contained membrane proteins with some surrounding lipid (29). Thus the membrane pellet contained membrane fragments with membrane proteins embedded and their associated proteins which remained bound (e.g., the RyR/Tri/Jun/CSQ complex or RyR/Tri/Jun complex). The solubilized membrane did not form vesicles, so that proteins like CSQ that dissociated from the luminal side of membrane were free to diffuse into the solution. The supernatant contained material that was not associated with (or had dissociated from) the membrane fragments.
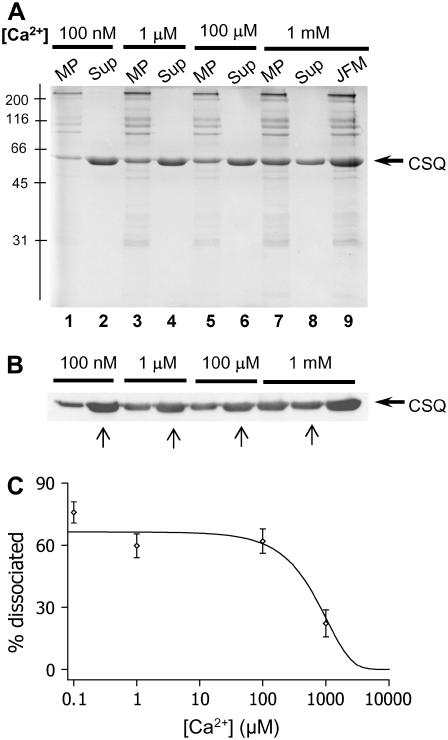
Solubilized junctional face membrane was exposed to [Ca2+]s of 1 mM, 100 μM, 1 μM, and 100 nM Ca2+ (Fig. 2, A and B) and the amounts of CSQ remaining in the membrane pellet and the amount dissociated (and present in the supernatant) compared with the amount found in the original fractions (Methods). Less CSQ was associated with the membrane pellet as the [Ca2+] fell (Fig. 2, A and C). Quantitation indicated that 20% of total CSQ was dissociated with 1 mM Ca2+, 62% with 100 μM Ca2+, 60% with 1 μM, and 76% with 100 nM Ca2+. The immunoblot analysis confirmed that the 55-kDa protein was CSQ (Fig. 2 B). Thus, although CSQ was removed from the RyR/Tri/Jun complex during exposure to low Ca2+ (particularly 100 nM Ca2+), a significant fraction remained associated with the membrane. This was in marked contrast to the >95% dissociation of CSQ from the junctional face membrane when ionic strength is increased to 500 mM or when Ca2+ is increased to >5 mM (25,26).
FIGURE 2.
CSQ is only partially dissociated from the RyR/Tri/Jun complex at low Ca2+. Solubilized junctional face membrane was exposed to different [Ca2+]s to determine the amount of CSQ dissociation. (A) 10% SDS polyacrylamide gel showing soluble protein which remained in the supernatant (Sup) and membrane bound protein, which was present in the resuspended membrane pellet (MP), obtained by centrifugation after the junctional face membrane (JFM) had been exposed to 100 nM (lanes 1 and 2), 1 μM (lanes 3 and 4), 100 μM (lanes 5 and 6), or 1 mM Ca2+ (lanes 7 and 8). Lane 9 shows the total JFM in the 1 mM Ca2+ buffer, before separation into soluble and membrane fractions, so that CSQ in this lane is the total CSQ (i.e., Sup plus MP). The position of the molecular mass markers are shown to the left of the gel. (B) Immunoblot of protein product shown in (A) immunostained with VIIIDl2 monoclonal anti-CSQ antibody. An equivolume (15 μls) of JFM (3 mg/ml), resuspended pellets and supernatant fractions (see Methods) were loaded to appropriate lanes for both the Coomassie blue stained gel and the immunoblot. The amount of CSQ in the supernatant increased and that associated with the pellet decreased as [Ca2+] was lowered. Arrows below the immunoblot show the supernatant lanes that were used for quantification. (C) Quantification (Methods) shows that the percentage of dissociated CSQ in the supernatant increased as [Ca2+] was lowered (n = 3–6). The percentage of dissociation was plotted as a function of [Ca2+] and was fitted with a sigmoid curve equation, which indicates 50% dissociation at ∼900 μM Ca2+. CSQ dissociation is presented as a percentage of the total CSQ present in the JFM before the exposure to different [Ca2+].
The reduction in the amount of CSQ associated with the junctional face membrane could have been due to either dissociation of all CSQ from a fraction of RyR/Tri/Jun complexes or to partial removal of CSQ from each RyR/Tri/Jun complex due to depolymerization of CSQ monomers not bound to the membrane complex. The second possibility is more likely because the data in Fig. 1 and Table 1 indicate that CSQ was largely depolymerized when luminal Ca2+ was 100 nM or 100 μM. Since depolymerized CSQ retains its ability to associate with triadin and junctin at low Ca2+ ((35) and N. A. Beard, M. Varsanyi, and A. F. Dulhunty, unpublished observation), it is likely that the residual terminal CSQ remained bound to the RyR/Tri/Jun complex, whereas the distal depolymerized CSQ dissociated from the complex.
Effects of prolonged exposure to low luminal Ca2+ on RyR channel activity
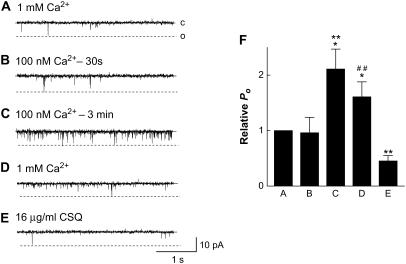
Native RyRs were incorporated into bilayers with 1 mM trans Ca2+ so that endogenous CSQ remained associated with the RyR/Tri/Jun complex and the CSQ polymer was stabilized (20,25,29,36). Channel activity can be recorded for 30 min or longer with multiple perfusions and addition of solutions lacking active compounds, with no consistent changes in activity (37). Therefore the changes in channel activity shown in Fig. 3 and subsequent figures are specific changes in response to changes in luminal [Ca2+]. When luminal (trans) Ca2+ was reduced to 100 nM, there was little initial change in channel activity during the subsequent 1-min recording period (Fig. 3 B). However, after ∼2–3 min, a sudden increase in activity was observed in 16 of 16 channels (Fig. 3 C). On average, there was an ∼2-fold increase in activity after >3-min exposure to 100 nM Ca2+. When trans Ca2+ was returned to 1 mM, channel activity fell but Po remained higher than control until 16 μg/ml of exogenous skeletal muscle CSQ was added to the solution (Fig. 3, D and E). The concentration of 16 μg/ml CSQ (∼0.39 μM) was chosen for several reasons. First, it is the minimal concentrations required to get observable regulation of the RyR (30). Second, higher concentration of CSQ had no further effect on channel activity (we tested up to 100 μg/ml, (30)). Finally, concentrations in this range have been found by others to regulate RyRs (15,38).
FIGURE 3.
Native RyR channels containing CSQ respond to low luminal Ca2+ with a delayed increase in activity. (A–E) Single channel records of 3 s of channel activity at −40 mV. Single channel opening is upward from zero current (c, continuous line) to maximum open conductance (o, broken line). Cytoplasmic (cis) [Ca2+] was 100 nM in each situation. (A) Control, with 1 mM trans Ca2+; (B) 30 s after lowering trans Ca2+ to 100 nM with 4.25 mM BAPTA; (C) 3 min after lowering trans Ca2+ to 100 nM; (D) after perfusing trans chamber with 1 mM Ca2+; (E) addition of 16 μg/ml CSQ; and (F) average data (n = 12–16) for relative open probability (relative Po) at +40 mV. Asterisks (*) indicate average values significantly different from control, and (**) and (##) indicate a significant difference from the previous condition (P < 0.05, Student's paired t-test (*, **), and sign test (##)).
Addition of purified recombinant CSQ to RyRs under the same conditions resulted in a similar drop in channel activity to control levels. The delayed increase in activity, with recovery only after addition of exogenous CSQ, was reminiscent of effects of CSQ dissociation from the RyR/Tri/Jun complex after increasing ionic strength or luminal Ca2+ (25,26). The similar result was surprising since dissociation of >95% CSQ from RyR/Tri/Jun complex with high ionic strength and polymer stabilization (25,30) is a very different physical process to polymer destabilization and dissociation of distal CSQ from residual CSQ that remains bound to triadin and junctin under low Ca2+ conditions. The delay before the increase in channel activity after reducing luminal Ca2+ was significantly longer than the delay after increasing luminal ionic strength or luminal Ca2+ (Table 2), supporting the hypothesis that different physical processes underpinned the increase in activity. The increase in activity suggests that even though ∼25% of CSQ remains bound, this residual CSQ no longer regulated the RyR even when the luminal [Ca2+] was restored to 1 mM. The fact that all channels showed a delayed increase in activity provides further evidence that some CSQ dissociated from each channel (see previous section) and hence that some CSQ also remained associated with each channel. We have defined RyRs that are no longer regulated by the residual CSQ as CSQ-deregulated RyRs.
TABLE 2.
Comparison of the delay before the increase in Po with different CSQ dissociation agents
| Procedure | N | Delay(s) | Mean ± SE(s) | Fraction dissociated (%) |
|---|---|---|---|---|
| High ionic strength500 mM Cs+ | 17 | 89 | 11 | >95 |
| High Ca2+5 mM | 9 | 110 | 17 | >90 |
| Low Ca2+100 nM | 11 | 203 | 23 | ∼76 |
A comparison of the delay before the increase in activity (Po) that signals deregulation of the RyR by complete or partial CSQ dissociation. The number of experiments (N) is given followed by the average time delay, the standard errors, and then the approximate fraction of CSQ dissociated from the junctional face membrane using high ionic strength (19), high Ca2+ (42), and low Ca2+ (Fig. 2, this study) detected by SDS-PAGE and immunoblot.
Raising [Ca2+] to 1 mM did not result in a full recovery of channel activity. This was presumably because the few molecules of CSQ that had dissociated were infinitely diluted in the 1.5-ml experimental solution and were unlikely to reassociate with the channel complex. Channel activity was not reduced to control levels until an appropriate CSQ concentration was achieved by adding excess exogenous CSQ to the solution. We previously reported that exogenous CSQ does not alter RyR activity when the endogenous CSQ polymer is already bound but reduces activity when it binds to the RyR/Tri/Jun complex lacking endogenous CSQ polymer (25,26). The significant decline in activity with CSQ addition in these experiments suggested that the exogenous CSQ was able to associate with the residual CSQ that remained bound to the RyR/Tri/Jun complex and restore the functional CSQ multimer. The small, but significant decline in activity when trans Ca2+ was initially increased to 1 mM is consistent with the effect of luminal Ca2+ per se on CSQ-deregulated RyRs (given in Results below).
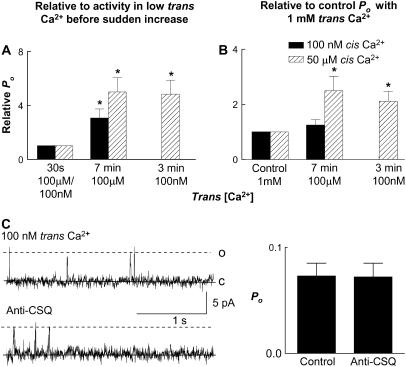
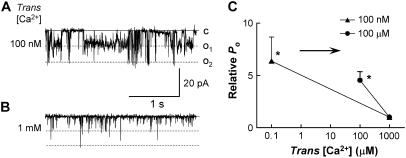
The data obtained from the stopped-flow, cross-linking, and analytical ultracentrifugation experiments suggested that conformational changes in CSQ occurred mostly in the [Ca2+] range between 100 μM to 1 mM. To determine whether the conformational changes in this range of [Ca2+]s also led to removal of CSQ regulation of RyRs, we examined the effect of reducing trans Ca2+ to 100 μM on single channel activity. RyR channels were incorporated at 1 mM trans Ca2+. There was a small initial decline in channel activity when trans Ca2+ was reduced to 100 μM (by adding BAPTA), which was greater with activating (50 μM) cis Ca2+ than with subactivating (100 nM) cis Ca2+ (see also Results below). After ∼7 min a sudden fourfold increase in activity was seen at both subactivating (100 nM) and activating (50 μM) cis Ca2+ (Fig. 4 A). There was a twofold increase relative to the initial control activity with 100 nM cis Ca2+ (Fig. 4 B). The immediate fall in Po when channels activated by 50 μM cis Ca2+ were exposed to 100 μM trans Ca2+ meant that the delayed fourfold increase did not raise Po above control values (Fig. 4 B). The sudden delayed increase in activity was consistent with the structural data and indicates that the conformational changes that lead to CSQ deregulation also occur when luminal Ca2+ is reduced to ≤100 μM.
FIGURE 4.
Delayed increase in channel activity with 100 μM trans Ca2+. An antibody test suggests CSQ deregulation of RyRs with low trans Ca2+. (A) Average relative open probability (relative Po). Po after 7-min exposure to 100 μM trans Ca2+ (after the delayed increase in activity), relative to Po with 100 μM trans Ca2+ before the delayed increase. Data are shown with 50 μM cis Ca2+ (solid bars, n = 14) and 100 nM cis Ca2+ (striped bars, n = 12). (B) Average relative Po, where Po after 7-min exposure to 100 μM trans Ca2+ is expressed relative to Po under control conditions with 1 mM trans Ca2+. The last column in A and B shows the late increase after 3-min exposure to 100 nM trans Ca2+ (included for comparison). (C) Channel records and average data using the anti-CSQ antibody to test for CSQ deregulation with 100 nM trans Ca2+. The left column shows 3-s records of channel activity at +40 mV. Single channel opening is upward from zero current (c, continuous line) to maximum open conductance (o, broken line). Cis [Ca2+] was 100 nM. The top panel shows channel activity after the delayed increase in activity with exposure to 100 nM trans Ca2+; the lower panel shows activity after addition of 6 μg/ml anti-CSQ antibody. The graph on the right shows average data (n = 8) for Po after the delayed increase in activity in 100 nM trans Ca2+ (first bin) and after the addition of the anti-CSQ antibody (second bin). There was no significant change in channel activity after antibody addition (Student's paired t-test).
It should be noted that lowering luminal Ca2+ from 1 mM to 100 μM or 100 nM resulted in an increase in conductance (apparent in Figs. 3–8) because of the removal of competition between Ca2+ and Cs+ within the pore, which is well known to reduce channel conductance (14).
FIGURE 5.
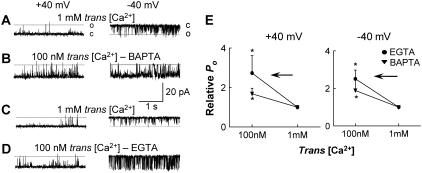
At subactivating 100 nM cis Ca2+, lowering trans [Ca2+] from 1 mM to 100 nM activates CSQ(-)RyRs. (A–D) Records of 3 s of single channel activity with channel opening upward from zero current (c, continuous line) to maximum open conductance (o, broken line) for the left panel (+40 mV) and downward from zero current (c) to maximal open conductance (o) in the right panel (−40 mV). Cis [Ca2+] was constant at 100 nM. Trans Cs+ was increased to 500 mM to dissociate CSQ and then lowered to 250 mM. (A) Control, with 1 mM trans Ca2+; (B) lowering trans Ca2+ to 100 nM with 4.25 mM BAPTA; (C–D) a repeat of A–B except with the use of 1.75 mM EGTA to lower trans Ca2+ from 1 mM to 100 nM; and (E) average data (n ≥ 5) for relative open probability (relative Po) for conditions shown in A–D at +40 mV (left panel) and −40 mV (right panel) for EGTA (•) and BAPTA (▾). The arrows in E indicate the direction of trans [Ca2+] change (from 1 mM to 100 nM). Asterisks (*) indicate average values significantly different from control (P < 0.05, Student's paired t-test).
FIGURE 6.
Lowering trans [Ca2+] from 1 mM to 100 nM activates CSQ(-) RyRs in channels activated by cis Ca2+. (A–C) Records of 3 s of channel activity at −40 mV. Single channel opening is downward from zero current (c, continuous line) to maximum open conductances (o, broken lines). Cis [Ca2+] was 50 μM. Trans Cs+ was increased to 500 mM to dissociate CSQ and then lowered to 250 mM. (A) Control, with 1 mM trans Ca2+; (B) after lowering trans Ca2+ to 1 μM with 1.07 mM EGTA; (C) after lowering trans Ca2+ to 100 nM by a further addition of 0.64 mM EGTA; and (D) average data (n = 6) for relative open probability (relative Po) at −40 mV (♦), and at +40 mV (▪), plotted as a function of trans [Ca2+]. Asterisk (*) indicate average values significantly different from control (P < 0.05, ANOVA). The arrow in D indicates the direction of trans [Ca2+] change.
FIGURE 7.
Increasing trans [Ca2+] from 100 nM or 100 μM to 1 mM results in inhibition of native CSQ-deregulated RyR channels activated by cis Ca2+. (A–C) Records of 3 s of channel activity at −40 mV. Single channel opening is downward from zero current (c, continuous line) to maximum open conductance (broken lines, o1 and o2). Cis [Ca2+] was constant at 50 μM. (A) Control, with 100 nM trans Ca2+; (B) after increasing trans Ca2+ to 1 mM by the addition of 1.28 mM CaCl2; and (C) average data for relative open probability (relative Po) after 100 nM trans Ca2+ incorporation (n = 14) (▴) and with 100 μM trans Ca2+ incorporation (n = 8)(•). Data are plotted as a function of trans [Ca2+]. A decrease in channel activity when trans Ca2+ was raised from 100 nM or 100 μM to 1 mM in these CSQ-deregulated RyRs shows the same response to luminal Ca2+ as CSQ(-)RyRs. Asterisks (*) indicate average values significantly different from control (P < 0.05, Student's paired t-test). The arrow shows the direction of the trans [Ca2+] change (in this case, from 100 nM or 100 μM to 1 mM).
FIGURE 8.
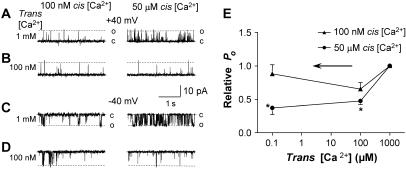
Lowering trans [Ca2+] to 100μM and 100 nM inhibits native untreated RyRs activated by 50 μM cis Ca2+. (A–D) Records of 3 s of single channel activity, at both +40mV (A–B) and −40 mV (C–D). Single channel opening is upward from zero current (c; continuous line) to maximum open conductance (o; broken line) at +40 mV and downward at −40 mV. Cis [Ca2+] was either 100 nM (left panels) or 50 μM (right panels). (A and C) Control, with 1 mM trans Ca2+ at +40 and −40 mV, respectively; (B and D) lowering trans Ca2+ to 100 nM with 4.25 mM BAPTA at +40 and −40 mV, respectively; and (E) average data for relative open probability (relative Po) as a function of trans [Ca2+]. Data obtained at subactivating (100 nM; ▴) and activating (50 μM; •) cis Ca2+ (n = 9–24). Asterisks (*) indicate average values significantly different from control (P < 0.05, Student's paired t-test). The arrow shows the direction of the trans [Ca2+] change (in this case, from 1 mM to 100 μM or 100 nM).
Anti-CSQ antibody was used as an additional probe for CSQ regulation of the RyR. The antibody reduces the activity of CSQ-regulated RyRs (25). After the delayed increase in channel activity during prolonged exposure to low luminal Ca2+, the antibody was added to the trans chamber and, in contrast to its effect on CSQ-regulated RyRs, it failed to alter the activity of channels (Po was 0.07 ± 0.003 before and 0.073 ± 0.004 after the addition of anti-CSQ antibody) (Fig. 4 C). This indicated that the RyRs were not regulated by the residual CSQ. The antibody binding to CSQ does not appear to be dependent on CSQ conformation. The antibody binds to unfolded CSQ in immunoblots (e.g., Fig. 2 B) as well as polymerized CSQ (25). Therefore its recognition of the CSQ epitope does not appear to depend on CSQ structure and we assume that it recognized the residual depolymerized CSQ remaining bound to triadin and junctin in low Ca2+ solutions. However in contrast to the RyR inhibition caused when the antibody binds to CSQ that is regulating RyR at 1 mM trans Ca2+, antibody binding to the residual CSQ that was not regulating RyR activity at 100 nM trans Ca2+ failed to alter RyR activity because there was no cross talk between the residual CSQ and the RyR.
Response of CSQ-depleted RyRs to a reduction in luminal [Ca2+]
To examine the effect of CSQ on the response of the RyR to lowering luminal Ca2+, control experiments were first conducted in the absence of CSQ. RyRs were treated with high ionic strength to fully dissociate CSQ (CSQ(-) RyRs) (25) and then the immediate effect of reducing trans Ca2+ from 1 mM to 100 nM examined. In this experiment, cytoplasmic [Ca2+] was 100 nM. Control Po was 0.0095 ± 0.003 (+40 mV) and 0.051 ± 0.037 (−40 mV). Addition of BAPTA, to reduce trans Ca2+, resulted in a rapid (within 1 min) 2.4-fold increase in relative Po at both positive and negative potentials (Fig. 5, A and B).
Since the increase in activity could have been due to the reduction in trans [Ca2+], or an effect of BAPTA per se, experiments were repeated using EGTA. Lowering [Ca2+] with EGTA also rapidly increased channel activity (Fig. 5, C and D). Control Po was 0.009 ± 0.02 (+40 mV) and 0.021 ± 0.007 (−40 mV) and there was a 3.5-fold increase within 1 min of reducing trans [Ca2+]. A small decrease in pH from 7.4 to 7.0 was measured when EGTA (buffered to a pH of 7.4 by 10 mM TES) was added; however this was not sufficient to alter RyR activity (33). There was no change in pH when BAPTA was added to the bilayer solutions (Methods). The similarity of the results obtained using both EGTA and BAPTA indicated that the rapid increase in the activity of CSQ(-) RyRs when Ca2+ was lowered was due to the fall in [Ca2+] and was independent of the Ca2+ buffer used.
Cytoplasmic [Ca2+] did not significantly alter the response of CSQ(-) RyRs to a fall in luminal [Ca2+]. The experiments were repeated with a cis [Ca2+] of 50 μM (activating [Ca2+]). The results in Fig. 6 again show an increase in CSQ(-) RyR activity when trans [Ca2+] was lowered. Activity immediately increased 2.1-fold from control levels after reducing Ca2+ to 1 μM (control Po was 0.016 ± 0.003 (+40 mV) and 0.082 ± 0.028 (−40 mV)). An additional, potential-independent increase in activity was observed after trans [Ca2+] was further lowered to 100 nM (Fig. 6). The subconductance activity apparent in the channel in Fig. 6 was typical of that seen in many RyR channels, particularly under conditions promoting long open times (cis Ca2+ of 1–100 μM and/or >0.5 mM ATP; A. F. Dulhunty, unpublished observations). Therefore the subconductance activity was more clearly seen when luminal Ca2+ fell and open times became longer (as is also seen in the channel in Fig. 3 above)—it did not appear to be specifically related to trans [Ca2+].
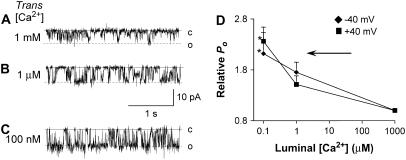
Next we examined how RyRs that were partially depleted of CSQ (and relieved of normal CSQ regulation by prolonged exposure to low luminal Ca2+) responded to changes in luminal [Ca2+]. RyRs were incorporated with a trans [Ca2+] of 100 nM at both activating (50 μM) and subactivating (100 nM) cis Ca2+. The usual delayed increase in channel activity, associated with the onset of CSQ deregulation, was observed ∼3 min after incorporation. RyRs relieved of CSQ regulation in this way responded to an increase in trans [Ca2+] to 1 mM with an immediate and sustained decrease in activity when cis Ca2+ was 50 μM (Fig. 7). A similar decrease in activity was seen in five experiments with 100 nM cis Ca2+ (data not shown). Although the fall in channel activity was significant at both +40 and −40 mV, the effects were most pronounced at negative potentials, with relative Po being reduced to almost 20% of that recorded in 100 nM Ca2+. Using a similar protocol, channels were incorporated at 100 μM trans Ca2+. In this case there was an increase in activity after ∼7 min, which suggested that CSQ regulation was removed. An increase in trans Ca2+ to 1 mM was then accompanied by decrease in channel activity to ∼30%, similar to the decrease seen when Ca2+ was increased to 1 mM after incorporation at 100 nM trans Ca2+ (Fig. 7 B). In conclusion, the response of CSQ-deregulated RyRs to changes in luminal Ca2+ between 100 nM (or 100 μM) and 1 mM was the same as the response of CSQ(-) RyRs. These experiments confirmed that, in the absence of an influence from CSQ, RyRs were more active at 100 nM or 100 μM trans Ca2+ than at 1 mM, and this occurred when trans Ca2+ was either reduced from high (1 mM) to low (100 μM or 100 nM) or increased from low (100 μM or 100 nM) to high (1 mM) concentrations.
Response to reduced luminal [Ca2+] when RyRs were not treated to dissociate CSQ
In the following experiments, RyRs were incorporated into bilayers with 1 mM trans Ca2+ to maintain the RyR/Tri/Jun/CSQ complex. The channels were not treated to dissociate CSQ (they were presumed to be associated with CSQ) and are referred to as “untreated” RyRs. Trans [Ca2+] was lowered to 100 μM or to 1 μM and then to 100 nM at both subactivating (100 nM) and activating (50 μM) cis [Ca2+]s. Although the low trans Ca2+ does dissociate ∼25% of CSQ, changes in channel activity associated with this process are slow (>3 min with 100 nM Ca2+ and >7 min with 100 μM Ca2+, data above), in contrast to the immediate luminal Ca2+-dependent changes in activity, which reflect the response of the RyR when it is regulated by CSQ. Therefore the response of channels to the change in trans Ca2+ was measured within ∼1 min of the addition of the Ca2+ chelator and a stirring step of 20 s, well before the delayed increase in channel activity was seen. In contrast to CSQ(-) RyRs, there was no immediate increase in the activity of these untreated RyRs when trans Ca2+ was reduced from 1 mM (to 100 μM or 100 nM) and the response of the untreated channels did depend on the cytoplasmic (cis) [Ca2+]. Channels examined with a cis Ca2+ of 100 nM had a Po of 0.078 ± 0.009 with 1 mM luminal Ca2+. Reducing luminal [Ca2+]s had little effect on their activity, with no significant effect on the average relative Po (Fig. 8, A–E, left). Two out of thirty-two channels with 100 nM cis Ca2+ responded in a potential-dependent manner (slight increase in activity at the negative potential, but a decrease at the positive potential), similar to the effect reported by Laver et al. (39). Nevertheless, overall there was no significant change when lowering trans Ca2+ from 1 mM to 100 μM at 100 nM cis Ca2+. In contrast, when the untreated channels were exposed to 50 μM cis Ca2+, lowering trans Ca2+ to 100 μM or to 100 nM caused a significant reduction in channel activity immediately after adding the Ca2+ chelating agents to the trans chamber (Fig. 8, A–E, right). When trans Ca2+ was lowered from 1 mM to 100 μM, there was a decrease in RyR activity with activating (50 μM) cis Ca2+ from Po of 0.19 ± 0.03 to 0.072 ± 0.009 (combined data at +40 and −40 mV).
In this series of experiments, a late increase in activity was again observed with 100 nM and 100 μM trans Ca2+ (data not shown), which presumably reflected CSQ depolymerization and partial dissociation from the RyR complex. Both the lack of a change in initial activity of the untreated channels when luminal Ca2+ was lowered in the presence of 100 nM cis Ca2+ and the reduction in activity observed with 50 μM cis Ca2+ were in marked contrast to the significant increase in activity seen when the same experiments were performed with CSQ(-) RyRs.
DISCUSSION
These novel results clearly demonstrate time-dependent conformational changes in CSQ when luminal Ca2+ is lowered from 1 mM to 100 μM, with little additional change when Ca2+ was further lowered to 100 nM. Consistent changes were observed in cross-linking, stopped-flow spectrophotometry, and sedimentation equilibrium coefficient experiments. These structural changes, likely to include depolymerization, occur under the same conditions as dissociation of a fraction of CSQ from the junctional face membrane. Nevertheless, a significant amount of the monomeric CSQ remained associated with the membrane. The destabilization of the polymer with low luminal Ca2+, like complete CSQ dissociation with high ionic strength, leads to an increase in RyR activity. We show for the first time that the response of the RyR to a fall in trans [Ca2+] to below normal physiological concentrations of 1 mM depends not only on the presence of CSQ but also on its structure. In the absence of CSQ or when CSQ is mostly in a monomeric form, the activity of RyRs increased rapidly (<1 min) when trans [Ca2+] was lowered to 100 μM, 1 μM, or 100 nM and did not change further during long exposures to the low trans [Ca2+]. In contrast, when CSQ had not been intentionally dissociated or structurally altered, a brief exposure to low trans [Ca2+] did not cause any change in channel activity when the cytoplasmic [Ca2+] was at resting levels (100 nM) and consistently reduced the activity of channels exposed to an activating cytoplasmic [Ca2+] of 50 μM. The results show clearly that CSQ multimer association with the Ca2+ release channel prevents the increase in channel activity that occurs in its absence and helps conserve Ca2+ when the store concentrations are low.
CSQ dissociation and depolymerization after exposure to low Ca2+
CSQ was partially dissociated from the solubilized junctional face membrane when [Ca2+] was lowered to 100 μM, 1 μM, or 100 nM. Indeed, CSQ has been reported to change from a random coil to a folded monomer, dimer, and polymer as the [Ca2+] rises from 0 to 1 mM (9,21,40,41) although the exact [Ca2+] which induces each conformation had not been determined. Our cross-linking data suggest that CSQ is mostly monomeric (with minimal amounts of dimers and tetramers) when [Ca2+] is between 100 nM and 100 μM and that significant dimerization, tetramerization, and higher molecular mass constructs form over a relatively narrow and physiological range of [Ca2+]s between 100 μM and 1 mM. Our results show conclusively that a), the majority of CSQ forms multimers at 1 mM Ca2+ but that the monomeric CSQ is stabilized at [Ca2+]s between 100 nM and 100 μM, b), ∼25% of the CSQ remains associated with the junctional face membrane even after exposure to [Ca2+]s as low as 100 nM, c), prolonged exposure to 100 μM or 100 nM Ca2+ causes a delayed rise in channel activity, d), this rise in activity can only be reversed by the addition of exogenous CSQ, and e), the change in activity is identical to that observed with full CSQ dissociation from the native skeletal RyR induced by high ionic strength or high Ca2+ (25,26). Shin et al. (35) reported that skeletal CSQ can associate with triadin and junctin at low Ca2+ levels, based on results from fusion protein affinity chromatography. We have confirmed this observation (N. A. Beard and A. F. Dulhunty, unpublished observations). Therefore we suggest that low luminal Ca2+ causes CSQ monomerization and dissociation of all but residual CSQ, which remains bound to the RyR/Tri/Jun complex. This differs from full CSQ dissociation with high ionic strength or [Ca2+]s >5 mM, where CSQ binding to triadin and junctin is disrupted, probably because the polymer is stabilized or supercompacted as it is with >1 mM Ca2+. Since both mechanisms produce the same changes in channel activity, we speculate the depolymerization (or monomer formation) prevented the residual CSQ from regulating RyR activity. This was confirmed when RyRs in this situation responded to changes in luminal Ca2+ in the same way as RyRs lacking any associated CSQ. The observation that a small fraction (<20%) of CSQ was dissociated at the physiological [Ca2+] (1 mM) could explain the observation that a small fraction of channels lack CSQ under control conditions (25).
The conformational changes in CSQ as a result of changing [Ca2+] between 100 μM or 100 nM and 1 mM in stopped-flow spectrophotometry experiments were two to three times faster than the changes seen in channel activity after changing [Ca2+]. The times are nevertheless within the same order of magnitude and the differences can be largely attributed to the very different mixing rates in the two types of experiments.
Comparison with previous studies of the RyR activity at low and physiological luminal Ca2+
Our results with CSQ-regulated RyRs (at 100 nM and 100 μM luminal Ca2+; Fig. 8) are consistent with results obtained in more intact preparations, which show an increase in Ca2+ release when store load is increased. This increase occurs over and above the changes in driving force on Ca2+ ions (4,8). We show for the first time that, under otherwise identical conditions, the effects of luminal Ca2+ differ depending on the presence or absence of regulation by the Ca2+-binding protein CSQ. The increase in CSQ-deregulated RyR activity observed when luminal Ca2+ was dropped from 1 mM to ≤100 μM is similar to the activation observed when purified RyRs (also lacking CSQ) are exposed to low luminal Ca2+ (10,11,42). However other divergent results obtained in different laboratories cannot be simply explained in terms of CSQ regulation, since activation and inhibition have been seen in both native RyRs, potentially containing CSQ/Tri/Jun (1,13,14,42), and purified RyRs where these associated proteins are likely to be absent (10,12,43,44). Therefore, some of the different reported effects of lowering luminal Ca2+ on RyR activity must be due to factors other than the presence or absence of CSQ.
Interestingly, and in contrast to the results shown here, Laver et al. (39) reported that untreated RyRs (presumably containing CSQ) did not respond to a fall in luminal Ca2+ to nM levels when cis Ca2+ was at activating concentrations (with other conditions similar to those in these experiments). However, in the majority of those experiments bilayers contained multiple channels which exhibited coupled gating under some conditions (39). The channels had much higher Po values than we report for experiments with one or at the most two active channels under similar conditions. It is possible that coupled and uncoupled channels respond quite differently to changes in luminal Ca2+. In addition, high Po channels may be oxidized (45,46). These possibilities remain to be investigated. The results support our contention that there are multiple Ca2+ sensors on the luminal side of the RyR channel complex (see below). Thus it is possible that different procedures employed in different laboratories allow a dominant effect of one or another of the Ca2+ sensors.
The location of the luminal Ca2+ sensors
We show that the activity of CSQ-deregulated RyRs increases when luminal [Ca2+] falls. This increase in activity must be due to an effect of the low [Ca2+] on the luminal side of the RyR/Jun/Tri complex or on some other luminal-associated proteins. Since the high ionic strength CSQ dissociation procedure and the low Ca2+ CSQ-deregulation process both leave triadin, junctin, and other proteins associated with the RyR in place (25), the Ca2+ sensor could either be on the luminal domain of the RyR, on triadin or junctin or another associated protein. It is possible that the Ca2+ sensor in this case corresponds to the luminal Ca2+ inhibition site detected after tryptic digestion of luminal proteins (14). In contrast to native skeletal preparations, purified cardiac RyRs (with added triadin and junctin) did not respond to changes in luminal Ca2+ from 20 μM to 5 mM until cardiac CSQ was added (15). This suggests either that an additional Ca2+-sensitive protein was removed during purification or that communication of store load to RyRs differs from that in skeletal muscle.
The physiological role of CSQ as a luminal SR Ca2+ sensor for the RyR
Our observation that channel activity is higher when luminal Ca2+ is 1 mM (than with 100 nM or 100 μM) in the presence of CSQ (with activating cis [Ca2+]s) is consistent with experiments in isolated vesicles and intact (skinned) fibers, which show that the amount of Ca2+ released increases as store load increases (4,8). Also consistent with these observations in vesicles and skinned fibers is the fact that RyR activity increases further when luminal [Ca2+] is increased above 1 mM (Fig. 9) (25,26). It is likely that CSQ depolymerization also occurs in skinned fiber and isolated SR when the SR is severely depleted of Ca2+ and free luminal [Ca2+] starts to drop (4,8). It was recently shown that SR Ca2+ levels transiently fall by as much as 10% after one Ca2+ release, initiated by an action potential and to levels close to 100 μM during prolonged release initiated by low Mg2+ (47) and this was attributed to CSQ depolymerization. Our results show for the first time, to our knowledge, that CSQ dissociation does occur within this range of [Ca2+] but suggest that it would occur as a result of (and not cause) the fall in [Ca2+] . The time course of our stopped-flow and single channel changes suggests that CSQ depolymerization is too slow to cause any significant changes after an action potential. The whole cell system differs from the bilayer system in that dissociated CSQ is contained within the small volume of the terminal cisternae and it is available to reassociate with the residual RyR/Tri/Jun-associated CSQ as soon as Ca2+ loading increases luminal Ca2+ to levels approaching 1 mM.
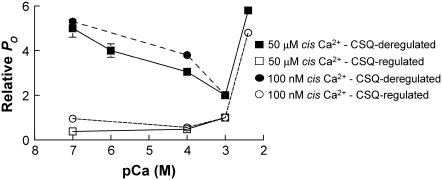
FIGURE 9.
Composite diagram of effects of increasing or decreasing luminal [Ca2+] on RyR activity in the presence or absence of CSQ. The figure provides a general summary of data presented in this article and in some previously published works (18,19,21,42). The data have been recalculated so that they are expressed relative to the activity of the CSQ-regulated RyRs in the presence of 1 mM luminal Ca2+. CSQ-deregulated RyRs have a higher open probability than CSQ-regulated RyRs under all conditions. CSQ regulation has the strongest effect on RyR activity when luminal Ca2+ is dropped below 1 mM. It is notable that cytoplasmic Ca2+ has a minimal effect on the response of the RyR to luminal Ca2+ regardless of whether the RyR is CSQ regulated or CSQ deregulated.
It is important to note that the activity of untreated channels that were activated by a cis [Ca2+] of 50 μM in fact fell significantly when the trans [Ca2+] was lowered. This suggests that CSQ may be particularly effective in reducing channel activity during skeletal excitation contraction coupling when the normal Mg2+ inhibition is relieved and channels can be activated by Ca2+ (48,49). Thus, the overall effect of the association of CSQ with the RyR is to store [Ca2+] and to prevent an increase in channel activity under conditions when the store is depleted (Fig. 9).
The situation in SR vesicles and in vivo is complicated by the fact that CSQ changes structure and stops regulating the RyR when luminal Ca2+ is lowered in a time-dependent manner. Thus it could be argued that CSQ regulation of the RyR can protect the SR from excess Ca2+ release only when Ca2+ is transiently depleted, not during prolonged depletion. However, this may not necessarily be the case because of the very different geometrical constraints on CSQ in the SR and in the bilayer situation. In addition, depolymerization is slower when [Ca2+] is depleted to 100 μM, for example, so that the store would be protected for correspondingly longer periods with less depletion.
The physiological significance of Ca2+ sensors other than CSQ, which produce an increase in channel activity when luminal Ca2+ falls, is not clear and is perhaps largely irrelevant for mature muscle fibers containing the normal complement of CSQ. An increase in channel activity when luminal Ca2+ is low would help maintain Ca2+ fluxes, but this would be detrimental in maintaining store load. It may well be that the Ca2+-dependent inhibition seen in CSQ-deregulated channels is a result of nonspecific conformational changes in luminal domains of the protein with the change in [Ca2+]. None of the effects of reducing luminal Ca2+ that we observe were voltage dependent (with the exception of 2 of 40 channels, see Results section) and thus cannot be attributed to luminal Ca2+ feeding through to bind to Ca2+ activation sites on the cytoplasmic domain of the RyR (12,43). Another situation in which we saw an increase in activity as luminal Ca2+ increased was under conditions in which the channels were already activated by a cytoplasmic [Ca2+] of 50 μM. Once again this cannot be attributed to a feed though effect. Clearly, the location of Ca2+ sensors that respond to changes in luminal [Ca2+] in the absence of CSQ regulation requires further investigation.
In conclusion, the results show that the response of native RyR channels to a fall in luminal [Ca2+] differs in the presence and absence of the Ca2+-binding protein, CSQ, and thus provides evidence that CSQ is a dominant luminal Ca2+ sensor for the RyR. However, this role of CSQ was maintained only if CSQ retained its normal multimeric conformation. The fact that RyR activity changes in response to luminal [Ca2+] in the absence of CSQ confirms that there are multiple luminal Ca2+ sensors. The existence of multiple sensors could potentially allow a complex response of the RyR to changes in luminal [Ca2+], which can be modified by a variety of conditions impinging on the Ca2+ release channel. The action of CSQ in suppressing an increase in RyR activity with store depletion is consistent with our hypothesis that CSQ acts to maintain store Ca2+ when the store is depleted.
Acknowledgments
We thank S. Pace and J. Stivala for help with SR vesicles preparation, Dr. J. Y. Fan for the assistance with cross-linking procedures and analytical ultracentrifugation, and Dr. M. Casarotto for assistance with stopped-flow techniques.
N.A.B. was supported by the Australian Research Council of Australia (project ID DP0344878), and L.W. was supported by an Australian National University PhD Scholarship. This work was supported by a Wellcome Trust equipment grant.
References
- 1.Gyorke, I., and S. Gyorke. 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75:2801–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukyanenko, V., I. Gyorke, and S. Gyorke. 1996. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 432:1047–1054. [DOI] [PubMed] [Google Scholar]
- 3.Lukyanenko, V., S. Viatchenko-Karpinski, A. Smirnov, T. F. Wiesner, and S. Gyorke. 2001. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+sensitive leak in rat ventricular myocytes. Biophys. J. 81:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamb, G. D., M. A. Cellini, and D. G. Stephenson. 2001. Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J. Physiol. 531:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasolato, C., B. Innocenti, and T. Pozzan. 1994. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol. Sci. 15:77–83. [DOI] [PubMed] [Google Scholar]
- 6.Hoth, M., and R. Penner. 1992. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 355:353–356. [DOI] [PubMed] [Google Scholar]
- 7.Zweifach, A., and R. S. Lewis. 1993. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA. 90:6295–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donoso, P., H. Prieto, and C. Hidalgo. 1995. Luminal calcium regulates calcium release in triads isolated from frog and rabbit skeletal muscle. Biophys. J. 68:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu, L., and G. Meissner. 1998. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+. Biophys. J. 75:2302–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szegedi, C., S. Sarkozi, A. Herzog, I. Jona, and M. Varsanyi. 1999. Calsequestrin: more than ‘only’ a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem. J. 337:19–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Ma, J., M. Fill, C. M. Knudson, K. P. Campbell, and R. Coronado. 1988. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 242:99–102. [DOI] [PubMed] [Google Scholar]
- 12.Tripathy, A., and G. Meissner. 1996. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys. J. 70:2600–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitsapesan, R., and A. J. Williams. 1995. The gating of the sheep skeletal sarcoplasmic reticulum Ca2+ release channel is regulated by luminal Ca2+. J. Membr. Biol. 146:133–144. [DOI] [PubMed] [Google Scholar]
- 14.Ching, L. L., A. J. Williams, and R. Sitsapesan. 2000. Evidence for Ca2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ. Res. 87:201–206. [DOI] [PubMed] [Google Scholar]
- 15.Gyorke, I., N. A. Hester, L. R. Jones, and S. Gyorke. 2004. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86:2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franzini-Armstrong, C., L. J. Kenney, and E. Varriano-Marston. 1987. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J. Cell Biol. 105:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, J., N. A. Maertz, A. J. Lokua, E. G. Kranias, and H. H. Valdivia. 2001. Regulation of cardiac ryanodine receptors activity by calsequestrin. Biophys. J. 80:590a (Abstr.) [Google Scholar]
- 18.Beard, N. A., D. R. Laver, and A. F. Dulhunty. 1999. Regulation of skeletal muscle ryanodine receptors by calsequestrin. Proc Aust Physiol Pharm Soc. 30:43 (abstr.). [Google Scholar]
- 19.Zhang, L., J. Kelley, G. Schmeisser, Y. M. Kobayashi, and L. R. Jones. 1997. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272:23389–23397. [DOI] [PubMed] [Google Scholar]
- 20.Wang, S., W. R. Trumble, H. Liao, C. R. Wesson, A. K. Dunker, and C. H. Kang. 1998. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol. 5:476–483. [DOI] [PubMed] [Google Scholar]
- 21.Beard, N. A., D. R. Laver, and A. F. Dulhunty. 2004. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog. Biophys. Mol. Biol. 155:33–69. [DOI] [PubMed] [Google Scholar]
- 22.Park, H., S. Wu, A. K. Dunker, and C. Kang. 2003. Polymerization of calsequestrin. Implications for Ca2+ regulation. J. Biol. Chem. 278:16176–16182. [DOI] [PubMed] [Google Scholar]
- 23.Fliegel, L., E. Newton, K. Burns, and M. Michalak. 1990. Molecular cloning of cDNA encoding a 55-kDa multifunctional thyroid hormone binding protein of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 265:15496–15502. [PubMed] [Google Scholar]
- 24.Scott, B. T., H. K. Simmerman, J. H. Collins, B. Nadal-Ginard, and L. R. Jones. 1988. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J. Biol. Chem. 263:8958–8964. [PubMed] [Google Scholar]
- 25.Beard, N. A., M. M. Sakowska, A. F. Dulhunty, and D. R. Laver. 2002. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys. J. 82:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beard, N. A., M. G. Casarotto, L. Wei, M. Varsanyi, D. R. Laver, and A. F. Dulhunty. 2005. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys. J. 88:3444–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito, A., S. Seiler, A. Chu, and S. Fleischer. 1984. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J. Cell Biol. 99:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahern, G. P., P. R. Junankar, and A. F. Dulhunty. 1994. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 352:369–374. [DOI] [PubMed] [Google Scholar]
- 29.Costello, B., C. Chadwick, A. Saito, A. Chu, A. Maurer, and S. Fleischer. 1986. Characterization of the junctional face membrane from terminal cisternae of sarcoplasmic reticulum. J. Cell Biol. 103:741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beard, N. A. 2003. Regulation of the skeletal muscle ryanodine receptor by calsequestrin. PhD thesis. Australian National University, Canberra.
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- 32.Towbin, H., T. Staehelin, and J. Gordon. 1992. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Biotechnology. 24:145–149. [PubMed] [Google Scholar]
- 33.Laver, D. R., K. R. Eager, L. Taoube, and G. D. Lamb. 2000. Effects of cytoplasmic and luminal pH on Ca2+ release channels from rabbit skeletal muscle. Biophys. J. 78:1835–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minium, E. W., B. M. King, and G. Bear. 1993. Statistical Reasoning in Psychology and Education. John Wiley & Sons, New York.
- 35.Shin, D. W., J. Ma, and D. H. Kim. 2000. The Asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin. FEBS Lett. 486:178–182. [DOI] [PubMed] [Google Scholar]
- 36.Guo, W., and K. P. Campbell. 1995. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J. Biol. Chem. 270:9027–9030. [DOI] [PubMed] [Google Scholar]
- 37.Dulhunty, A. F., D. R. Laver, E. M. Gallant, M. G. Casarotto, S. M. Pace, and S. Curtis. 1999. Activation and inhibition of skeletal RyR channels by a part of the skeletal DHPR II-III loop: effects of DHPR Ser687 and FKBP12. Biophys. J. 77:189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohkura, M., K. Furukawa, H. Fujimori, A. Kuruma, S. Kawano, M. Hiraoka, A. Kuniyasu, H. Nakayama, and Y. Ohizumi. 1998. Dual regulation of the skeletal muscle ryanodine receptor by triadin and calsequestrin. Biochemistry. 37:12987–12993. [DOI] [PubMed] [Google Scholar]
- 39.Laver, D. R., E. R. O'Neill, and G. D. Lamb. 2004. Luminal Ca2+-regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J. Gen. Physiol. 124:741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostwald, T. J., D. H. MacLennan, and K. J. Dorrington. 1974. Effects of cation binding on the conformation of calsequestrin and the high affinity calcium-binding protein of sarcoplasmic reticulum. J. Biol. Chem. 249:5867–5871. [PubMed] [Google Scholar]
- 41.Ikemoto, N., G. M. Bhatnagar, B. Nagy, and J. Gergely. 1972. Interaction of divalent cations with the 55,000-dalton protein component of the sarcoplasmic reticulum. Studies of fluorescence and circular dichroism. J. Biol. Chem. 247:7835–7837. [PubMed] [Google Scholar]
- 42.Fill, M., R. Coronado, J. R. Mickelson, J. Vilven, J. J. Ma, B. A. Jacobson, and C. F. Louis. 1990. Abnormal ryanodine receptor channels in malignant hyperthermia. Biophys. J. 57:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrmann-Frank, A., and F. Lehmann-Horn. 1996. Regulation of the purified Ca2+ release channel/ryanodine receptor complex of skeletal muscle sarcoplasmic reticulum by luminal calcium. Pflugers Arch. 432:155–157. [DOI] [PubMed] [Google Scholar]
- 44.Xu, L., A. Tripathy, D. A. Pasek, and G. Meissner. 1999. Ruthenium red modifies the cardiac and skeletal muscle Ca2+ release channels (ryanodine receptors) by multiple mechanisms. J. Biol. Chem. 274:32680–32691. [DOI] [PubMed] [Google Scholar]
- 45.Marengo, J. J., C. Hidalgo, and R. Bull. 1998. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys. J. 74:1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pessah, I. N., K. H. Kim, and W. Feng. 2002. Redox sensing properties of the ryanodine receptor complex. Front. Biosci. 7:a72–a79. [DOI] [PubMed] [Google Scholar]
- 47.Launikonis, B. S., J. Zhou, L. Royer, T. R. Shannon, G. Brum, and E. Rios. 2006. Depletion “skraps” and dynamic buffering inside the cellular calcium store. Proc. Natl. Acad. Sci. USA. 103:2982–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamb, G. D. 2000. Excitation-contraction coupling in skeletal muscle: comparisons with cardiac muscle. Clin. Exp. Pharmacol. Physiol. 27:216–224. [DOI] [PubMed] [Google Scholar]
- 49.Laver, D. R., T. M. Baynes, and A. F. Dulhunty. 1997. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J. Membr. Biol. 156:213–229. [DOI] [PubMed] [Google Scholar]