Abstract
Restrictive cardiomyopathy (RCM) is an uncommon heart muscle disorder characterized by impaired filling of the ventricles with reduced volume in the presence of normal or near normal wall thickness and systolic function. The disease may be associated with systemic disease but is most often idiopathic. We recognized a large family in which individuals were affected by either idiopathic RCM or hypertrophic cardiomyopathy (HCM). Linkage analysis to selected sarcomeric contractile protein genes identified cardiac troponin I (TNNI3) as the likely disease gene. Subsequent mutation analysis revealed a novel missense mutation, which cosegregated with the disease in the family (lod score: 4.8). To determine if idiopathic RCM is part of the clinical expression of TNNI3 mutations, genetic investigations of the gene were performed in an additional nine unrelated RCM patients with restrictive filling patterns, bi-atrial dilatation, normal systolic function, and normal wall thickness. TNNI3 mutations were identified in six of these nine RCM patients. Two of the mutations identified in young individuals were de novo mutations. All mutations appeared in conserved and functionally important domains of the gene.
Introduction
Restrictive cardiomyopathy (RCM) is a myocardial disease characterized by impaired ventricular filling and reduced diastolic volume in the presence of normal systolic function and normal or near normal myocardial thickness (1). The disease is characterized by symptoms of progressive left- and right-sided heart failure. The overall prognosis is poor, especially when onset is in childhood, and patients often require cardiac transplantation (2, 3). Although several inherited and acquired disorders may cause RCM, many cases remain idiopathic (4, 5). Familial RCM has been reported, but whether a distinct genetic entity exists remains uncertain (6).
A child and his mother were diagnosed with idiopathic RCM in our cardiomyopathy clinic. Their family history revealed that 12 individuals died suddenly and that several surviving relatives had symptoms of heart disease. Clinical investigation of the family (H640) identified another individual with RCM and nine individuals with hypertrophic cardiomyopathy (HCM). Genetic investigations identified a disease-causing mutation within a highly conserved region of the cardiac troponin I gene (TNNI3). To test the hypothesis that RCM is part of the clinical expression of TNNI3 mutations, we performed genetic investigations of the gene in nine additional idiopathic RCM patients. This paper presents the results of clinical and genetic investigations in ten unrelated patients diagnosed with idiopathic RCM and their relatives, including family H640 mentioned above.
Methods
This study was approved by the local research ethics committee, and informed consent was obtained from all participants.
Patients.
Clinical investigations of the initial family (H640) comprised the proband and 32 of his surviving relatives. The remaining study cohort consisted of nine additional unrelated patients diagnosed with idiopathic RCM over a 6-year period. Relatives of these RCM patients were also invited to participate in the study. All patients and those relatives who agreed to participate underwent physical examination, 12-lead ECG, and transthoracic two-dimensional echocardiography and Doppler studies. Selected patients underwent cardiac catheterization and right ventricular biopsy (H417, H906, H816, H974, H38).
Echocardiography.
Standard views for M-mode and two-dimensional studies were obtained. End-diastolic left ventricular wall thickness was recorded at the level of the mitral valve and papillary muscle in the anterior and posterior septum and the lateral and posterior wall using short-axis two-dimensional images. Anterior and posterior septal thickness at the apex was assessed from apical four-chamber and parasternal short-axis views. The maximum left ventricular wall thickness was defined as the maximal measurement recorded in any of the myocardial segments studied. Left ventricular wall thickness and left ventricular end-diastolic dimension was adjusted for age and body surface area in RCM patients (Tables 1 and 2) (7, 8). Septal/posterior wall ratio was obtained from measurements in the parasternal long-axis view, the short-axis view at the mitral valve level, or the short-axis view at the papillary muscle level. Table 2 reports the ratio that was the greater of the two. Left ventricle in- and outflow velocities were determined using continuous and pulse wave Doppler echocardiography.
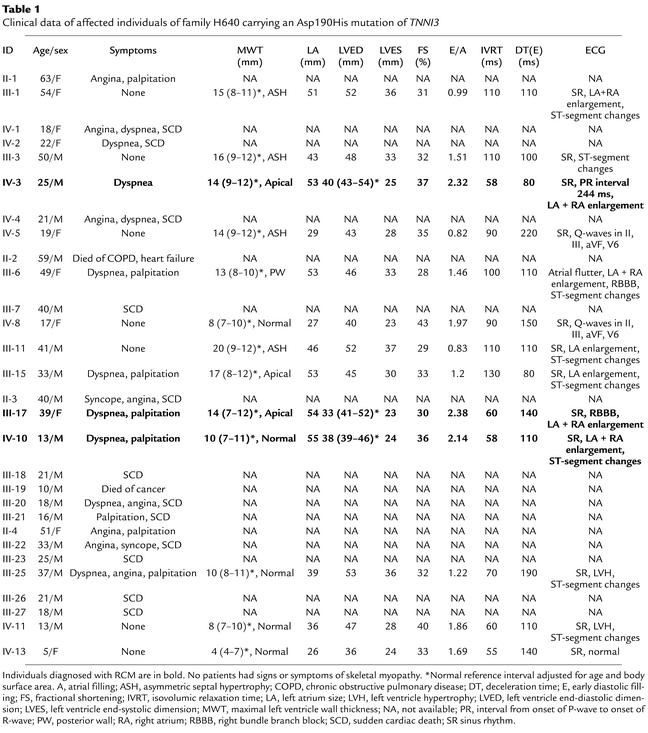
Table 1.
Clinical data of affected individuals of family H640 carrying an Asp190His mutation of TNNI3
Table 2.
Clinical data of patients diagnosed with idiopathic RCM
Restrictive cardiomyopathy.
Individuals were diagnosed with RCM in accordance with previous established criteria when echocardiography revealed the following (1, 4, 5): Doppler measurements consistent with restrictive left ventricular filling pattern (increased ratio of early diastolic filling [E] to atrial filling [A] ≥ 2; decreased deceleration time [DT] ≤ 150 ms; decreased isovolumic relaxation time [IVRT] ≤ 70 ms); reduced or low-normal left ventricular end diastolic dimension (LVED); normal or near normal left ventricular wall thickness and systolic function.
Hypertrophic cardiomyopathy.
Individuals were diagnosed with HCM when echocardiography identified unexplained left ventricle hypertrophy greater than or equal to 13 mm, or they fulfilled proposed diagnostic criteria for HCM within the context of familial disease, i.e., echocardiographic criteria and/or one of the following ECG abnormalities (9, 10): left ventricular hypertrophy (Romhilt-Estes score ≥4); Q-waves (duration >0.04 sec and/or a depth >1/4 of ensuring R wave in at least two leads); and marked repolarization abnormalities (T-wave inversion in at least two leads).
DNA extraction.
Genomic DNA was extracted from peripheral blood samples using a kit from QIAGEN Inc. (no. 51306; West Sussex, United Kingdom).
Linkage, haplotype, and paternity analysis.
Linkage analysis of recognized HCM genes in family H640 were carried out using microsatellite markers and the LINKAGE 5.1 package (http://linkage.rockefeller.edu/index.html), as described previously (11). Haplotype analysis of pedigree H816 and H805 was performed using microsatellite markers defining the TNNI3 locus (D19S254, D19S210, D19S877, and D19S887) (12). Paternity analysis was performed in pedigree H417 and H906 using 13 microsatellite markers (D1S1656, D3S1578, D11S1344, D7S483, D12S1583, MyoI-CA, MyoII-CA, HTMα-CA, D15S1036, D15S974, D15S153, D19S926, D19S877, and D19S887).
Mutation analysis.
The eight protein-encoding exons of TNNI3 were amplified using intronic primers designed according to the genomic sequence of the gene using the OLIGO 4.1 primer analysis software (MedProbe AS, Oslo, Norway) (13). Primer sequences and PCR conditions are available upon request.
PCR products were purified prior to cycle sequencing using a kit from Amersham Pharmacia Biotech (no. 25-6902-01; Amersham, United Kingdom) and cycle sequencing was performed using a BigDye Terminator Cycle Sequencing Kit from Applied Biosystems Inc. (no. 4314414; Foster City, California, USA). The sequences were analyzed on an ABI Prism 3100 Sequencer in accordance with the manual of the manufacturer.
When a mutation was identified, a second blood sample from the affected patient was reanalyzed to confirm the finding by direct sequencing and conventional SSCP analysis (standard 10% polyacrylamide gel electrophoresis in 1×TBE buffer at room temperature and postelectrophoresis silver staining). Three of the mutations identified were predicted to change restriction enzyme sites according to computer analysis using Webcutter software (http://bio.lundberg.gu.se/index.html). These findings were confirmed by PCR amplification of the relevant exon restriction enzyme digest, followed by size fractionation using either 3% agarose or 12% PAGE gel electrophoresis. Mutation Asp190Gly in pedigree H640 abolished a BsrI site, mutation Arg192His in pedigree H417 abolished a HhaI site, and mutation Arg145Trp in pedigree H816 and H805 created an AciI site.
Results
Clinical investigations of family H640.
The proband of family H640, individual IV-10 (Figure 1, Table 1), had onset of heart failure symptoms at the age of 11 years. Echocardiography revealed severe bi-atrial enlargement accompanied by a restrictive filling pattern, normal systolic function, and normal left ventricular wall thickness. His ECG showed prominent P-waves in all leads consistent with bi-atrial enlargement. His mother (III-17) had symptoms of heart failure from the age of 29 years. Her investigations also demonstrated restrictive left ventricular physiology with normal wall thickness measurements, except for localized apical thickening (14 mm).
Figure 1.
Pedigree drawings of restrictive cardiomyopathy families affected by cardiac troponin I mutations. Squares indicate male family members; circles, female family members; symbols with slash, deceased individuals; open symbols, unaffected individuals; filled symbols, individuals affected by RCM (indicated by arrows) or HCM; checkered symbols, individuals who died suddenly; question mark, unknown clinical status. Plus sign indicates presence of mutation, whereas minus sign indicates absence of mutation. Generation II of family H640 consisted of a total of 13 siblings, of which 9 were available for this investigation. Four generation-II siblings were obligate carriers of the mutation (included in the pedigree drawing with their offspring), while five remaining siblings were shown not to carry the mutation. Two of the obligate carriers (III-7, II-3) died suddenly at the age of 40. The remaining obligate carriers (II-1, II-2, II-4), reached the age of 63, 59, and 51 years, respectively. Ten sudden deaths appeared in young individuals who had a 50% chance of having inherited the disease (IV-1/2/4; III-18/20/21/22/23/26/27). Their average age of death was 25 years (16–33 years), and most had symptoms of cardiac disease prior to their death. Individual IV-13 was shown to be a healthy carrier of the mutation at the age of 5 years.
The family history revealed several relatives with symptoms of heart disease and nine individuals who died suddenly at less than 30 years of age. A cousin of the proband, individual IV-3, had already been diagnosed previously with restrictive cardiomyopathy following echocardiography and ECG-recording (Table 1).
Nine living relatives within pedigree H640 fulfilled diagnostic criteria for HCM within the context of familial disease (7, 8). Six of these individuals (III-1, III-3, IV-5, III-6, III-11, III-15) had left ventricular wall hypertrophy of 13–20 mm, including three individuals with a left atrial size of more than 50 mm (III-1, III-6, III-15). The diastolic filling patterns of these individuals were mixed, showing features of restriction as well as impaired diastolic relaxation with a shortened DT, but normal or prolonged IVRT, and diminished to normal E/A ratio in the majority. Three individuals had a normal echocardiogram, but an abnormal ECG (IV-8, III-25, IV-11).
Genetic investigation of family H640.
Linkage analysis of recognized HCM genes was performed, and TNNI3 was identified as the likely disease gene. Mutation analysis of TNNI3 by direct sequencing identified a 87A→G nucleotide substitution of exon 8 resulting in an Asp190His amino acid substitution that segregated with the disease in the family (maximal two-point lod score: 4.8).
Clinical and genetic investigation of other RCM patients.
Following the identification of a disease causing TNNI3 mutation within family H640, investigation of the gene was performed in an additional nine unrelated patients who had been diagnosed with idiopathic RCM. The following six patients were shown to carry TNNI3 mutations.
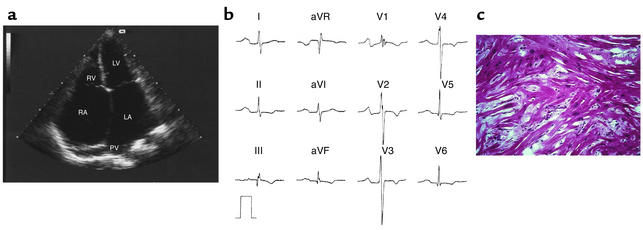
H417 was diagnosed at the age of 16 following a stroke associated with an episode of paroxysmal atrial fibrillation. Echocardiography showed severe bi-atrial enlargement accompanied by normal left ventricular wall thickness and normal systolic function (Table 2 and Figure 2a). ECG showed abnormal P-waves in all leads (Figure 2b). He died of heart failure at the age of 19 awaiting cardiac transplantation. Microscopy of right ventricular (RV) endomyocardial biopsies obtained antemortem showed nonspecific interstitial fibrosis without myocyte hypertrophy or myocyte disarray. Postmortem micro-scopy, however, revealed myofiber disarray, myocyte hypertrophy, and fibrosis similar to the findings in HCM patients (Figure 2c) (14, 15).
Figure 2.
Clinical features of restrictive cardiomyopathy in individual H417 affected by a de novo mutation in the gene for cardiac troponin I (Arg192His). (a) Apical four-chamber echocardiogram in systole of H417 at the age of 18 with marked bi-atrial dilatation (left atrial [LA] size, 64 mm), dilatation of pulmonary veins (PV), normal-sized ventricles and normal wall thickness. LV, left ventricle; RV, right ventricle; RA, right atrium. (b) 12-lead ECG in sinus rhythm with prominent P-waves in all leads, T-wave inversions, and incomplete right bundle branch block. (c) Microscopy of heart tissue obtained postmortem with myocyte hypertrophy, abundant interstitial fibrosis, and myofibril disarray equivalent to the histological findings in HCM patients (14, 15) (hematoxylin and eosin staining, ×40).
Direct sequencing of TNNI3 identified a 92C→A nucleotide substitution of exon 8, which resulted in an Arg192His amino acid substitution. The mutation was not present in either of his parents. Paternity was confirmed following haplotype analysis of 13 micro-satellite markers and the mutation was therefore considered to be a de novo mutation.
H906 had failed to thrive since the age of 3 years and had a body weight of only 15 kg when idiopathic RCM was diagnosed at the age of 6 years. She had suffered from recurrent chest infections and frequent episodes of abdominal pain. Her walking distance was less than 100 m. Echocardiography and ECG recordings showed typical features of RCM (Table 2). Microscopy of RV endomyocardial biopsies showed nonspecific interstitial fibrosis as in biopsies from patient H417. The patient is currently awaiting cardiac transplantation. Mutation analysis of TNNI3 identified an 886A→G nucleotide substitution of exon 7, which resulted in a Lys178Glu amino acid substitution. The mutation was not present in her parents, and paternity was confirmed following haplotype analysis. This mutation was also considered to be a de novo mutation.
H805, H816, and H974 had typical features of RCM diagnosed in their late fifties (Table 2, and Figure 1). H805 and H816 had symptoms of heart failure, while H974 was diagnosed following an embolic stroke. Micro-scopy of RV endomyocardial biopsies from H974 showed interstitial fibrosis with mild myocyte hypertrophy, but no myocyte or myofibrillar disarray. Mutation analysis of TNNI3 in both H805 and H816 identified the same 799C→T nucleotide substitution of exon 7 that lead to an Arg145Trp amino acid substitution. Haplotype analysis with respect to the TNNI3 locus on chromosome 19 did not suggest a common founder effect of the mutation. Their offspring were not carriers of the mutation, while remaining relatives were unwilling to participate in the study at present. Mutation analysis of H974 identified an 856G→A nucleotide substitution of exon 7 that led to an Ala171Thr amino acid substitution. He was an only child, and no clinical data or DNA was available on his deceased parents. Subsequent mutation analysis of his children did not reveal further carriers.
H38 developed symptoms of heart failure at the age of 17 years. Her echocardiogram was typical of RCM, except for a very localized mid-septal bulge (13 mm) without outflow tract obstruction. She died of heart failure at the age of 31 awaiting cardiac transplantation. Postmortem microscopy of heart tissue showed myocyte and myofibrillar disarray, myocyte hypertrophy, and fibrosis similar to the findings in HCM patients (14, 15). Mutation analysis of TNNI3 identified a 797T→A nucleotide substitution of exon 7, which led to a Leu144Gln amino acid substitution. Several members of her family were known to have died suddenly, but lived abroad and were not available for investigation.
Mutation analysis.
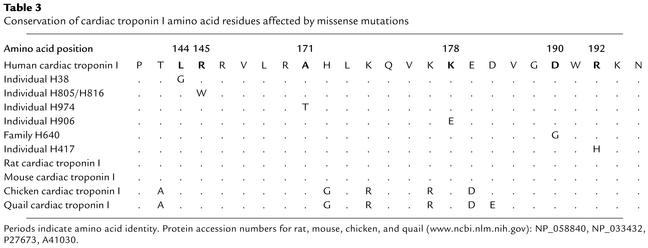
All mutations in the present study were identified in patients of Caucasian descent and believed to be disease causing based upon (a) a significant lod score in family H640 (Asp190His); (b) the appearance of the same mutation in two unrelated individuals, H816 and H805 (Arg145Trp); (c) the appearance of de novo mutations in affected individuals, H417 and H906 (Lys178Glu and Arg192His); (d) absence of sequence variations leading to amino acid substitutions in 200 chromosomes from Caucasian control individuals; and (e) the location of all mutations in functionally important and conserved regions of the gene (Table 3 and Figure 3).
Table 3.
Conservation of cardiac troponin I amino acid residues affected by missense mutations
Figure 3.
Schematic representation of the human cardiac troponin I gene with the number of corresponding amino acids encoded by the gene and interaction sites with other sarcomeric contractile proteins (13, 28). The stars indicate mutations identified in patients with restrictive cardiomyopathy in numerical order: Leu144Gln identified in H38, Arg145Trp identified in H816 and H805, Ala171Thr identified in H974, Lys178Glu identified in H906, Asp190His identified in H640, and Arg192His identified in H417.
Discussion
Prior to the genetic era of cardiomyopathies beginning with the identification of the first disease gene in HCM in 1990, it was believed that HCM, RCM, and the inherited form of dilated cardiomyopathy (DCM) were separate and distinct clinical and pathophysiological entities (16, 17). Much has been learned over the past decade, and it has been shown that sarcomeric gene mutations may be associated with both HCM and DCM (18). These findings suggest that variation in the location of mutations within specific functional domains of sarcomeric disease genes can result in different clinical phenotypes (19, 20). The results of the current investigation show that RCM may also be part of the spectrum of hereditary sarcomeric contractile protein disease.
The clinical expression of HCM in family H640 was unusual. The majority of affected HCM individuals had enlarged atria and/or evidence of restrictive filling with shortened DT in the presence of only mild to moderate left ventricle hypertrophy and normal cavity dimensions. Three gene carriers from family H640 had symptomatic clinical presentation that was dominated by restrictive filling pattern, marked atrial enlargement, and reduced left ventricular end-diastolic dimension (Table 1). Individual IV-10 had normal ventricular wall thickness, while individual IV-3 and III-17 had a small localized area of thickening at the apex. The phenotype of IV-3 and III-17 was characterized by RCM, and the severity of the changes was not readily explained by the small area of localized apical thickening (Table 1). The number of premature sudden deaths in the family suggests that the Asp190His amino acid substitution identified is associated with an adverse phenotype. The mixed appearance of HCM and RCM in H640 have previously only been reported in three small families in which no genetic investigations were performed (21, 22). Previous studies have identified TNNI3 mutations in a small number of HCM families with insufficient phenotype information to determine whether these mutations were associated with a specific disease expression (23–25).
The finding of three patients in family H640 with a phenotype dominated by restrictive physiology lead us to hypothesize that idiopathic RCM could be part of the clinical expression of TNNI3 mutations. Another nine patients fulfilling RCM diagnostic criteria were investigated for mutations in TNNI3. Their clinical presentation was with embolic stroke, heart failure, or for transplant evaluation. They had severe restrictive filling on Doppler, marked left atrial dilatation, and low-normal or reduced left ventricular end-diastolic dimension with normal left ventricular wall thickness (Table 2). Two young individuals (H417, H906) had severe and early onset of disease consistent with them being carriers of de novo mutations. The histology of RV endomyocardial biopsies from three patients (H417, H906, H974) showed nonspecific interstitial fibrosis without myocyte or myofiber disarray. Postmortem microscopy of cardiac tissue from two patients (H906, H38), however, showed typical HCM histology (Figure 2b). These apparent differences may reflect difficulties in obtaining representative myocardial tissue by biopsy as reported previously (26). The finding of typical HCM histology when the whole heart was available for evaluation confirms previous observations of hearts from patients with a clinical diagnosis of RCM (27).
The genetic investigations of the current study identified six novel TNNI3 mutations, which were all localized in conserved and functional important regions of the gene (Table 3 and Figure 3). It is well established that regulation of muscle contraction is primarily dependent on the intracellular concentration of Ca2+. The major sensor of the intracellular Ca2+ level is the troponin complex, which is composed of three subunits, troponin C, troponin I, and troponin T. All three troponins are located within the thin muscle filament, and their primary function is to control the interaction between the thick and thin filament during muscle contraction and relaxation. Troponin I can bind to actin-tropomyosin and prevent muscle contraction by inhibition of actin-tropomyosin–activated myosin (actomyosin) ATPase activity. The inhibitory effect of troponin I is reversed by troponin C following binding of Ca2+, which subsequently introduces conformational changes in the entire troponin complex leading to muscle contraction (28). Previous studies have defined different functional domains of TNNI3 (Figure 3) (28). It has been shown that the sequence required for inhibition of human cardiac troponin I actomyosin ATPase activity consists of 21 amino acid residues from number 137 to 148 (29). Two of the RCM mutations identified, Leu144Gln and Arg145Trp, are localized within this important region of TNNI3. The remaining RCM mutations are localized in proximity to or within other important domains of troponin I necessary for the normal function of the troponin complex. The Ala171Thr and Lys178Glu mutations may influence the inhibitory function through actin binding since previous studies have shown that amino acids number 173–181 bind to actin and increase the inhibitory effect of troponin I. The Asp190His and Arg192His mutations are both localized within the conserved C-terminal region of the protein, which is also required for normal inhibitory function of troponin I.
Several recent in vitro expression studies have investigated previous reported TNNI3 mutations associated with HCM (30–32). These HCM mutations were found to be associated with increased Ca2+ sensitivity and diminished inhibition of the actomyosin ATPase activity. Similar findings were reported following analysis of transgenic mice expressing an Arg145Gly HCM mutation (33). The hearts of transgenic mice showed increased contractility, whereas relaxation was impaired.
The diversity of the phenotypic expression of TNNI3 mutations in families with a mixed appearance of HCM and RCM is not explained by the current knowledge of the molecular and functional impact of TNNI3 mutations and suggest that additional genetic and environmental factors influence disease expression. However, the finding of TNNI3 mutations in a substantial proportion of patients diagnosed with idiopathic RCM suggest a causal relationship between gene abnormality and disease. This is further strengthened by the fact that de novo mutations were identified in patients with severe and early onset RCM. Previous expression studies of TNNI3 mutations in HCM reveal alterations in Ca2+ sensitivity and inhibitory function that may also influence the development of RCM. The identification of TNNI3 mutations in RCM patients may serve as a model for the development of a similar phenotype in transgenic animals to further investigate pathophysiology and diastolic abnormalities (filling, relaxation, compliance) in the presence of normal systolic function and wall thickness.
In summary, in patients with idiopathic RCM evaluation of the family or histology from the whole heart may reveal clinical or pathological features of HCM. The fact that TNNI3 mutations were identified in a significant proportion of such patients indicates that idiopathic RCM is part of the clinical expression of sarcomeric contractile protein disease and of HCM. Troponin I disease should be considered in patients with apparent idiopathic RCM and family investigation for both restrictive and hypertrophic cardiomyopathy should be offered to relatives of these patients.
Acknowledgments
We would like to thank the families and physicians who made this study possible. We would also like to thank Ulla Steffensen and Annie O’Donoghue for assistance in the laboratory and clinical investigations, respectively, and Michael J. Davies and Sian Hughes for providing histology of heart tissue. The study was supported by grants from the British Heart Foundation, The Danish Medical Research Council, and The Danish Heart Foundation.
Footnotes
See the related Commentary beginning on page 175.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: restrictive cardiomyopathy (RCM); troponin I gene (TNNI3); hypertrophic cardiomyopathy (HCM); early diastolic filling (E); atrial filling (A); deceleration time (DT); isovolumic relaxation time (IVRT); left ventricular end-diastolic dimension (LVED); right ventricular (RV); dilated cardiomyopathy (DCM).
References
- 1.Richardson P, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 2.Rivenes SM, Kearney DL, Smith EO, Towbin JA, Denfield SW. Sudden death and cardiovascular collapse in children with restrictive cardiomyopathy. Circulation. 2002;102:876–882. doi: 10.1161/01.cir.102.8.876. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RJ, Shah PK, Fishbein MC. Idiopathic restrictive cardiomyopathy. Circulation. 1984;70:165–169. doi: 10.1161/01.cir.70.2.165. [DOI] [PubMed] [Google Scholar]
- 4.Keren A, Popp RL. Assignment of patients into the classification of cardiomyopathies. Circulation. 1992;86:1622–1633. doi: 10.1161/01.cir.86.5.1622. [DOI] [PubMed] [Google Scholar]
- 5.Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. N. Engl. J. Med. 1997;336:267–276. doi: 10.1056/NEJM199701233360407. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick AP, Shapiro LM, Rickards AF, Poole-Wilson PA. Familial restrictive cardiomyopathy with atrioventricular block and skeletal myopathy. Br. Heart J. 1990;63:114–118. doi: 10.1136/hrt.63.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardin JM, et al. Echocardiographic measurements in normal subjects: evaluation of an adult population without clinically apparent heart disease. J. Clin. Ultrasound. 1979;7:439–447. doi: 10.1002/jcu.1870070606. [DOI] [PubMed] [Google Scholar]
- 8.Henry WL, et al. Echocardiographic measurements in normal subjects. Growth-related changes that occur between infancy and early adulthood. Circulation. 1978;57:278–285. doi: 10.1161/01.cir.57.2.278. [DOI] [PubMed] [Google Scholar]
- 9.Charron P, et al. Diagnostic value of electrocardiography and echocardiography for familial hypertrophic cardiomyopathy in a genotyped adult population. Circulation. 1997;96:214–219. doi: 10.1161/01.cir.96.1.214. [DOI] [PubMed] [Google Scholar]
- 10.McKenna WJ, Spirito P, Desnos M, Dubourg O, Komajda M. Experience from clinical genetics in hypertrophic cardiomyopathy: proposal for new diagnostic criteria in adult members of affected families. Heart. 1997;77:130–132. doi: 10.1136/hrt.77.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mogensen J, et al. Development and application of linkage analysis in genetic diagnosis of familial hypertrophic cardiomyopathy. J. Med. Genet. 2001;38:193–198. doi: 10.1136/jmg.38.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogensen J, Kruse TA, Borglum AD. Assignment of the human cardiac troponin I gene (TNNI3) to chromosome 19q13.4 by radiation hybrid mapping. Cytogenet. Cell Genet. 1997;79:272–273. doi: 10.1159/000134740. [DOI] [PubMed] [Google Scholar]
- 13.Bhavsar PK, Brand NJ, Yacoub MH, Barton PJR. Isolation and characterization of the human cardiac troponin I gene (TNNI3) Genomics. 1996;35:11–23. doi: 10.1006/geno.1996.0317. [DOI] [PubMed] [Google Scholar]
- 14.Maron BJ, Ferrans VJ. Ultrastructural features of hypertrophied human ventricular myocardium. Prog. Cardiovasc. Dis. 1978;21:207–238. doi: 10.1016/0033-0620(78)90026-9. [DOI] [PubMed] [Google Scholar]
- 15.Varnava AM, et al. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104:1380–1384. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 16.Geisterfer-Lowrance AA, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 17.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 18.Kamisago M, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N. Engl. J. Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 19.Mogensen J, et al. Alpha-cardiac actin is a novel disease gene in familial hypertrophic cardiomyopathy. J. Clin. Invest. 1999;103:R39–R43. doi: 10.1172/JCI6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Chien KR. Complexity in simplicity: monogenic disorders and complex cardiomyopathies. J. Clin. Invest. 1999;103:1483–1485. doi: 10.1172/JCI7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feld S, Caspi A. Familial cardiomyopathy with variable hypertrophic and restrictive features and common HLA haplotype. Isr. J. Med. Sci. 1992;28:277–280. [PubMed] [Google Scholar]
- 22.Angelini A, et al. Morphologic spectrum of primary restrictive cardiomyopathy. Am. J. Cardiol. 1997;80:1046–1050. doi: 10.1016/s0002-9149(97)00601-2. [DOI] [PubMed] [Google Scholar]
- 23.Kimura A, et al. Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat. Genet. 1997;16:379–382. doi: 10.1038/ng0897-379. [DOI] [PubMed] [Google Scholar]
- 24.Kokado H, et al. Clinical features of hypertrophic cardiomyopathy caused by a Lys183 deletion mutation in the cardiac troponin I gene. Circulation. 2000;102:663–669. doi: 10.1161/01.cir.102.6.663. [DOI] [PubMed] [Google Scholar]
- 25.Morner S, et al. Deletion in the cardiac troponin I gene in a family from northern Sweden with hypertrophic cardiomyopathy. J. Mol. Cell Cardiol. 2000;32:521–525. doi: 10.1006/jmcc.1999.1099. [DOI] [PubMed] [Google Scholar]
- 26.Tazelaar HD, Billingham ME. The surgical pathology of hypertrophic cardiomyopathy. Arch. Pathol. Lab. Med. 1987;111:257–260. [PubMed] [Google Scholar]
- 27.Angelini A, et al. Morphologic spectrum of primary restrictive cardiomyopathy. Am. J. Cardiol. 1997;80:1046–1050. doi: 10.1016/s0002-9149(97)00601-2. [DOI] [PubMed] [Google Scholar]
- 28.Perry SV. Troponin I: inhibitor or facilitator. Mol. Cell Biochem. 1999;190:9–32. [PubMed] [Google Scholar]
- 29.Syska H, Wilkinson JM, Grand RJ, Perry SV. The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit. Biochem. J. 1976;153:375–387. doi: 10.1042/bj1530375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott K, Watkins H, Redwood CS. Altered regulatory properties of human cardiac troponin I mutants that cause hypertrophic cardiomyopathy. J. Biol. Chem. 2000;275:22069–22074. doi: 10.1074/jbc.M002502200. [DOI] [PubMed] [Google Scholar]
- 31.Lang R, et al. Functional analysis of a troponin I (R145G) mutation associated with familial hypertrophic cardiomyopathy. J. Biol. Chem. 2002;277:11670–11678. doi: 10.1074/jbc.M108912200. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi-Yanaga F, et al. Functional consequences of the mutations in human cardiac troponin I gene found in familial hypertrophic cardiomyopathy. J. Mol. Cell Cardiol. 2001;33:2095–2107. doi: 10.1006/jmcc.2001.1473. [DOI] [PubMed] [Google Scholar]
- 33.James J. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ. Res. 2000;87:805–811. doi: 10.1161/01.res.87.9.805. [DOI] [PubMed] [Google Scholar]