Abstract
The synthetic random amino acid copolymer Copolymer 1 (Cop 1, Copaxone, glatiramer acetate) suppresses experimental autoimmune encephalomyelitis, slows the progression of disability, and reduces relapse rate in multiple sclerosis (MS). Cop 1 binds to various class II major histocompatibility complex (MHC) molecules and inhibits the T cell responses to several myelin antigens. In this study we attempted to find out whether, in addition to MHC blocking, Cop 1, which is immunologically cross-reactive with myelin basic protein (MBP), inhibits the response to this autoantigen by T cell receptor (TCR) antagonism. Two experimental systems, “prepulse assay” and “split APC assay,” were used to discriminate between competition for MHC molecules and TCR antagonism. The results in both systems using T cell lines/clones from mouse and human origin indicated that Cop 1 is a TCR antagonist of the 82–100 epitope of MBP. In contrast to the broad specificity of the MHC blocking induced by Cop 1, its TCR antagonistic activity was restricted to the 82–100 determinant of MBP and could not be demonstrated for proteolipid protein peptide or even for other MBP epitopes. Yet, it was shown for all the MBP 82–100-specific T cell lines/clones tested that were derived from mice as well as from an MS patient. The ability of Cop 1 to act as altered peptide and induce TCR antagonistic effect on the MBP p82–100 immunodominant determinant response elucidates further the mechanism of Cop 1 therapeutic activity in experimental autoimmune encephalomyelitis and MS.
Keywords: immunoregulation, experimental autoimmune encephalomyelitis, multiple sclerosis, altered peptide
T cell activation occurs when a T cell receptor (TCR) recognizes an autologous major histocompatibility complex (MHC) molecule carrying a specific peptide and induces a cascade of molecular events of secondary signals (1, 2). Binding of the MHC–antigen complex to the TCR therefore has been the focus of attention for various therapeutic approaches in autoimmune diseases in an attempt to interfere in the recognition of self-antigens by the autoreactive T cells (3, 4). MHC blockade by synthetic peptides that are either unrelated or similar to the pathogenic autoantigen, and thus compete with self-peptides for binding to MHC class II molecules, have been tested extensively (5–8). This approach, however, is limited by the lack of specificity and the interference with other essential immune responses linked to the same MHC. Yet, for certain analogs of the native disease-inducing peptide, it was found that MHC blockade is not the sole mechanism responsible for disease prevention and that the analogs also interfere in T cell activation by binding to the autoreactive TCR (8–11). Recent studies demonstrated that these peptide–MHC complexes engage the TCR without inducing all the adequate second signals in a manner that results in functional receptor inactivation (TCR antagonism) or differential signaling (partial agonism). Such nonstimulatory antigen-altered peptides were shown to be powerful and selective inhibitors of T cell activity in vitro and in vivo. In experimental autoimmune encephalomyelitis (EAE), which is considered a model of multiple sclerosis (MS), analogs of both myelin basic protein (MBP) regions 1–11 (12, 13) and 87–99 (14) and proteolipid protein (PLP) region 139–151 (15, 16) have shown to exert TCR antagonistic effect and thereby to ameliorate the disease.
The synthetic amino acid copolymer Copolymer 1 (Cop 1), composed of l-Ala, l-Glu, l-Lys, and l-Tyr, exerts a marked suppressive and protective effect on EAE in various species including primates, as well as on chronic relapsing EAE (17, 18). Cop 1 also was shown to slow the progression of disability and reduce relapse rate in MS patients (19), and it is currently an authorized drug for the treatment of MS under the trade name of Copaxone (glatiramer acetate). The mechanism of Cop 1 activity involves, on the one hand, induction of T helper 2 (Th2) suppressor cells that down-regulate the disease (20–22) and, on the other hand, interference with the encephalitogenic T cells that mediate the disease (23–25). It was demonstrated previously that Cop 1 inhibited the response to MBP (23, 24) and to other myelin antigens (25, 26) via competition at the MHC level because of its high-affinity and promiscuous binding to various class II MHC molecules (27, 28). However, Cop 1 does not interfere in vivo with unrelated immune responses or induce general immune deficiency (18), as expected for antigens that operate solely through MHC blocking, suggesting that although MHC binding is a necessary step for Cop 1 activity, it is not the only mode by which Cop 1 exerts its therapeutic effect on EAE and MS. Additional steps involving antigen-specific processes such as induction of cross-reactive T cell tolerance or T cell receptor antagonism should play a role.
The immunological crossreactivity between Cop 1 and the natural autoantigen MBP has been demonstrated previously at both the cellular (29) and humoral (30) levels. Moreover, this crossreactivity was shown to correlate with Cop 1-suppressive activity in vivo (31). It therefore is possible that complexes of Cop 1/class II antigens compete with complexes of MBP-derived peptides/class II antigens for binding to the T cell receptor. In the present study we tested whether Cop 1 indeed can induce a T cell receptor antagonistic effect on the response to MBP, specifically on the response to the 82–100 peptide of MBP, which has been found as the immunodominant region in SJL mice and in humans. Here we demonstrate that Cop 1 inhibits the response to MBP p82–100 in mice and humans not only by competing for MHC binding, but also at the T cell level as a T cell receptor antagonist.
MATERIALS AND METHODS
Mice.
SJL/J and B10PL mice were purchased from The Jackson Laboratory. Female mice, 7–12 weeks old, were used for all experiments.
Antigens.
Cop 1, glatiramer acetate, is a synthetic random basic polymer, prepared by polymerization of the N-carboxyanhydrides of l-alanine, γ-benzyl-l-glutamate, ɛ,N-trifluoroacetyl l-lysine, and l-tyrosine (17) followed by removal of blocking groups. Two Cop 1 batches obtained from Teva Pharmaceutical Industries (Petach Tikva, Israel) were used throughout the study. Batches 55495 and 2997 had average molecular masses of 5,800 and 6,000 kDa, respectively. MBP was isolated from spinal cords of guinea pigs, as described previously (32). The synthetic peptides MBP p82–100 (DENPVVHFFKNIVTPRTPP); MBP p1–11 (AcASQKRPSQRHG); PLP p139–151 (HSLGKWLGHPDKF); KM-core, a core peptide of ovalbumin 327–332 with lysine and methionine extensions at both sides (KMKMVHAAHAKMKM); and hen egg white lysozyme (HEL) p46–61 (NTDGSDYGILQINSR) were synthesized by the Merrifield solid-phase method (33), using the peptide synthesizer model 430A of Applied Biosystems, and purified by HPLC. MBP p91A (p87–99 with alanine substitution in position 91) was a gift from L. Steinman (Weizmann Institute of Science).
T Cell Lines and Clones.
Murine T cell lines were derived according to Teitelbaum et al. (23) from spleens of SJL/J mice immunized with MBP p82–100 or PLP p139–151 and B10.PL mice immunized with MBP p1–11 (20 μg/mouse) emulsified in complete Freund’s adjuvant (CFA, Difco). Cells were selected in vitro by using the homologous antigen in RPMI medium 1640 supplemented with 1% autologous serum. Every 14–21 days cells were exposed to the antigen (10–20 μg/ml) presented on syngeneic irradiated (3,000 rad) spleen cells (5 × 106/ml) for 3 days in 10% fetal calf serum (FCS) culture medium, followed by propagation in 10% supernatant of Con A activated normal mouse spleen cells as T cell growth factor (TCGF). Cloning of T cell lines was performed by limiting dilution at 0.3 cells per well.
Human T cell clones were derived according to Teitelbaum et al. (24) from peripheral blood mononuclear cells of an MS patient of HLA DR217 by incubating 5 × 106 cells in a 24-well culture plate with p82–100 of MBP (5 μg/ml) or guinea pig MBP (50 μg/ml) in culture medium, supplemented with 10% heat-inactivated autologous serum. After 7 days, the cells were transferred to culture medium containing 10% FCS and recombinant human interleukin 2 (20 units/ml). The cells were grown continuously in this medium with periodic exposure to antigen presented on irradiated (3,000 rad), autologous mononuclear cells every 14–18 days.
Epstein–Barr Virus (EBV) Transformed B Cell Lines.
These lines were initiated as described (24) by culturing 20 × 106 peripheral blood mononuclear cells with B95.8 cell line supernatant for 1 h at 37°C. The cells then were washed and cultured in RPMI 1640 medium with 10% FCS and cyclosporin A (10 μg/ml) to deplete T cells.
Prepulse Assay.
This method was performed according to De Magistris et al. (9) with some modifications. Irradiated antigen-presenting cells (APC), either murine spleen cells (3 × 106/ml) or human EBV-transformed cells (0.3 × 106/ml), were incubated with a suboptimal dose of the antigen (2–10 μg/ml) at 37°C. After 3 h, cells were washed extensively and recultured in individual wells of 96-well microculture plates (5 × 105/well and 5 × 104/well for mouse and human cells, respectively) with T cells from lines/clones (1.5 × 104/well). Cop 1 or control peptide inhibitors (3–15 μM) were either preincubated in the first stage with the antigen to inhibit MHC binding or added in the second stage with the T cells to inhibit binding to the TCR. At the end of 48 h of incubation, cultures were pulsed with 1 μCi [3H]thymidine and harvested 12 h later. Results are expressed as mean cpm thymidine incorporation for triplicate cultures. SDs were less than 20% of the mean cpm.
Split APC Assay.
In this system the antigenic peptide and the inhibitor (3–15 μM) were either coincubated on the same APC competing for MHC binding or presented on different APC, which were mixed only when added to the T cells competing on binding to the TCR. APC (either 3 × 106/ml mouse splenic cells or 0.3 × 106/ml human EBV-transformed cells) were incubated with the indicated peptides or with PBS (unpulsed APC) for 3 h at 37°C, then washed and plated in 96-well microplates. The APC that had been pulsed with the specific antigen were mixed with APC pulsed with inhibitor or with unpulsed APC. The APC that were coincubated with both antigen and inhibitor were mixed with unpulsed APC to adjust to the same APC number per well (1 × 106/well and 1 × 105/well for mouse and human cells, respectively). T cells from murine or human lines/clones (1.5 × 104/well) were then added and cultured for 2 additional days. Cells then were pulsed with 1 μCi [3H]thymidine and harvested 12 h later. Results are expressed as mean cpm thymidine incorporation for triplicate cultures. SDs were less than 20% of the mean cpm.
RESULTS
Two experimental systems were used to discriminate between MHC blocking and TCR antagonism. In the “prepulse assay,” APC were first preincubated with a suboptimal dose of the antigen and then incubated with T cells. The tested inhibitor was added either at the first stage with the antigen to inhibit MHC binding or at the second stage to inhibit the binding to the TCR. In the other system, “split APC assay,” the antigenic peptide and the inhibitor were either coincubated with the same APC, competing for MHC binding, or presented on different APC, which were mixed only when added to the T cells, competing on the TCR binding level. These two systems were used to study the effect of Cop 1 on various T cell lines and clones of different specificities from both mouse and human origin.
The Effect of Cop 1 on Murine T Cell Lines and Clones
Cop 1 was tested for its effect on the response to two distinct encephalitogenic determinants of MBP, the peptides 82–100 and 1–11, as well as on the response to a non-MBP peptide, PLP p139–151. For this purpose, MBP p82–100- and PLP p139–151-specific T cell lines and clones from SJL/J (H-2s) mice and MBP p1–11-specific clones from B10PL (H-2u) mice were used. We studied the inhibition of the proliferative response of these clones to their respective antigen, which is induced by Cop 1 as well as by different control peptides.
Prepulse Assay.
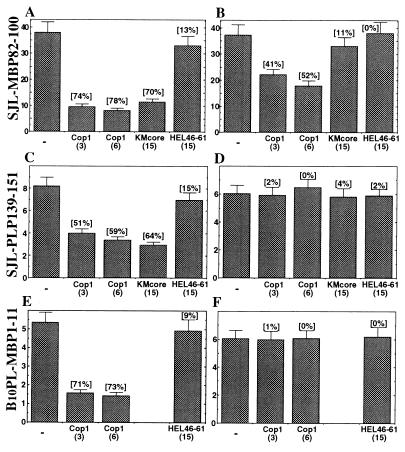
The response of three representative T cell clones in the presence of Cop 1 or two other peptides, KMcore and HEL p46–61, in a prepulse assay is demonstrated in Fig. 1. When Cop 1 was added with the antigenic peptides to compete for MHC binding, it inhibited proliferation of all the T cell clones tested, i.e., MBP p82–100-specific clone (Fig. 1A), PLP p139–151-specific clone (Fig. 1C), and MBP p1–11-specific clone (Fig. 1E). However, when Cop 1 was added at the second stage of the reaction, with the T cells, to compete at the TCR level, it inhibited only the response of the MBP p82–100-specific clone (Fig. 1B). Considerable inhibition (41 and 52% inhibition by 3 μM and 6 μM of Cop 1, respectively) was obtained, although lower than the inhibition of the MHC-binding stage (74 and 78%). Cop 1 did not induce any TCR antagonistic effect on the response of the other two clones either from SJL/J origin specific to PLP (Fig. 1D) or from B10PL origin specific to MBP p1–11 (Fig. 1F), even though all these clones were inhibited by Cop 1 at the MHC level.
Figure 1.
Antagonistic effect of Cop 1 in a prepulse assay on murine T cell lines and clones. (A) MHC blocking of SJL/J MBP p82–100 clone 3. (B) TCR antagonism of SJL/J MBP p82–100 clone 3. (C) MHC blocking of SJL/J PLP p139–151 clone III. (D) TCR antagonism of SJL/J PLP p139–151 clone III. (E) MHC blocking of B10.PL MBP p1–11 clone 2. (F) TCR antagonism of B10.PL MBP p1–11 clone 2. Irradiated (3,000 rad) spleen cells (3 × 106/ml) were incubated with a suboptimal dose of the antigen (2–10 μg/ml) at 37°C. After 3 h, cells were washed and recultured in individual wells of 96-well microculture plates (5 × 105/well) with T cells (1.5 × 104/well). Cop 1 or control peptide inhibitor were either preincubated in the first stage with the antigen to inhibit MHC binding or added in the second stage with the T cells to inhibit binding to the TCR. Results are expressed as mean cpm thymidine incorporation for triplicate cultures ×10−3 and represent one of five independent experiments.
The control peptide KMcore, which had been shown to bind to H-2s MHC, but does not cross-react with MBP (34), inhibited both SJL/J clones when preincubated with the APC (Fig. 1 A and C), but had no effect when added with the T cells (Fig. 1 B and D), indicating that it acts solely as MHC-blocking peptide. The other control peptide, HEL p46–61, with low affinity for H-2s (35), did not inhibit the response of any of the tested lines either as MHC or TCR blocker.
The effect of Cop 1 versus control peptides in prepulse assay was tested by using two additional SJL MBP p82–100 lines/clones and one additional B10PL MBP p1–11 clone (data not shown). Patterns of inhibition similar to those described above were obtained.
Split APC Assay.
The inhibition induced by Cop 1 and by control MBP-related peptides on the proliferation of six lines/clones of three specificities in a split APC assay is shown in Table 1. When Cop 1 was coincubated together with the antigen on the same APC as MHC blocker, it markedly inhibited the response to all three peptides. Thus, 95–98%, 84–92%, and 65% inhibition of the response to MBP p82–100, MBP p1–11, and PLP p139–151, respectively, was obtained. On the other hand, when Cop 1 was presented to the TCR on different APC populations as TCR antagonist, it inhibited the response of the three SJL/J-derived T cell lines/clones to MBP p82–100 (37–41%), but not the response of the two B10PL-derived T cell clones to the other MBP peptide 1–11 (0–2%) or the other SJL/J clone specific to PLP p139–151 (8–9%).
Table 1.
Antagonistic effect of Cop 1 on murine T cell lines/clones in a split APC assay
| T cell Clone | Inhibitor | Coincubation, cpm (% inhibition) | Split incubation, cpm (% inhibition) |
|---|---|---|---|
| SJL-MBP p82-100 | — | 6,865 | 6,906 |
| Clone 3 | Cop 1 | 151 (97) | 4,083 (41) |
| MBP p91A | 3,003 (54) | 4,569 (34) | |
| MBP p1–11 | 1,016 (85) | 6,892 (0) | |
| PLP p139–151 | 4,363 (36) | 6,702 (3) | |
| SJL-MBP p82–100 | — | 3,222 | 3,301 |
| Line 1 | Cop 1 | 156 (95) | 2,075 (37) |
| MBP p91A | 1,299 (60) | 2,217 (33) | |
| MBP p1–11 | 1,085 (66) | 3,624 (0) | |
| PLP p139–151 | 2,170 (32) | 3,317 (0) | |
| SJL-MBP p82–100 | — | 2,532 | 2,556 |
| Clone 6 | Cop 1 | 45 (98) | 1,506 (41) |
| B10PL-MBP p1–11 | — | 6,752 | 6,799 |
| Clone 2 | Cop 1 | 1,072 (84) | 6,636 (2) |
| MBP p82–100 | 3,686 (45) | 6,534 (4) | |
| B10PL-MBP p1–11 | — | 1,692 | 1,926 |
| Clone 20 | Cop 1 | 123 (92) | 2,102 (0) |
| MBP p82–100 | 906 (46) | 1,842 (4) | |
| SJL-PLP p139–151 | — | 8,534 | 8,622 |
| Clone III | Cop 1 | 3,006 (65) | 7,896 (8) |
| MBP p82–100 | 3,446 (60) | 8,049 (9) |
Irradiated spleen cells (3 × 106/ml) were incubated with 3 μM of antigenic peptide: MBP p82–100, MBP p1–11, or PLP p139–151. Inhibitor peptide: Cop 1 (6 μM), MBP p82–100, MBP p91A, MBP p1–11, or PLP p139–151 (15 μM) was either coincubated on the same APC competing for MHC binding (coincubation) or presented on different APC, which were mixed only when added to the T cells, competing on binding to the TCR (split incubation). After 3 h, cells were washed and plated in 96-well microplates with T cells (1.5 × 104/well) for 2 additional days. Results are expressed as mean cpm thymidine incorporation for triplicate cultures. SDs were less than 20% of the mean cpm. Results represent one of four independent experiments.
Several control MBP-related peptides were also tested for their ability to inhibit these lines. Peptide 91A, which was shown as a TCR antagonist of H-2s-restricted T cells specific to MBP 82–100 (15), exhibited an inhibition pattern similar to that demonstrated by Cop 1. Thus, it inhibited the response to MBP p82–100 when presented with the antigenic peptide either on the same APC (54–60% inhibition) or on different APC (33–34% inhibition). On the other hand, MBP p1–11 and PLP p139–151, which were shown to bind to H-2s haplotype (25, 36), inhibited the response to MBP p82–100 only when presented on the same APC as MHC-blocking peptides (85, 66, and 36% inhibition, respectively), but not when presented on different APC as TCR antagonists (0–3% inhibition). The MBP p82–100 inhibited the B10PL-MBP p1–11 clones and the SJL/J-PLP p139–151 clone only as MHC-blocking peptide (45, 46, and 60%, respectively), but induced no significant inhibition as TCR antagonist (4 and 9% respectively).
Both inhibitors that induced TCR antagonistic effect on the MBP p82–100 response, i.e., Cop 1 and peptide 91A, were more effective when tested as MHC blockers than as TCR antagonists.
The Effect of Cop 1 on Human T Cell Clones
To test whether the activity of Cop 1 as TCR antagonist is limited to H-2s-restricted immune response to MBP p82–100 or valid also for other MHC-restricted responses to this epitope, we studied the effect of Cop 1 on human T cell clones that had been established from an MS patient and were restricted to HLA-DR2. The activity of Cop 1 as MHC blocker and as TCR antagonist on human cells was studied both in the prepulse and split APC assays.
Prepulse Assay.
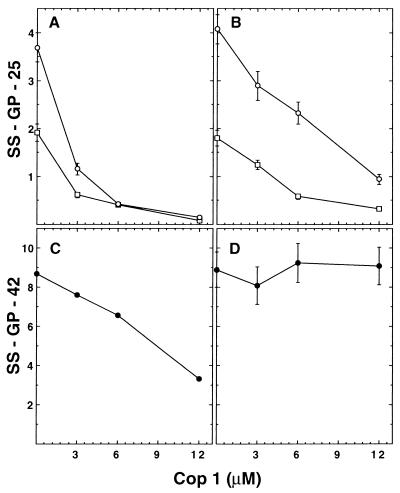
The effect of Cop 1 first was tested by using the human SS-GP-25 T cell clone, which is specific to the 82–100 epitope. Cop 1 inhibited the proliferation of this clone to its specific antigen, both when preincubated with p82–100 competing for MHC binding (Fig. 2A) and when added with the T cells competing for the TCR (Fig. 2B). This inhibition was demonstrated at several concentrations of Cop 1, in a dose-dependent way, and for two doses of the inhibited antigen—MBP p82–100. However, the inhibition obtained by Cop 1 when added at the MHC-binding stage was always higher than the inhibition of the TCR-binding stage. Thus, 68 and 89% inhibition were obtained in equimolar concentrations for 3 and 6 μM, respectively, in MHC blocking, whereas 31 and 43% inhibition were obtained by TCR antagonism. The specificity of Cop 1 effect in the human system was then tested by using another MBP-specific T cell clone from the same MS patient, SS-GP-42. This clone also was restricted to DR2 but did not recognize the 82–100 epitope and therefore was activated by the whole MBP molecule. Cop 1 inhibited the proliferation of this clone when preincubated with MBP competing for MHC binding (Fig. 2C), although to a lesser extent than the inhibition obtained for the 82–100-specific clone under the same conditions (Fig. 2A). On the other hand, Cop 1 did not exert any inhibition of this clone when added with the T cells to compete for the binding to the TCR (Fig. 2D), thus indicating that the inhibitory effect induced by Cop 1 as TCR antagonist is restricted to the 82–100-specific T cell receptor.
Figure 2.
Antagonistic effect of Cop 1 in a prepulse assay on human T cell clones. (A) MHC blocking of SS-GP-25 clone specific to p82–100 of MBP. (B) TCR antagonism of SS-GP-25 clone specific to p82–100 of MBP. (C) MHC blocking of SS-GP-42 clone unresponsive to p82–100 of MBP. (D) TCR antagonism of SS-GP-42 clone unresponsive to p82–100 of MBP. Irradiated (10,000 rad), EBV-transformed cells (0.3 × 106/ml) were incubated with: peptide 82–100 of MBP [3 μM (□)] and [6 μM (○)] or MBP [1.5 μM (•)]. After 3 h, cells were washed extensively and recultured in individual wells of 96-well microculture plates (5 × 104/well) with T cells (1.5 × 104/well). Cop 1 was either preincubated in the first stage with the antigen to inhibit MHC binding or added in the second stage with the T cells to inhibit binding to the TCR. Results are expressed as mean cpm thymidine incorporation for triplicate cultures ×10−3 and represent one of three independent experiments.
Split APC Assay.
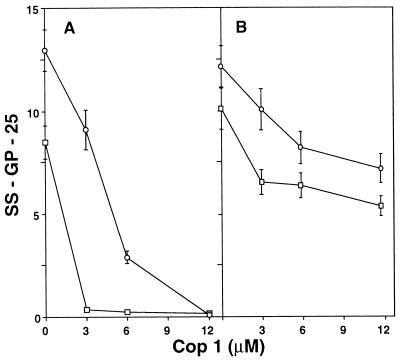
The effect of Cop 1 on SS-GP-25, the p82–100-specific human clone, also was tested in the split APC assay (Fig. 3). In this system, too, Cop 1 inhibited proliferation both when coincubated with the 82–100 peptide as MHC blocker (Fig. 3A) and when presented to the TCR on different APC as TCR antagonist (Fig. 3B). This inhibition was demonstrated for two antigen concentrations and various Cop 1 concentrations in a dose-dependent way. Similar to the results obtained in the other systems, Cop 1 inhibited the response to p82–100 more efficiently when added as MHC blocker (100 and 78% inhibition in equimolar concentrations of 3 and 6 μM, respectively) than when used as TCR antagonist (35 and 39% inhibition).
Figure 3.
Antagonistic effect of Cop 1 in a split APC assay on SS-GP-25 human T cell clone specific to MBP p82–100. (A) MHC blocking. (B) TCR antagonism. Peptide 82–100 of MBP [3 μM (□) and 6 μM (○)] and Cop 1 (3–12 μM) was either coincubated on the same APC (EBV-transformed cells, 0.3 × 106/ml), competing for MHC binding, or presented on different APC, which were mixed only when added to the T cells, competing on binding to the TCR. After 3 h of incubation, the EBV cells were washed and added to the T cells (1.5 × 104/well). Cultures were pulsed with 1 μCi [3H]thymidine at the end of 48 h of incubation. Results are expressed as mean cpm thymidine incorporation for triplicate cultures ×10−3 obtained in one of two independent experiments.
Thus, the findings in both the prepulse and the split APC assays indicate that, in addition to the competition at the MHC level obtained irrespective of the T cell origin and specificity, Cop 1 acts as an altered peptide of MBP p82–100 and inhibits the response to this determinant as a specific TCR antagonist.
DISCUSSION
This study demonstrates that Cop 1 acts as a TCR antagonist of the 82–100 epitope of MBP by employing both murine- and human-specific T cell lines in two experimental systems. Various studies using synthetic analogs of native autoantigens have led to the fine dissection of the MHC–antigen–TCR interaction (1–11). While MHC binding is a stage of broad specificity, recognition of antigens by the TCR has been considered to be of exquisite specificity. Recent data have indicated flexibility in this recognition since analogues of T cell epitopes have been shown to bind to the TCR and either block T cell activation or induce differential T cell response (8–16). Such nonstimulatory antigen analogs, TCR antagonists, offer antigen-specific immunointervention in autoimmune diseases. Cop 1, which suppresses EAE and MS, was shown previously to inhibit in vitro the response of various T cell lines and clones (18, 23–25). In the present study we tested whether the inhibition induced by Cop 1 of the response to three myelin antigenic epitopes that have been implicated in the pathogenesis of EAE and MS results solely from MHC blocking or whether Cop 1 is also a TCR antagonist. To differentiate MHC blockade from TCR antagonism, the two sequential steps, i.e., binding of antigenic peptide to the MHC versus binding of the peptide fragment presented on MHC to the TCR, ought to be disassociated. To this end we used the well established “prepulse assay” that previously was used to demonstrate TCR antagonism for several antigens (9). In this system, MHC binding was discriminated from TCR binding in time–antigen pulse before the addition of the competitor (Figs. 1 and 2). To further confirm the results, an additional system was designed—the “split APC assay.” In this assay, MHC and TCR binding were separated by presenting the antigen and the tested inhibitor on different APC populations (Table 1 and Fig. 3). The results obtained in both experimental systems by using T cell lines and clones from both human and mouse indicated that Cop 1 is a TCR antagonist of the 82–100 epitope of MBP (Figs. 1B, 2B, and 3B; Table 1). The TCR antagonistic activity of Cop 1 was restricted to MBP p82–100 and could not be demonstrated for PLP peptide (Fig. 1D and Table 1) or even for other MBP epitopes (Figs. 1F and 2D; Table 1); yet, it was shown for both murine (Fig. 1 and Table 1) and human (Figs. 2 and 3) MBP p82–100-specific T cell lines/clones. In the latter case the TCR antagonistic effect was exerted on T cells from an MS patient, suggesting that this effect may be related to the therapeutic value of Cop 1. In addition, it was demonstrated again that Cop 1 is a very effective competitor for MHC binding (Figs. 1 A, C, and E, 2A, and 3A; Table 1). In contrast to the restriction of the TCR antagonistic activity to one epitope, Cop 1 exhibited broad specificity in MHC blocking and inhibited the response to both MBP and PLP lines irrespective of their origin and peptide specificity, as demonstrated before (23–25). The MHC blocking induced by Cop 1 results from its promiscuous and efficient binding to various class II MHC molecules from mouse and human origin (26–28).
To verify that Cop 1 did not function in these assays only by its capacity to bind MHC molecules, various control MHC-binding peptides also were tested as MHC blockers or TCR antagonists. The results obtained in both systems indicated that peptides unrelated to the T cell-specific antigen tested, i.e., KMcore (Fig. 1 A and C), p1–11 of MBP, and p139–151 of PLP (Table 1), which had been shown to bind to the H-2s haplotype (25, 34, 36), acted as MHC blockers with all the SJL/J T cell clones tested. Yet, they were devoid of any activity when tested for TCR antagonism (Fig. 1 B and D; Table 1). In contrast, peptide 91A (p87–99 with alanine substitution in position 91), which is a TCR antagonist of the MBP 82–100 epitope (14), inhibited the response of the MBP p82–100-specific T cell lines/clones at both the MHC and the TCR levels, exhibiting an inhibition pattern similar to that demonstrated for Cop 1 (Table 1). These cumulative results indicate that the dual-inhibition capability of Cop 1 both as MHC blocker and TCR antagonist was not obtained because of artifact in the experimental systems and that Cop 1 is indeed a TCR antagonist of the 82–100 epitope of MBP.
In all the experiments, the inhibition induced by Cop 1 as well as by peptide 91A was significantly lower when tested as TCR antagonists (Table 1; Figs. 1B, 2B, and 3B) than as MHC blockers (Table 1; Figs. 1A, 2A, and 3A). This could result from inferior efficiency of Cop 1 in binding to the MBP 82–100-specific TCR than to MHC molecules. Another possible explanation is that by testing Cop 1’s ability to inhibit antigen presentation, both its activity as MHC blocker and as TCR antagonist were measured, while only the net effect of Cop 1 as TCR antagonist was obtained when Cop 1 inhibited the TCR-binding stage.
The TCR antagonistic effect induced by Cop 1 is characterized on the one hand by the restriction to the 82–100 epitope of MBP and on the other hand by the broad range of 82–100-specific T cell lines/clones affected from both murine and human origin. The TCR contact motifs of encephalitogenic epitopes involved in the pathology of EAE and MS recently were elucidated by employing various peptide analogues. Hence, Try-144 and His-147 are the TCR contact residues critical for the recognition of PLP p139–151 (15, 16), while the glutamine and proline in positions 3 and 6 of MBP p1–11 are essential for the T cell recognition of this epitope (12, 13). In the recognition of the 82–100 epitope by T cells from mouse (14), rat (37), as well as human (38) origin, lysine at position 91 was always demonstrated as a critical TCR contact residue. Interestingly, lysine is an abundant constituent of Cop 1 while tryptophan, histidine, glutamine, and proline are not included (17, 18). This may provide an explanation for the confinement of Cop 1 TCR antagonistic activity to the MBP p82–100-specific T cell receptor. The requirement for the same TCR contact residues by different clones can explain the feasibility of a single inhibitor to induce TCR antagonism on various p82–100 MBP-specific T cell lines/clones that originated in different species. In addition, it was found that T cells specific to the 82–100 epitope from rodents and man shared TCR rearrangement motifs (39).
This study elucidates further the mechanism of Cop 1 activity in EAE and MS. Hence, Cop 1 competes for MHC binding with several myelin-associated antigens such as MBP and PLP. This competition results in inhibition of the pathological effector functions induced by these encephalitogens (i.e., proliferation, interleukin secretion, and cytotoxicity). Binding of Cop 1 to the MHC class II molecules is the least specific step. Yet, because MHC binding is essential for antigen recognition, it is a prerequisite for Cop 1 effect by any mechanism. Since Cop 1 does not induce nonspecific blockade of unrelated immune responses in vivo (18) as expected for immunomodulators that operate mainly through MHC blocking, additional steps involving antigen-specific mechanisms apparently should proceed. In this study we showed that in the case of the immunodominant determinant 82–100 of MBP, an additional, more specific mechanism for Cop 1 activity is involved. In this case, competition also occurs at the level of the T cell receptor, between complex of MBP p82–100 with class II MHC and complex of Cop 1 with class II MHC molecules. The exact mechanism by which binding of the Cop 1-MHC complexes to the TCR results with functional receptor inactivation was not revealed in this study. Whether Cop 1 acts as complete antagonist or a partial agonist that triggers only selected T cell functions is not yet clear. Direct TCR antagonism leading to prevention of MBP p82–100-specific T cell activation probably accounts partially for Cop 1’s ability to block EAE when given at the onset of the disease. Furthermore, it has been shown recently that several TCR antagonists operate in vivo not only by direct antagonism but also by induction of T helper 2 (Th2) regulatory cells that exert bystander suppression for a number of autoantigens present in the target organ (14, 40). Cop 1 is a potent inducer of Th2 regulatory cells that recognize determinants shared between the native encephalitogen, MBP, and Cop 1 (20, 21). We recently demonstrated that Th2 suppressor lines and clones induced by Cop 1 ameliorated EAE in vivo and even mediated bystander suppression of the response to unrelated PLP peptides (22). The relationship between this Cop 1-induced Th2 response and the activity of Cop 1 as altered peptide of MBP p82–100, as demonstrated in this study, should be investigated further.
The T cell response to the 82–100 region of MBP is of particular importance in the MS disease process. Predominant T cell response to this region was found in MS patients, particularly in those of the HLA DR2 haplotype that accounts for more than 60% of the general MS population (38, 41, 42). This immunodominant epitope is a major target of T cells in brain lesions of MS patients as demonstrated by analyzing TCR from the site of the MS plaque (41, 42). The ability of Cop 1 to inhibit this specific response therefore is of therapeutic significance, revealing the mechanism of its beneficial effect in the treatment of MS.
Acknowledgments
This work was supported by a grant from Teva Pharmaceutical Industries, Ltd. (Israel), to D.T., R.A., and M.S.
ABBREVIATIONS
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- MBP
myelin basic protein
- Cop 1
copolymer 1
- TCR
T cell receptor
- APC
antigen-presenting cells
- EBV
Epstein–Barr virus
- MHC
major histocompatibility complex
- PLP
proteolipid protein
- Th
T helper
References
- 1.Altman A, Coggeshall K M, Mustelin T. Adv Immunol. 1990;48:227–360. doi: 10.1016/s0065-2776(08)60756-7. [DOI] [PubMed] [Google Scholar]
- 2.Ullman K S, Northrop J P, Verweil C L, Crabtree G R. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- 3.Smilek D E, Lock C B, McDevitt H O. Immunol Rev. 1990;118:37–71. doi: 10.1111/j.1600-065x.1990.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 4.Aharoni R, Teitelbaum D, Arnon R, Puri Y. Nature (London) 1991;352:147–150. doi: 10.1038/351147a0. [DOI] [PubMed] [Google Scholar]
- 5.Sakai K, Zamvil S S, Mitchell D J, Hodgkinson S, Rothbard J B, Steinmann L. Proc Natl Acad Sci USA. 1989;86:9470–9474. doi: 10.1073/pnas.86.23.9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wraite D C, Smilek D E, Mitchell D J, Steinmann L, McDevitt H O. Cell. 1989;57:709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- 7.Lamont A G, Sette A, Fujinami R, Colon S M, Miles C, Grey H M. J Immunol. 1990;145:1687–1693. [PubMed] [Google Scholar]
- 8.Guery J C, Adorini L. Crit Rev Immunol. 1993;13:195–206. [PubMed] [Google Scholar]
- 9.De Magistris M T, Alexander J, Coggeshall M, Altman A, Gaeta C A, Grey H M, Sette A. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 10.Evavold B D, Sloan-Lancaster J, Allen P M. Immunol Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 11.Sette A, Alexander J, Ruppert J, Snoke K, Franco A, Ishioka G, Grey H M. Immunol Rev. 1994;12:413–431. doi: 10.1146/annurev.iy.12.040194.002213. [DOI] [PubMed] [Google Scholar]
- 12.Wraith D C, Smilek D E, Mitchell D J, Steinmann L, McDevitt H O. Cell. 1989;59:247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- 13.Smilek D E, Wraith D C, Hodkinson S, Dwivedy S, Steinmann L, McDevitt H O. Proc Natl Acad Sci USA. 1991;88:9633–9637. doi: 10.1073/pnas.88.21.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaur A, Boehme S A, Chalmers D, Crowe P D, Pahuja A, Ling N, Brocke S, Steinman L, Conlon P. J Immunol. 1997;74:149–158. doi: 10.1016/s0165-5728(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 15.Kuchroo V K, Greer J M, Kaul D, Ishioka G, Franco A, Sette A, Sobel R A, Lee B. J Immunol. 1994;153:3326–3336. [PubMed] [Google Scholar]
- 16.Nicholson L B, Greer J M, Sobel R A, Lee B, Kuchroo V K. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 17.Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- 18.Teitelbaum D, Aharoni R, Fridkis-Hareli M, Arnon R, Sela M. In: The Decade of Autoimmunity. Shoenfeld Y, editor. New York: Elsevier; 1998. pp. 183–188. [Google Scholar]
- 19.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Sciffer R B, et al. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 20.Aharoni R, Teitelbaum D, Arnon R. Eur J Immunol. 1993;23:17–25. doi: 10.1002/eji.1830230105. [DOI] [PubMed] [Google Scholar]
- 21.Aharoni R, Teitelbaum D, Sela M, Arnon R. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aharoni, R., Teitelbaum, D., Sela, M. & Arnon, R. (1998) J. Neuroimmunol., in press. [DOI] [PubMed]
- 23.Teitelbaum D, Aharoni R, Arnon R, Sela M. Proc Natl Acad Sci USA. 1988;85:9724–9728. doi: 10.1073/pnas.85.24.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teitelbaum D, Milo R, Arnon R, Sela M. Proc Natl Acad Sci USA. 1992;89:137–141. doi: 10.1073/pnas.89.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teitelbaum D, Fridkis-Hareli M, Arnon R, Sela M. J Neuroimmunol. 1996;64:209–217. doi: 10.1016/0165-5728(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 26.Fridkis-Hareli M, Teitelbaum D, Kerlero de Rosbo N, Arnon R, Sela M. J Neurochem. 1994;63:S61. (abstr.). [Google Scholar]
- 27.Fridkis-Hareli M, Teitelbaum D, Gurevich E, Pecht I, Brautbar C, Kwon O J, Brenner T, Arnon R, Sela M. Proc Natl Acad Sci USA. 1994;91:4872–4876. doi: 10.1073/pnas.91.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fridkis-Hareli M, Strominger J L. J Immunol. 1998;160:4386–4397. [PubMed] [Google Scholar]
- 29.Webb C, Teitelbaum D, Arnon R, Sela M. Eur J Immunol. 1973;3:279–286. doi: 10.1002/eji.1830030506. [DOI] [PubMed] [Google Scholar]
- 30.Teitelbaum D, Aharoni R, Sela M, Arnon R. Proc Natl Acad Sci USA. 1991;88:9528–9532. doi: 10.1073/pnas.88.21.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb C, Teitelbaum D, Herz A, Arnon R, Sela M. Immunochemistry. 1976;13:333–337. doi: 10.1016/0019-2791(76)90344-x. [DOI] [PubMed] [Google Scholar]
- 32.Hirschfeld H, Teitelbaum D, Arnon R, Sela M. FEBS Lett. 1970;7:317–320. doi: 10.1016/0014-5793(70)80193-4. [DOI] [PubMed] [Google Scholar]
- 33.Merrifield R B. Science. 1965;150:178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- 34.Sette A, Lamont A G, Buus S, Colon S M, Miles C, Grey H M. J Immunol. 1989;143:1268–1273. [PubMed] [Google Scholar]
- 35.Hunt D F, Michel H, Dickinson T A, Shabanowitz J, Cox A, Sakaguchi K, Appella E, Grey H M, Sette A. Science. 1992;256:1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- 36.Wall M, Southwood S, Sidney J, Oseroff C, Lamont A G, Colon S M, Arrhenius T, Gaeta F C A, Sette A. International Immunol. 1992;4:773–777. doi: 10.1093/intimm/4.7.773. [DOI] [PubMed] [Google Scholar]
- 37.Karin N, Mitchell D J, Brocke S, Ling N, Steinman L. J Exp Med. 1994;180:2227–2237. doi: 10.1084/jem.180.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozovska M, Zang Y C Q, Aebischer I, Lnu S, Rivera V M, Crowe P D, Boehem S A, Zhang J Z. Eur J Immunol. 1998;28:1894–1901. doi: 10.1002/(SICI)1521-4141(199806)28:06<1894::AID-IMMU1894>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 39.Gold D P, Vainiene M, Celnik B, Wiley S, Gibbs C, Hashim G A, Vandenbark A A, Offner H. J Immunol. 1992;148:1712–1717. [PubMed] [Google Scholar]
- 40.Nicholson L B, Murtaza A, Hafler B P, Sette A, Kuchroo V K. Proc Natl Acad Sci USA. 1997;94:9279–9284. doi: 10.1073/pnas.94.17.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hafler D A, Saadeh M G, Kuchroo V K, Milford E, Steinman L. Immunol Today. 1996;17:152–153. doi: 10.1016/0167-5699(96)80611-6. [DOI] [PubMed] [Google Scholar]
- 42.Steinman L, Waisman A, Altmann D. Mol Med Today. 1995;1:179–184. doi: 10.1016/s1357-4310(95)92366-7. [DOI] [PubMed] [Google Scholar]