Abstract
Although gene therapy can cure patients with severe combined immunodeficiency (SCID) syndromes, the clinical occurrence of T cell malignancies due to insertional mutagenesis has raised concerns about the safety of gene therapy. Several key questions have remained unanswered: (i) are there unique risk factors for X-linked SCID (XSCID) gene therapy that increase the risk of insertional mutagenesis; (ii) what other genetic lesions may contribute to transformation; and (iii) what systems can be used to test different vectors for their relative safety? To address these questions, we have developed an XSCID mouse model in which both the Arf tumor-suppressor gene and the γc gene were ablated. Gene therapy in this animal model recapitulates the high incidence of integration-dependent, T cell tumors that was seen in the clinical trial. Ligation-mediated PCR analysis showed integration sites near or within established protooncogenes (Chd9, Slamf6, Tde1, Camk2b, and Ly6e), demonstrating that T cell transformation was associated with targeting of oncogene loci; however, no integrations within the Lmo2 locus were identified. The X-SCID background in transplanted cells was required for high rate transformation and was associated with expansion of primitive hematopoietic cells that may serve tumor precursors. This model should be useful for testing safety-modified vectors and for further exploring the risk factors leading to insertional mutagenesis in gene therapy trials.
Keywords: Arf, stem cells, immunodeficiency, leukemia, vector
Retroviral vector-mediated gene transfer into hematopoietic stem cells (HSCs) is an attractive strategy to correct inherited and acquired hematological disorders and has achieved clinical success in a number of cases (1–3). In a French gene therapy trial, 9 of 11 infants born with X-linked severe combined immunodeficiency (XSCID) were successfully treated with autologous bone marrow cells transduced ex vivo with a retroviral vector expressing a normal copy of the γc (common γ-chain) gene, which is mutated in XSCID patients (4). Before these studies, it had been estimated that the risk of an insertional event within 10 kb of a potential protooncogene ranged between 10−2 and 10−3 (5). Despite this estimated low risk, three of the XSCID patients treated in the French gene therapy trial developed clonal T cell leukemias (6–8). In these cases, vector integration occurred within the LMO2 locus, which codes for a known human T cell oncogene (9), and led to aberrant LMO2 expression and tumorigenesis. This unexpected high rate of leukemia has raised serious concerns regarding the safety of gene therapy for not only the XSCID disorders but for other hematologic disorders as well (10).
Although 3 of 11 patients in the French XSCID trial have developed leukemia, other patients enrolled in gene therapy trials for XSCID and other immune system disorders have shown clinical benefit without hematologic abnormalities (1, 3, 11). More follow-up may be necessary to fully assess the risk; for instance, benign clonal proliferations have been observed in patients with chronic granulomatous disease treated with gene therapy (12). An important question that remains is whether there are unique risk factors for XSCID gene therapy that are not present in other gene therapy applications. Possible XSCID-specific factors could include (i) an expanded target population of lymphoid progenitors that are susceptible to vector-induced mutagenesis; (ii) the presence of severe immunodeficiency in the host recipients and lack of antitumor immune responses; and (iii) a strong proliferative advantage for γc-transduced lymphoid progenitors that predisposes to transformation.
Another question is whether a system can be developed that allows testing of newer vectors designed to provide an improved safety profile. Although replication-incompetent retroviral vectors can induce tumors in mice (13, 14), tumors occur too infrequently to provide a quantitative transformation assay. To increase the sensitivity for detecting vector-induced mutagenesis, we generated a mouse model of XSCID gene therapy in which both the Arf tumor-suppressor gene and the γc gene were ablated. Arf-null mice were chosen because they are prone to develop T cell malignancies (15) and because Arf mutations occur in a high proportion of human T cell malignancies (16). Arf-null mice are highly tumor prone, with 72% of the animals dying from tumor formation within 1 year of birth. The majority of these malignancies are various solid tumors, and 29% of the tumors are lymphomas. There are no reports of tumor development in unmanipulated γc-null mice. We have used the resulting Arf−/−γc−/− mice as bone marrow cell donors to create a mouse model of XSCID gene therapy that reproduces the high incidence of T cell malignancies seen in the clinical trial.
Results
Development of T Cell Lymphomas in XSCID Mice Treated with the γc Vector.
Bone marrow cells from Arf−/−γc−/− mice were transduced with a murine stem cell virus (MSCV)-γc-internal ribosome entry site (IRES)-GFP or with a control MSCV-GFP vector and transplanted into lethally irradiated, CD45.1+ wild-type recipients. Reconstitution of T and B lymphopoiesis was seen in all cases where the MSCV-γc-IRES-GFP vector was used and in no cases where the GFP control vector was used. After 55 weeks of follow-up, 13 of 15 mice in the γc-transplanted group developed hematopoietic malignancies that had either a leukemic presentation, with circulating tumor cells in the peripheral blood, or as a lymphoma presentation, with infiltration of tumor cells into solid organs. In the first experiment, 11 tumors were T cell, and 2 were B cell phenotype (Fig. 1A and Table 2, which is published as supporting information on the PNAS web site). In contrast, only three lymphomas occurred in a total of 17 animals in the GFP control group (Fig. 1A). Very similar results were obtained in a second independent experiment in which 15 of 18 mice developed tumors in the γc-transplanted group, whereas only 4 of 17 animals in the GFP control group developed tumors (Fig. 1B and Table 2).
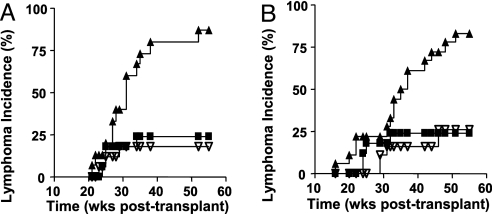
Fig. 1.
Incidence of lymphoma in mice undergoing XSCID gene therapy. (A) The lymphoma incidence in the first transplant experiment is plotted for the MSCV-γc vector transplant group (n = 15; filled triangle) and the GFP vector control group (n = 17; open triangle). In both of these series, Arf−/−γc−/− bone marrow cells were transduced and transplanted. The third curve was derived by transplanting mice with Arf−/−γc+/+ bone marrow cells that had been transduced with a MFG-γc vector transplant group (n = 18; filled squares). (B) The lymphoma incidence in a second independent transplant experiment is shown. As in A, the γc vector transplant group (n = 18; filled triangle) and the GFP vector control group (n = 17; open triangle) are shown using Arf−/−γc−/− bone marrow cells. The third curve was obtained from mice transplanted with Arf−/−γc+/+ bone marrow cells that had been transduced with a MSCV-γc vector (n = 19, filled square). Time is shown as weeks after transplant.
To determine whether the XSCID background was contributing to the high transformation rate, we also evaluated the tumor frequency seen with transplant of Arf−/−γc+/+ bone marrow cells that had been transduced with γc vectors. Bone marrow cells from Arf−/−γc+/+ mice were transduced with either a MFG-γc or the MSCV-γc-IRES-GFP vector and transplanted into lethally irradiated wild-type recipients (Fig. 1 A and B, respectively). In the first experiment, only 4 of 18 mice transplanted with transduced Arf−/−γc+/+ cells developed lymphomas during the 1-year follow-up period (Fig. 1A). This rate was significantly lower than what we observed using Arf−/−γc−/− mice donor bone marrow cells (P = 0.0001 by log-rank test). The second experiment used the MSCV-γc-IRES-GFP vector and gave essentially the same results (Fig. 1B). These data indicate that the XSCID background was an important contributor to the risk for tumorigenesis.
Histological analysis revealed an aggressive tumor morphology, in which the architecture of thymus, spleen, bone marrow, and lymph nodes was completely replaced with tumor cells and with invasion of tumor cells into the central nervous system (Fig. 2A). In uncorrected Arf−/−γc−/− animals, normal lymphoid development is blocked because of the absence of γc. Therefore, it was not surprising that, in the group transplanted with Arf−/−γc−/− cells transduced with the control MSCV-GFP vector, all lymphomas were GFP-negative and were derived from recipient rather than donor cells (Fig. 2B). In contrast, in the group transplanted with Arf−/−γc−/− bone marrow cells transduced with the γc vector, all lymphoma cells were derived from donor cells, and all of them except one were predominantly comprised of GFP+ cells (Fig. 2B and Table 2).
Fig. 2.
Characterization of lymphomas in XSCID gene therapy mice. (A) Histologic appearance of tumors from the γc group. Note the infiltration of lymphoblastic cells in the thymus (i), spleen (ii), lymph node (iii), and bone marrow (iv). (Aiv) Infiltration of the meninges with tumor cells (arrow). (B) FACS profiles of representative lymphoma cells from a GFP control case (Left) and γc vector case (Right). Cells from the control tumor were CD45.1-positive and GFP-negative (Upper Left), demonstrating that they were not transduced and were derived from the transplant recipient. In contrast, lymphoma cells from the γc group were CD45.1-negative and GFP-positive, showing that they were derived from transduced donor cells. In both cases, lymphoma cells were CD4+(Lower, y axis). (C) T cell phenotypes of lymphomas from different mice. Lymphocytes were stained with CD4 and CD8 antibodies as shown (Upper). Compared with thymocytes from a normal mouse (Control), lymphomas seen with the γc vector were either CD8+ (#645), CD4+ (#650), or CD4+CD8+ (#664). Lymphomas are highly GFP-positive, as shown (Lower).
In the French XSCID trial, the three cases of T cell leukemia were phenotypically distinct. One case showed a γδ T cell receptor (TCR) rearrangement, whereas three different TCR αβ T cell clones (Vβ1, Vβ2, and Vβ23) were identified in the second patient (8). We also saw phenotypically diverse T cell neoplasms in our mouse model. Lymphoma cells from individual cases expressed a variety of T cell marker patterns including CD8+, CD4+, or CD4+CD8+ (Fig. 2C and Table 2).
Evidence for Insertional Mutagenesis Involving Protooncogene Loci.
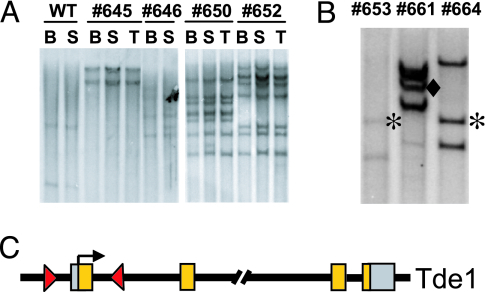
Southern blot analysis of tumors from different organs within individual mice showed a clonal pattern of integration sites, with two to seven vector copies per clone (Fig. 3A). We then used linear-amplification-mediated (LAM)-PCR to map 58 different insertions from 12 lymphoma samples in the γc-transplanted group. Five integrations occurred within or near genes that are represented in the Retroviral Tagged Cancer Gene Database (RTCGD) (17) (Table 1, asterisks). This database contains ≈300 common insertion sites that have been associated with murine tumors induced with wild-type Moloney retrovirus (http://RTCGD.ncifcrf.gov). We also detected insertions near or within genes that are not in the RTCGD but that have previously been demonstrated to have a role in oncogenesis (Table 1). For instance, Mela and Trim36 were identified in our screen and are highly expressed in murine melanoma (18) or in human prostate cancer (19), respectively. Of the 42 loci we detected that contained vector integrations, 12 were identified that contained multiple independent insertions that occurred in different regions of the gene. For instance, we identified two different integrations in the Tde1 (tumor differentially expressed 1) locus (Fig. 3B and Table 1). One insertion was in the sense orientation and mapped 1.4 kb upstream of the transcription start site, and the other was in an antisense orientation in the first intron, 1.8 kb downstream from the transcription start site (Fig. 3C). Interestingly, this finding mirrors the general insertion pattern into the LMO2 locus seen in the first two patients reported in the XSCID clinical trial and is consistent with a selective advantage for clonogenic tumor cells that have activation of specific oncogenes.
Fig. 3.
Vector integration site analysis in tumor cells. (A) Southern blot analysis of genomic DNA from bone marrow (BM), spleen (S), and thymus (T) of mice with lymphoma in the γc vector-transduced group. DNA was digested with EcoRI, which cuts once in the vector, and probed for a GFP fragment to identify individual integration events. Genomic DNA from a GFP vector-transduced wild-type recipient was used as a probe control. DNA from a normal mouse (WT) without transplantation is shown as a negative control. (B) EcoRI-digested DNA from three lymphomas (numbers above columns) were analyzed by Southern blot. Fragments are labeled as follows: ∗, Tde-1 integration no. 1; ♦, Tde-1 integration no. 2. (C) Schematic illustration of the two Tde-1 integration sites. The arrowheads show the orientation of the integrated vector, and the arrow shows the transcriptional start site.
Table 1.
Vector insertions in loci of oncogenes
| Locus | Gene ID | Chromosome | Position to TSS (intron) | Orientation | Occurrence in tumors |
|---|---|---|---|---|---|
| Chd9* | 109151 | 8C4 | 45651 | F | 645 |
| Slamf6* | 30925 | 1H3 | 14808 | R | 645, 646, 653, 657, 660 |
| Tde1* (hit 1) | 26943 | 2H3 | 1794 (1) | R | 653, 660, 664 |
| Tde1* (hit 2) | 26943 | 2H3 | −1371 | F | 661 |
| Camk2b* | 12323 | 11A1 | 54021 (3) | R | 661 |
| Ly6e* | 17069 | 15D3 | 24092 | R | 646, 650, 58, 661, 664 |
| Mela (hit 1) | 17276 | 8E2 | −5639 | F | 650 |
| Mela (hit 2) | 17276 | 8E2 | 723 (1) | F | 658 |
| Mela (hit 3) | 17276 | 8E2 | 1086 (1) | F | 657 |
| Trim36 | 28105 | 18C | 121105 | R | 653 |
Insertion sites recovered by LAM-PCR from lymphoma samples in the γc-transplanted group. Genomic locations and sequence codes (Gene ID) are determined according to the National Center for Biotechnology Information (NCBI) mouse genome database. Insertions are defined with respect to the transcription start sites (TSS) of neighboring genes. Asterisks indicate loci that are listed in the RTCGD database. The individual animals that had each of these insertions are listed in the rightmost column. F, forward orientation with respect to the gene’s transcription direction; R, reverse orientation with respect to the gene’s transcription direction.
We also identified specific integrations occurring in different tumors. For instance, six tumors contained a specific integration 15 kb upstream of the Slamf6 locus (Table 1), a gene known to be involved in lymphocyte signaling (20). The presence of this integration in multiple tumors suggests that this integration conferred a proliferative advantage to this clone during the in vitro culture phase before transplant, causing at least three cell doublings in 48 h. This result is consistent with a recent report showing that vector integrations into oncogenes can result in immortalization of mouse bone marrow cells grown in vitro (21). This result also suggests that in vitro culture conditions (transduction period, cytokine combination) may be implicated in the rate of transformation by selecting for specific integration events.
An Expanded Population of Bone Marrow Progenitors in XSCID Mice.
Our data show that the incidence of tumors in cohorts treated with the γc vector was much lower when Arf−/−γc+/+ cells were used versus Arf−/−γc−/− cells (Fig. 1 A and B), indicating that the XSCID background is a significant risk factor for tumorigenesis. One important implication of this result is that the risk of insertional mutagenesis may be uniquely elevated for XSCID gene therapy and less in other hematopoietic disorders, such as those affecting myeloid cells. We hypothesized that a potential risk factor could be an expanded pool of hematopoietic progenitor cells resulting from the developmental block induced by the γc mutation, and, if so, this increased progenitor cell population could increase the target size for vector-induced insertional mutagenesis. To explore this possibility, we analyzed bone marrow cells from XSCID mice for their relative content of primitive cells with the c-kit+Sca-1+Lin− (KSL) and side population (SP) phenotype. Cells with the KSL phenotype are highly enriched for progenitor and stem cells, and the subset of KSL cells with the SP phenotype is further enriched for pluripotent hematopoietic repopulating cells (22, 23). We observed a reproducible 3- to 5-fold expansion of KSL cells in bone marrow from XSCID mice (Fig. 4A, which is published as supporting information on the PNAS web site). The majority of these expanded KSL cells lacked the SP phenotype, indicating that they were not HSCs but, instead, more committed progenitor cells. Competitive repopulation experiments confirmed the lack of expansion of myeloid repopulating cells in XSCID mice (Fig. 4B). We are currently evaluating the functional characteristics of this expanded population. One interesting possibility is that these cells could be early lymphocyte progenitors; however, the lack of γc expression in these cells confounds a phenotypic analysis for IL7R expression.
Discussion
We have generated an XSCID mouse model that reproduces the T cell transformation arising from insertional mutagenesis that was seen in the French XSCID gene therapy trial. The system is highly efficient and resulted in >90% of the experimental animals developing lymphomas within 1 year after transplant. This high rate of transformation was, in part, due to the loss of tumor-suppressor p19Arf. It is known that the Arf pathway becomes activated and induces apoptosis when deregulated oncogene expression induces cellular proliferation. For instance, when cMyc is overexpressed in lymphoid cells, most cells undergo apoptosis due to activation of the Arf pathway (24). In the minority of clones that survive and proliferate, loss of the Arf pathway was an obligate requirement for transformation (25). Our data indicate a similar mechanism may apply to insertional mutagenesis in XSCID gene therapy. In this model, the initiating event would be clonal proliferation due to insertional activation of an oncogene. This proliferation would be further augmented by the proliferative advantage associated with restoration of IL7-dependent signaling. Most of these clones would undergo apoptosis and be eliminated because of activation of the Arf pathway; however, those with mutations in the Arf/p53 pathway would survive and continue to proliferate. This model is consistent with the 3-year latency seen in the clinical cases and raises the question as to whether loss of the Arf signaling pathway was also present in the French XSCID cases or, indeed, may be required.
Our data also show that the frequency of T cell transformation was significantly increased when using donor cells from XSCID versus immunocompetent mice, despite Arf deletion in both cases. There are at least three aspects of the XSCID background that could contribute to this increased incidence of transformation. First, it is known that immunodeficiency in mice leads to an increased rate of tumor formation because of the lack of antitumor immune surveillance (26). In particular, XSCID mice and patients have a drastic reduction in the number of natural killer (NK) cells, an important component of the antitumor immune response. Therefore, recipients may be at greater risk for developing tumors, given the delay in NK cell reconstitution. In contrast, immediate adoptive transfer of NK cells occurs when Arf−/−γc+/+ donor cells are used.
The second possible risk factor is the strong selective advantage for transduced T cell progenitors resulting from acquisition of prethymic IL7 responses. Even when low proportions of HSCs are transduced with the vector, all lymphoid cells are derived from a small subpopulation of transduced cells. This nonphysiologic degree of proliferation could contribute to genetic damage and clonal selection. A third possible risk factor that is suggested by our data is the presence of an expanded population of primitive progenitors that exists in XSCID bone marrow. This population does not contain an expanded pool of HSCs, as evidenced by our phenotypic and repopulation studies but, rather, may be due to an expansion of lymphoid progenitors. If this is the case, this “stalled target cell population” could directly increase the number of clones that harbor growth-promoting integration events. Further studies are needed to define this population and its role in transformation.
Another possible mechanism for the occurrence of T cell malignancies in XSCID gene therapy is that deregulated expression of the vector-encoded γc gene could directly contribute to transformation. A recent study showed that 33% of mice developed T cell lymphoma after transplant with either XSCID or wild-type bone marrow cells that were transduced with a lentiviral vector encoding γc (27). In contrast, our own data show that deregulated γc gene expression was not a dominant factor, because the rate of lymphoma development was relatively low in mice transplanted with Arf-null, γc wild-type cells transduced with the γc vector. Furthermore, other studies have shown no adverse effects when oncoretroviral vectors were used to overexpress γc in mouse models of XSCID gene therapy (28–30). One possible explanation for these discrepancies is that the study by Woods et al. (27) used a lentiviral vector, whereas our own and other studies used oncoretroviral vectors based on Moloney leukemia virus. However, it is not clear why lentiviral vectors would be more likely to cause transformation, given the known differences in integration-site patterns. The higher tumor formation rate could be due to higher lentiviral versus oncoretroviral copy numbers, although the copy number was not reported in the recent study (27). To gain a better understanding of these issues, a direct comparison of the oncogenicity of lenti- versus onco-retroviral vectors in an XSCID gene therapy model will be necessary.
Our LAM-PCR data show that vector integration near oncogenes or growth-promoting genes was associated with clonal transformation. Six insertions in CIS/protooncogenes have been identified. We also found that several oncogenes contained multiple independent insertions that occurred in different tumor samples, suggesting that certain loci are preferred targets for integrations and/or that forced expression of these genes causes a dominant growth advantage. Vector integration near the LMO2 locus has been show in all three cases of leukemia in the French XSCID trial; however, in our Arf−/−γc−/− animal model, we did not detect any insertion within the LMO2 locus. Assuming that Arf is not involved in the clinical cases, although it may be, one possibility is that our model is biased toward the activation of oncogenes cooperating with loss of Arf function. Another potential explanation for the lack of LMO2 insertions is that we used bone marrow cells from young adults, whereas the clinical studies used neonatal bone marrow cells. Furthermore, we targeted whole bone marrow cells in contrast to CD34+-selected cells in the clinical cases. These methodological differences could lead to changes in retroviral integration patterns at different developmental stages.
In summary, our data show that the key requirements for transformation are transduction with the γc vector, presence of the XSCID background in donor cells, selection of insertion sites near or within cellular protooncogenes, and biallelic deletion of Arf. Because these risk factors are lacking in other gene therapy approaches, such as in the treatment of myeloid disorders, the risk of insertional mutagenesis in these cases may be much less than that seen in XSCID gene therapy. This prediction is consistent with the lack of insertional oncogenesis in other human and large-animal studies (31), with the exception of a recent single documented case (32). Our other conclusion is that this model should allow for quantitative assessment of vector modifications designed to increase safety. We now can test whether lentiviral vectors that lack viral enhancer and promoter sequences, or that include flanking chromatin insulators (33), will result in a decreased rate of transformation. Another variable that can be tested is the effect of using different populations of donor cells derived by alternate isolation strategies intended to reduce the number of vulnerable target cells. These studies may support new clinical strategies that will enhance the safety and efficacy of XSCID gene therapy.
Materials and Methods
Animals.
Arf-null (34) and γc-null (35) mice were kindly provided by Dr. Charles Sherr’s (St. Jude Children’s Research Hospital) and Dr. Warren Leonard’s (National Heart, Lung, and Blood Institute) laboratories, respectively. Arf−/−γc−/− mice were produced by interbreeding of single-null mice and genotyping as described previously. All mice were maintained on a C57B6 background and were used for bone marrow harvest between 8 and 12 weeks of age. All experimental procedures were approved by the institutional animal care and use committee of St. Jude Children’s Research Hospital.
Bone Marrow Transplantation.
Generation of MSCV-IRES-GFP vector was described in ref. 36. Human γc cDNA was generated by RT-PCR from mRNA of human peripheral blood and inserted into either a MFG or MSCV-IRES-GFP vector backbone. The vector plasmid was then transfected with the pPAM3 (-E) and pVSV-G plasmids into 293T cells, and supernatant was harvested and used to infect GP+E86 cells three times repeatedly over 3 days. The transduced GP+E86 cells were sorted with FACSVantage (Becton Dickinson, San Jose, CA) and subsequently expanded. Retroviral transduction and transplantation of primary bone marrow cells was performed as described (36). Briefly, bone marrow cells were harvested from Arf−/−γc−/− mice and prestimulated in DMEM with 15% heat-inactivated FBS, 50 ng/ml rat stem cell factor, 20 ng/ml mIL-3, and 50 ng/ml hIL-6 (Amgen, Thousand Oaks, CA) for 48 h. The prestimulated bone marrow cells were then cocultured with irradiated (1,500 cGy) GP+E86 producer cells with 6 μg/ml polybrene for an additional 48 h before injection into lethally irradiated congenic CD45.1 mice. Transduction efficiency was determined by flow-cytometry analysis of peripheral blood cells 9 weeks after transplantation. Transplanted mice were followed up for 1 year with regular complete blood count examinations.
Analysis of Leukemia.
Transplanted mice were observed daily and killed when they showed signs of illness or became moribund. Various tissues were collected, fixed in 4% formalin, and paraffin-embedded for histologic examination. Cells from bone marrow, spleen, and thymus were stained with phycoerythrin-conjugated CD45.1, CD4, and B220 and allophycocyanin-conjugated Gr-1, CD8, and Mac-1 antibodies and subjected to flow cytometry. Genomic DNA was extracted from spleen, thymus, and bone marrow samples for further analysis.
Southern Blot and LAM-PCR.
For Southern blot analysis, 10 μg of genomic DNA was digested with EcoRI and hybridized with a probe corresponding to a partial GFP sequence. LAM-PCR was performed as described (37). For linear amplification of the 3′ LTR-genomic junction site, 100 ng of DNA was amplified by repeated primer extension by using a MSCV-LTR specific 5′-biotinylated primer (LTR1): 5′-CTGGGGACCATCTGTTCTTGGCCCT. The biotinylated extension products were selected with 200 μg of magnetic beads (Dynal, Oslo, Norway), digested with Tsp509I (New England Biolabs, Beverly, MA), and ligated to an asymmetric linker cassette (5′-AATTCTCTAGTATGCTACTCGCACCGATTATCTCCGCTGTCAGT and 5′-ACTGACAGCGGAGATAATCGGTGCGAGTAGCATACTAGAG). Ligation products were then amplified with a vector-specific primer LTR2 (5′-GACTTGTGGTCTCGCTGTTCCTTGG) and a linker cassette primer LC1 (5′-ACTGACAGCGGAGATAATCG). Nested PCR with internal primers LTR3: (5′-GGTCTCCTCTGAGTGATTGACTACC) and LC2: (5′-GTGCGAGTAGCATACTAGAG) was done to further amplify the products. The final PCR products were separated on a high-resolution Spreadex gel (Elchrom Scientific, Cham, Switzerland). Specific bands were excised and reamplified with primer pair LTR3 and LC2 and then cycle-sequenced directly.
Analysis of SP-KSL-CD34− Cells.
Bone marrow cells from 6- to 8-week-old mice were stained with 5 μg/ml Hoechst 33342 (Sigma) at 37°C for 90 min. After two washes, cells were stained with phycoerythrin-conjugated anti-Sca-1, allophycocyanin (APC)-conjugated anti-c-Kit, FITC-conjugated anti-CD34, and biotinylated lineage-antibody mixture consisting of anti-Gr-1, Mac-1, B220, CD3, and Ter119 mAbs for 30 min on ice and were further stained with APC-Cy7-conjugated streptavidin. Four-color analysis was performed on a FACSVantage (Becton Dickinson).
Supplementary Material
Acknowledgments
We thank Dr. Kumar Srivastava for help with statistical analysis, Dr. Charles Sherr for helpful discussions and providing Arf-null mice, Dr. Kelli Boyd for performing the immunohistochemistry analysis, and Dr. Geoffrey Neale for help in analyzing integration-site data. This work was supported by National Heart, Lung, and Blood Institute Grant P01 HL 53749, Cancer Center Support Grant P30 CA 21765, the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities.
Abbreviations
- γc
common γ-chain
- HSC
hematopoietic stem cell
- IRES
internal ribosome entry site
- KSL
c-kit+Sca-1+Lin−
- LAM
linear-amplification-mediated
- MSCV
murine stem cell virus
- SCID
severe combined immunodeficiency
- XSCID
X-linked SCID.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Aiuti A., Slavin S., Aker M., Ficara F., Deola S., Mortellaro A., Morecki S., Andolfi G., Tabucchi A., Carlucci F., et al. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M., Hacein-Bey S., De Saint B. G., Gross F., Yvon E., Nusbaum P., Selz F., Hue C., Certain S., Casanova J. L., et al. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 3.Gaspar H. B., Parsley K. L., Howe S., King D., Gilmour K. C., Sinclair J., Brouns G., Schmidt M., von Kalle C., Barington T., et al. Lancet. 2004;364:2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 4.Hacein-Bey-Abina S., Le Deist F., Carlier F., Bouneaud C., Hue C., de Villartay J. P., Thrasher A. J., Wulffraat N., Sorensen R., Dupuis-Girod S., et al. N. Engl. J. Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 5.Baum C., Dullmann J., Li Z., Fehse B., Meyer J., Williams D. A., von Kalle C. Blood. 2003;101:2099–2114. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- 6.Fischer A., Abina S. H., Thrasher A., von Kalle C., Cavazzana-Calvo M. N. Engl. J. Med. 2004;350:2526–2527. doi: 10.1056/NEJM200406103502422. [DOI] [PubMed] [Google Scholar]
- 7.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J. L., Fraser C. C., Cavazzana-Calvo M., et al. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 8.Hacein-Bey-Abina S., von Kalle C., Schmidt M., McCormack M. P., Wulffraat N., Leboulch P., Lim A., Osborne C. S., Pawliuk R., Morillon E., et al. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 9.McCormack M. P., Rabbitts T. H. N. Engl. J. Med. 2004;350:913–922. doi: 10.1056/NEJMra032207. [DOI] [PubMed] [Google Scholar]
- 10.Nienhuis A. W., Dunbar C. E., Sorrentino B. P. Mol. Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Bonini C., Grez M., Traversari C., Ciceri F., Marktel S., Ferrari G., Dinauer M., Sadat M., Aiuti A., Deola S., et al. Nat. Med. 2003;9:367–369. doi: 10.1038/nm0403-367. [DOI] [PubMed] [Google Scholar]
- 12.Ott M. G., Schmidt M., Schwarzwaelder K., Stein S., Siler U., Koehl U., Glimm H., Kuhlcke K., Schilz A., Kunkel H., et al. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 13.Modlich U., Kustikova O. S., Schmidt M., Rudolph C., Meyer J., Li Z., Kamino K., von Neuhoff N., Schlegelberger B., Kuehlcke K., et al. Blood. 2005;105:4235–4246. doi: 10.1182/blood-2004-11-4535. [DOI] [PubMed] [Google Scholar]
- 14.Li Z., Dullmann J., Schiedlmeier B., Schmidt M., von Kalle C., Meyer J., Forster M., Stocking C., Wahlers A., Frank O., et al. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- 15.Kamijo T., Bodner S., van de Kamp E., Randle D. H., Sherr C. J. Cancer Res. 1999;59:2217–2222. [PubMed] [Google Scholar]
- 16.Gardie B., Cayuela J. M., Martini S., Sigaux F. Blood. 1998;91:1016–1020. [PubMed] [Google Scholar]
- 17.Akagi K., Suzuki T., Stephens R. M., Jenkins N. A., Copeland N. G. Nucleic Acids Res. 2004;32:D523–D527. doi: 10.1093/nar/gkh013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi H., Matsubara H., Yokota T., Kuwabara I., Kanno M., Koseki H., Isono K., Asano T., Taniguchi M. J. Immunol. 1992;149:1223–1229. [PubMed] [Google Scholar]
- 19.Balint I., Muller A., Nagy A., Kovacs G. Gene. 2004;332:45–50. doi: 10.1016/j.gene.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 20.Veillette A., Cruz-Munoz M. E., Zhong M. C. Trends Immunol. 2006;27:228–234. doi: 10.1016/j.it.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Du Y., Jenkins N. A., Copeland N. G. Blood. 2005;106:3932–3939. doi: 10.1182/blood-2005-03-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou S., Morris J. J., Barnes Y., Lan L., Schuetz J. D., Sorrentino B. P. Proc. Natl. Acad. Sci. USA. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tadjali M., Zhou S., Rehg J., Sorrentino B. P. Stem Cells. 2006;24:1556–1563. doi: 10.1634/stemcells.2005-0562. [DOI] [PubMed] [Google Scholar]
- 24.Zindy F., Eischen C. M., Randle D. H., Kamijo T., Cleveland J. L., Sherr C. J., Roussel M. F. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eischen C. M., Weber J. D., Roussel M. F., Sherr C. J., Cleveland J. L. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oppenheim D. E., Roberts S. J., Clarke S. L., Filler R., Lewis J. M., Tigelaar R. E., Girardi M., Hayday A. C. Nat. Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 27.Woods N. B., Bottero V., Schmidt M., von Kalle C., Verma I. M. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
- 28.Lo M., Bloom M. L., Imada K., Berg M., Bollenbacher J. M., Bloom E. T., Kelsall B. L., Leonard W. J. Blood. 1999;94:3027–3036. [PubMed] [Google Scholar]
- 29.Otsu M., Anderson S. M., Bodine D. M., Puck J. M., O’Shea J. J., Candotti F. Mol. Ther. 2000;1:145–153. doi: 10.1006/mthe.1999.0020. [DOI] [PubMed] [Google Scholar]
- 30.Soudais C., Shiho T., Sharara L. I., Guy-Grand D., Taniguchi T., Fischer A., Di Santo J. P. Blood. 2000;95:3071–3077. [PubMed] [Google Scholar]
- 31.Kiem H. P., Sellers S., Thomasson B., Morris J. C., Tisdale J. F., Horn P. A., Hematti P., Adler R., Kuramoto K., Calmels B., et al. Mol. Ther. 2004;9:389–395. doi: 10.1016/j.ymthe.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Seggewiss R., Pittaluga S., Adler R. L., Guenaga F. J., Ferguson C., Pilz I. H., Ryu B., Sorrentino B. P., Young W. S., III, Donahue R. E., et al. Blood. 2006;107:3865–3867. doi: 10.1182/blood-2005-10-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emery D. W., Yannaki E., Tubb J., Stamatoyannopoulos G. Proc. Natl. Acad. Sci. USA. 2000;97:9150–9155. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamijo T., Zindy F., Roussel M. F., Quelle D. E., Downing J. R., Ashmun R. A., Grosveld G., Sherr C. J. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 35.Cao X., Shores E. W., Hu-Li J., Anver M. R., Kelsall B. L., Russell S. M., Drago J., Noguchi M., Grinberg A., Bloom E. T., et al. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 36.Sawai N., Zhou S., Vanin E. F., Houghton P., Brent T. P., Sorrentino B. P. Mol. Ther. 2001;3:78–87. doi: 10.1006/mthe.2000.0223. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M., Zickler P., Hoffmann G., Haas S., Wissler M., Muessig A., Tisdale J. F., Kuramoto K., Andrews R. G., Wu T., et al. Blood. 2002;100:2737–2743. doi: 10.1182/blood-2002-02-0407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.