Abstract
Although augmented prostaglandin E2 (PGE2) synthesis and accumulation have been demonstrated in the lesion sites of rodent transient focal ischemia models, the role of PGE2 in neuronal survival has been controversial, showing both protective and toxic effects. Here we demonstrate the induction of microsomal PGE synthase 1 (mPGES-1), an inducible terminal enzyme for PGE2 synthesis, in neurons, microglia, and endothelial cells in the cerebral cortex after transient focal ischemia. In mPGES-1 knockout (KO) mice, in which the postischemic PGE2 production in the cortex was completely absent, the infarction, edema, apoptotic cell death, and caspase-3 activation in the cortex after ischemia were all reduced compared with those in wild-type (WT) mice. Furthermore, the behavioral neurological dysfunctions observed after ischemia in WT mice were significantly ameliorated in KO mice. The ameliorated symptoms observed in KO mice after ischemia were reversed to almost the same severity as WT mice by intracerebroventricular injection of PGE2 into KO mice. Our observations suggest that mPGES-1 may be a critical determinant of postischemic neurological dysfunctions and a valuable therapeutic target for treatment of human stroke.
Keywords: apoptosis, cyclooxygenase-2, inflammation, ischemia, prostaglandin E2
Stroke remains a major cause of death and neuronal disability worldwide. Although effective stroke treatments based on thrombolysis and restoration of blood flow have been developed (1), these therapies are effective only during the first few hours after the onset of the stroke (2). At later times after ischemia, many kinds of gene induction occur (3), some of which are known to be involved in the brain inflammation that is a major factor in the progression of the injury (4, 5).
Prostaglandin E2 (PGE2), one of the most likely candidates for propagation of inflammation, is known to be accumulated at the lesion sites of the postischemic brain (6, 7). PGE2 is sequentially synthesized from arachidonic acid by cyclooxygenase (COX) and PGE2 synthase (PGES). Among the COX isoforms, COX-2 is the inducible form; however, it has been immunohistochemically detected in neurons in the normal brain (8). COX-2 has been demonstrated to be up-regulated after transient ischemia in neurons (9–12) and has recently been identified in nonneuronal cells as well at some lesion sites, e.g., in microglia in the brains of patients with multiple sclerosis and chronic cerebral ischemia (13, 14). The genetic disruption and chemical inhibition of COX-2 have been shown to ameliorate neuronal death after transient forebrain ischemia, suggesting that the PGE2 accumulated through COX-2 induction mediates the toxic effects in the brain (7, 10, 15). Conversely, the genetic disruption of EP2, one of the PGE2 receptors expressed in the brain, has been shown to exacerbate neuronal death after transient forebrain ischemia, suggesting that PGE2 has a neuroprotective effect on postischemic injury (16). In in vitro studies, the effect of PGE2 has also been controversial, with results showing both toxic and protective effects on neuronal survival (17, 18). Therefore, a study of PGES, a terminal enzyme for PGE2 synthesis, should provide a considerable amount of information that could help to resolve these discrepancies.
Three major isoforms of PGES were recently isolated: cytosolic PGES (cPGES), microsomal PGES 1 (mPGES-1), and mPGES-2. Whereas cPGES and mPGES-2 are constitutively expressed in various cells and tissues, mPGES-1 is induced by proinflammatory stimuli and in various models of inflammation and is functionally coupled to COX-2 (19, 20). The profile of the mPGES-1 knockout (KO) mice strongly supports the idea that mPGES-1 plays an important role in the inflammatory PGE2 production and in the inflammation in animal models of pain, arthritis, and pyresis (21–23). Nonetheless, the behaviors and roles of PGES in brain inflammation have not yet been established. Recently, we demonstrated that the activation of microglia by lipopolysaccharide contributes to PGE2 production through the mPGES-1 induction at sites of inflammation of the brain parenchyma (24). Here we show that the coinduction of mPGES-1 and COX-2 in the neurons, microglia, and endothelial cells in the cerebral cortex contributes not only to the postischemic PGE2 production, but also to the edema, infarction, apoptotic cell death, and even the behavioral neurological dysfunctions observed after ischemia.
Results
Induction of mPGES-1 After Cerebral Ischemia.
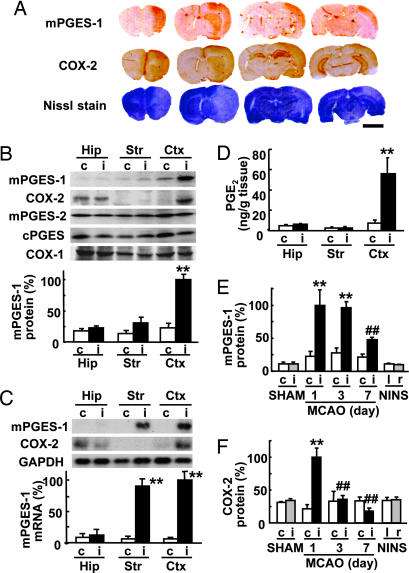
The immunostaining for mPGES-1 and COX-2 of rat brains after 2 h of focal ischemia followed by 24 h of reperfusion showed ipsilateral coinduction of mPGES-1 and COX-2 in the postischemic lesion site (the pale area in the Nissl staining; Fig. 1A). In sham-operated rats and no-ischemia, no-surgery (NINS) rats, the induction of mPGES-1 was detected in neither the ipsilateral (right) nor contralateral (left) cortex (see Fig. 7A, which is published as supporting information on the PNAS web site). The immunostaining of mPGES-1 observed in the cortex and striatum after ischemia was abolished when a preabsorbed antibody with an antigen peptide for mPGES-1 was used (Fig. 7B). The Western blot analysis again demonstrated that mPGES-1 protein and COX-2 protein, but not mPGES-2, cPGES, or COX-1 protein, were potently induced in the ipsilateral cerebral cortex 24 h after transient ischemia (Fig. 1B). Slight mPGES-1 induction in the striatum also was detected. As was the case for mPGES-1 protein induction, the mRNA of mPGES-1 was undetectable in the contralateral hemisphere and was increased in the ipsilateral (postischemic) striatum and cortex, but not in the hippocampus (Fig. 1C). COX-2 mRNA also was expressed in the bilateral hippocampus and induced in the postischemic cortex. A significant accumulation of PGE2 was detected only in the ipsilateral cortex (Fig. 1D). We next investigated the time course of induction of mPGES-1 and COX-2 proteins. In the postischemic cortex, but not in the cortex of sham-operated animals, mPGES-1 protein was dramatically increased after 1 day, and by 1–3 days, the expression had reached a maximal level that was ≈10-fold higher than that in the no-ischemia, no-surgery (NINS) normal rats (Fig. 1E). In the contralateral hemisphere, slight mPGES-1 up-regulation was observed after ischemia as compared with sham-operated or NINS rats, but this effect was not significant. The induction of the COX-2 protein after ischemia was more transient than that of mPGES-1 (Fig. 1F). The basal expressions of mPGES-1 and COX-2 in the hippocampus and striatum did not change by sham operation (Fig. 7 C–F). The content of PGE2 in ipsilateral cortex was increased 1 day after ischemia (ipsilateral, 52.23 ± 11.21 ng/g vs. contralateral, 3.46 ± 0.73 ng/g; n = 4; P < 0.01), then decreased to the basal level 3 days after ischemia (ipsilateral, 6.73 ± 2.30 ng/g vs. contralateral, 2.20 ± 0.13 ng/g; n = 4).
Fig. 1.
mPGES-1 induction in the rat brain after transient ischemia. (A) Immunostaining for mPGES-1 and COX-2 and Nissle staining of a coronal brain slice 24 h after ischemia. Representative data from six animals are presented. (Scale bar, 5 mm.) (B) (Upper) Western blot analysis of the expression of mPGES-1, COX-2, mPGES-2, cPGES, and COX-1 in the hippocampus (Hip), striatum (Str), and cortex (Ctx) of the ipsilateral (i) or contralateral (c) hemisphere 24 h after ischemia. (Lower) Quantitated data from immunoblotting with mPGES-1 antibody were scaled to a percentage of the maximal response. (C) (Upper) Northern blot analysis of the expression of mPGES-1 and COX-2 in the brain tissue 6 h after ischemia. GAPDH signals were used as loading controls. (Lower) Quantitated data from blotting with mPGES-1 probe were normalized to GAPDH and scaled to a percentage of the maximal response. (D) The amount of PGE2 in the brain tissue 24 h after ischemia was measured by using an enzyme immunoassay kit. (E and F) Time course of mPGES-1 (E) and COX-2 (F) protein expression in the cortex of MCAO animals, sham-operated animals killed 24 h after surgery (SHAM), and animals without surgery and ischemia (no-ischemia, no-surgery; NINS). Quantitated data from immunoblotting with mPGES-1 and COX-2 antibody were scaled to a percentage of the maximal response. n = 4 animals per group; ∗∗, P < 0.01 vs. the contralateral tissue; ##, P < 0.01; and #, P < 0.05 vs. the ipsilateral cortex (day 1).
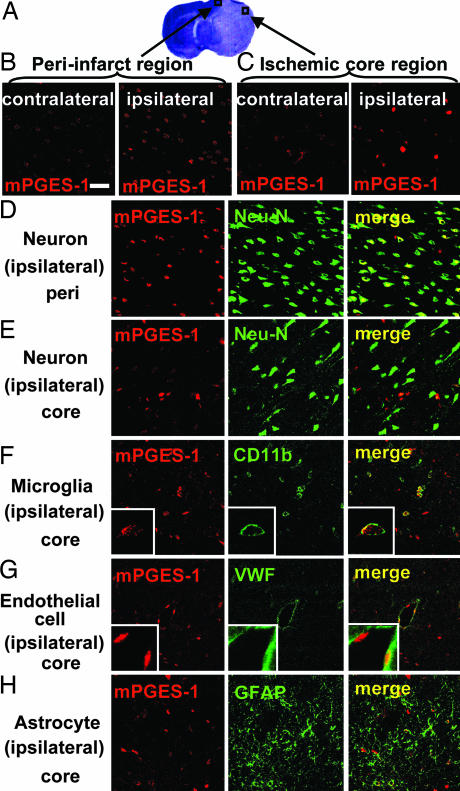
Immunostaining of rat brain slices for mPGES-1 revealed induction of mPGES-1 in the peri-infarct and core region of the postischemic cortex (Fig. 2A). In the peri-infarct region of the postischemic cortex, there were numerous mPGES-1-positive cells (43.33 ± 1.64 cells/0.054 mm2, n = 9) with moderate expression (Fig. 2B). Most of these cells showed a morphology resembling neurons that have dendritic processes and are positive for neuron-specific nuclear protein (Neu-N), a specific marker for neurons (Fig. 2D). Conversely, in the ischemic core region, there were fewer mPGES-1-positive cells (17.11 ± 1.51 cells/0.054 mm2; n = 9; P < 0.01 vs. the peri-infarct region) with abundant expression (Fig. 2C). These cells were not positive for Neu-N (Fig. 2E) but were positive for CD-11b or von Willebrand factor (VWF), a specific marker for microglia or endothelial cells, respectively (Fig. 2 F and G). High magnifications clearly show that mPGES-1 and either CD-11b or VWF are expressed in the same cells (Fig. 2 F Inset and G Inset). None of the mPGES-1-positive cells were positive for glial fibrillary acidic protein (GFAP), a specific marker for astrocytes (Fig. 2H).
Fig. 2.
Induction of mPGES-1 in neurons, microglia, and endothelial cells in the cortex after ischemia. (A) The predesignated areas in the peri-infarct and ischemic core regions of the postischemic cortex for microscopic examination. (B and C) The immunostaining of mPGES-1 in the peri-infarct region (B) and ischemic core region (C) of ipsilateral postischemic cortex and contralateral cortex. (D–H) The double-immunostaining of mPGES-1 (red) and cell-type-specific marker proteins (green) in the peri-infarct (D) and core (E–H) regions of the cortex. Neurons (D and E), microglia (F), endothelial cells (G), and astrocytes (H) were recognized by antibodies for Neu-N, CD11b, von Willebrand factor (VWF), and GFAP, respectively. Insets show high magnification of each staining. The photographs shown are representative examples from three separate experiments. (Scale bars: main images, 40 μm; Insets, 10 μm.)
Necessity of mPGES-1 for Postischemic PGE2 Production.
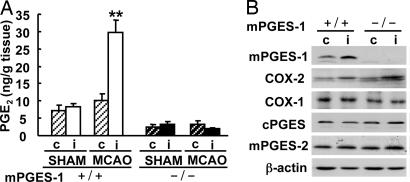
To evaluate the contribution of mPGES-1 to postischemic PGE2 production, we used mPGES-1 KO mice. The postischemic PGE2 production observed in the ipsilateral cortex of wild-type (WT) mice was completely absent in that of mPGES-1 KO mice (Fig. 3A). The expressions of mPGES-2, cPGES, and COX-1 and the inducible expression of COX-2 were similar in WT and KO mice (Fig. 3B). There were no significant differences between mPGES-1 KO and WT mice in mean arterial pressure, pH, pCO2, or pO2 levels and changes of cerebral blood flow before, during, or after middle cerebral artery occlusion (MCAO) and in anatomy of the circle of Willis or the origins of the cerebral arteries (see Table 1 and Fig. 8, which are published as supporting information on the PNAS web site).
Fig. 3.
mPGES-1 is an essential component for PGE2 production in the postischemic cortex. (A) The production of PGE2 in the ipsilateral (i) or contralateral (c) cortex of mPGES-1 KO (−/−) and WT (+/+) mice 24 h after MCAO and sham operation. n = 8 animals per group; ∗∗, P < 0.01 vs. another sample. (B) Western blot analyses for mPGES-1, COX-2, COX-1, cPGES, and mPGES-2 in the cortex 24 h after transient ischemia. Representative immunoblots from three separate experiments are presented.
mPGES-1 Contributes to Edema, Infarction, Neurological Dysfunctions, and Neuronal Apoptosis Observed After Ischemia.
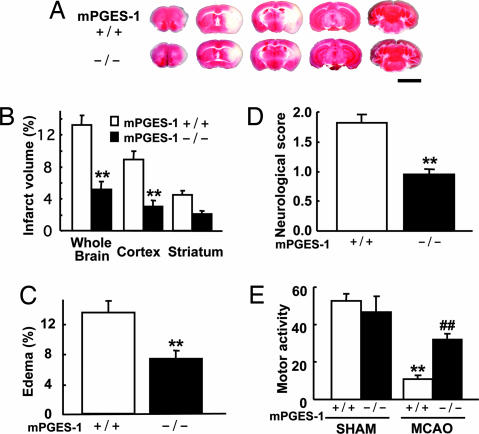
To investigate the role of mPGES-1 in postischemic insult, we carried out studies in mPGES-1 KO mice. We observed smaller infarct size in mPGES-1 KO mice compared with WT mice (Fig. 4A and B); the infarct volume in both the whole brain and cortex of mPGES-1 KO mice was <40% that of WT. The infarct volume in the striatum of KO mice was <50% that of WT mice, but the difference was not significant. The degree of edema of mPGES-1 KO mice was also significantly smaller, almost 50% that of WT mice (Fig. 4C). To determine the functional role of mPGES-1 in behavioral symptoms, we investigated the neurological dysfunction observed after MCAO. Mean neurological deficits of mPGES-1 KO mice were significantly lower than those of WT mice (Fig. 4D). The difference in spontaneous motor activity after ischemia was also evident, whereas there was no phenotypic difference between sham-operated WT and KO mice (Fig. 4E).
Fig. 4.
Deletion of the mPGES-1 resulted in marked amelioration of the infarction, edema, and behavioral neurological dysfunctions observed after ischemia. (A) Representative 2,3,5-triphenyltetrazolium chloride (TTC)-stained coronal sections at −2, 0, +2, +4, and +8 mm from the bregma of the mPGES-1 KO (−/−) and WT (+/+) mouse. (Scale bar: 5 mm.) (B) The volume of infarcted brain tissue 24 h after ischemia was estimated and expressed as a percentage of the corrected tissue volume. n = 10 animals per group; ∗∗, P < 0.01 vs. WT mice. (C) The corrected edema percentage in the mPGES-1 KO (−/−) and WT (+/+) mice. n = 10 animals per group; ∗∗, P < 0.01 vs. WT mice. (D) Improvement of neurological dysfunction in mPGES-1 KO mice. The neurological score was measured 24 h after ischemia. n = 21–22 animals per group; ∗∗, P < 0.01 vs. WT mice. (E) The motor activity of MCAO or sham-operated (SHAM) mice. n = 9–10 animals for SHAM and n = 19–20 animals for MCAO; ∗∗, P < 0.01 vs. SHAM WT mice; ##, P < 0.01 vs. MCAO-treated WT mice.
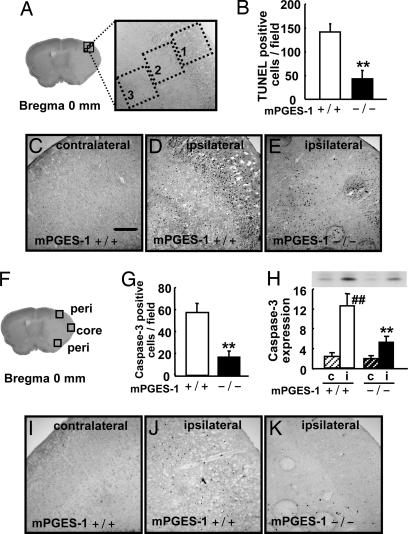
To explore the nature of differences in the infarction volume between mPGES-1 KO and WT mice, we examined the apoptotic reaction in the penumbra. Although the penumbra in KO was not exactly in the same place as that in WT because of smaller size of ischemic core region, we investigated three predesignated areas in the cerebral cortex (Fig. 5A and F). In WT mice, significant TUNEL staining was observed in the ipsilateral cortex at 24 h after ischemia, whereas the staining was completely negative in the contralateral cortex (Fig. 5 C and D). The mPGES-1 KO penumbra showed less in apoptosis compared with WT (Fig. 5 B and E). Furthermore, immunoreactivity for caspase-3 was not detectable in the contralateral hemisphere (Fig. 5I). The caspases-3 activation became pronounced in the middle cerebral artery (MCA) territory (Fig. 5J). The number of the caspase-3 positive cells was significantly fewer in the cortex of mPGES-1 KO than in that of WT (Fig. 5 G and K). The Western blot analysis from whole cerebral cortex samples also demonstrated a significant reduction of caspase-3 in the cortex of mPGES-1 KO mice compared with that in WT mice (Fig. 5H). There were fewer caspase-3-positive cells than TUNEL-positive ones. Caspase-3 activation would be down-regulated at this time point, possibly because caspase-3 activation is an event that appears to occur before DNA fragmentation. The TUNEL and caspase-3-positive cells were also positive for Neu-N (data not shown), indicating neuronal apoptosis in the postischemic cortex.
Fig. 5.
Deletion of the mPGES-1 gene resulted in a marked decrease in the apoptotic neuronal death in the postischemic cortex. (A) Three predesignated areas in the penumbra of the postischemic cortex for counting TUNEL-positive cells. (B) The average number of TUNEL-positive cells per unit area (A) of mPGES-1 KO (−/−) and WT (+/+) mice. n = 7 animals per group; ∗∗, P < 0.01 vs. WT mice. (C–E) Representative results of TUNEL staining in the contralateral or ipsilateral cortex of the mPGES-1 KO and WT mouse. (Scale bar: 100 μm.) (F) Three predesignated areas in peri-infarct (peri) and core regions for counting caspase-3-positive cells in the cortex. (G) The average number of caspase-3-positive cells per unit area (F) after transient ischemia. n = 7 animals per group; ∗∗, P < 0.01 vs. WT mice. (H) The tissue lysates from the contralateral (c) or ipsilateral (i) cortex were subjected to Western blot analysis for caspase-3. The densities of immunoblots were measured. n = 5 animals per group; ##, P < 0.01 vs. contralateral cortex. (I–K) Representative caspase-3 immunostaining in the cortex.
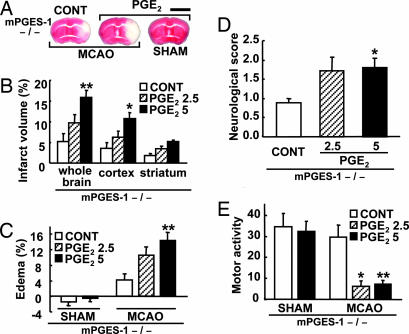
To investigate whether or not the improved symptoms in mPGES-1 KO mice after ischemia resulted from absence of PGE2 production in the brain, we injected PGE2 into the KO mice brain. In the WT ipsilateral cortex, ≈3 ng of PGE2 was produced after ischemia (Fig. 3) at 24 h after operation. Therefore, 2.5 or 5 ng of PGE2 was injected into the bilateral cerebral ventricle (1.25 or 2.5 ng each side) just before MCAO. The intracerebroventricular (i.c.v.) injections of PGE2 significantly increased infarct volume and edema percentage in the MCAO KO mice (Fig. 6A–C) to the same level as in the MCAO WT mice (Fig. 4 A–C). Moreover, PGE2 significantly exaggerated the neurological dysfunctions in KO mice (Fig. 6 D and E) to the same level as in the MCAO WT mice (Fig. 4 D and E). The i.c.v. injections of PGE2 caused no infarction (Fig. 6A), edema (Fig. 6C), neurological dysfunction (data not shown), or reduction of motor activity (Fig. 6E) in sham-operated KO mice.
Fig. 6.
i.c.v. injection of PGE2 exaggerated postischemic symptoms in mPGES-1 KO mice. (A) Representative 2,3,5-triphenyltetrazolium chloride (TTC)-stained coronal sections (3 mm from the prefrontal) from PGE2-injected (5 ng) or vehicle-injected (CONT) mice brain 24 h after ischemia (MCAO) or sham operation. (Scale bar: 5 mm.) (B and C) The corrected volume of infarcted brain tissue (B) and the corrected edema percentage (C) were measured from PGE2-injected (2.5 or 5 ng) or vehicle-injected (CONT) injected mice brain 24 h after ischemia (MCAO) or sham operation and expressed as a percentage of the total volume. (D and E) The neurological score (D) and the motor activity (E) were measured 24 h after ischemia (MCAO) or sham operation. For B–E, n = 7–10; ∗∗, P < 0.01; ∗, P < 0.05 vs. CONT.
Discussion
Here we have shown that the production of PGE2 through mPGES-1 induction plays a key role in the formation of postischemic brain infarction and neurological dysfunction in a rodent model of ischemia.
The expression of mPGES-1 was markedly induced in the cerebral cortex after transient ischemia. Although we found no apparent changes in the expression of cPGES or mPGES-2 after transient ischemia, the possibility that other types of PGES contribute to postischemic PGE2 production could not be excluded. However, the cortices of the mPGES-1-KO mice were unable to produce PGE2, even after transient ischemia, indicating that mPGES-1 plays a predominant role in postischemic PGE2 production in the brain. There was no compensatory up-regulation of other enzymes implicated in PGE2 synthesis in mPGES-1 KO mice. By using these mice, we showed that genetic disruption of mPGES-1 ameliorated cortical infarction, edema, and apoptotic neuronal death. Moreover, the neurological dysfunctions after transient ischemia also were improved by genetic disruption of mPGES-1. Because the reduction in cerebral blood flow produced by MCAO, physiological parameters, as well as the anatomy in the circle of Willis did not differ between the genotypes, the amelioration of symptoms observed in mPGES-1 KO mice cannot be attributed to differences in the severity of the ischemic insult. Together, these results suggest that mPGES-1 is a critical factor in the formation of the brain infarction and neurological dysfunction observed after ischemia. In recent studies, COX-2-deficient mice showed attenuated ischemia-induced neurotoxicity and DNA fragmentation as well as PGE2 production (7, 15). The phenotype in the postischemic insult of mPGES-1 KO mice resembles that of COX-2-deficient mice. This finding suggests that coordinate induction of mPGES-1 and COX-2 is required for postischemic PGE2 production, which causes inflammation and then ischemia-induced neuronal death. In fact, in our rat transient ischemia model, prominent PGE2 production was observed only when and where the expressions of both mPGES-1 and COX-2 were observed, i.e., in the cortex 1 day after ischemia, indicating again the necessity of the coexpression of mPGES-1 and COX-2 in postischemic PGE2 production. The time courses of the expression of mPGES-1 and COX-2 in the cortex differed from each other. The increased mPGES-1 expression was maintained at least 3 days after ischemia, whereas the enhanced expression of COX-2 was more transient, suggesting a difference of the mechanisms underlying the induction of mPGES-1 and COX-2. We have reported a similar difference in time course between these proteins in cultured microglia (24) and fibroblasts (25). The coexpressions of mPGES-1 and COX-2 protein were both preceded by mRNA induction, indicating that the induction of mPGES-1, as well as that of COX-2, is regulated at least in part at the transcription level.
The double immunostaining for mPGES-1 and each brain cell-specific marker protein shows that the mPGES-1 induction occurred in neurons in the peri-infarct region and microglia and vascular endothelial cells in the ischemic core region of the cortex. Because neuronal COX-2 induction has been reported in the peri-infarct region (12) and neuronal overexpression of COX-2 increases cerebral infarction (26), the coinduction of mPGES-1 and COX-2 in neurons in the peri-infarct region may contribute to postischemic brain injury. Moreover, because microglial and endothelial COX-2 inductions after ischemia and multiple sclerosis also have been reported (13, 14), PGE2 production through coinduction of mPGES-1 and COX-2 in microglia and endothelial cells is suggested to play an important role in postischemic inflammation and microcirculatory disturbances, which in turn contribute to neuronal death and the expansion of cerebral infarction (5, 27). Although we cannot exclude the possibility that the mPGES-1 induction observed in the cortex is due to the infiltrating monocytes, it was shown recently that microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia (28). Our results suggest that neurons, microglia, and endothelial cells are predominant sources of postischemic PGE2 production. Indeed, we recently identified a microglia-specific mPGES-1 induction that contributed to PGE2 production in an animal model of brain inflammation induced by intraparenchymal injection of lipopolysaccharide (24). In addition, the endothelial mPGES-1 induction is well known in the brains of several animal models (29–31).
The involvement of PGE2 under induction of mPGES-1 in expansion of postischemic brain injury was confirmed by the ability of injected PGE2 to increase postischemic injury in mPGES-1 KO mice. Although the PGE2 injected i.c.v. may not remain elevated for a whole 24 h after ischemia, and further study will be needed to quantify the optimal amount of injected PGE2 and its time of action, PGE2 diffusing from the cerebral ventricle to the striatum and/or cerebral cortex at an even earlier time point than 24 h can be thought to exacerbate the postischemic injury. PGE2 can efflux by simple diffusion after synthesis and activate four receptor subtypes (EP1–4) with quite different signaling cascades. Using EP2-deficient mice, it recently was shown that the neuroprotective function of the EP2 receptor in cerebral ischemia depends on cAMP signaling (16). How then is the toxicity of mPGES-1 enzymatic activity mediated? The toxic effects also could be mediated by PG receptor subtypes that are not positively coupled to cAMP formation: for example, by EP3, which is thought to mediate effects in a manner opposite to the mediation by EP2; by EP1, which may enhance the elevation of intracellular calcium concentrations in neurons after ischemia; or by receptors of other prostanoids converted from PGE2, such as PGF2α, which promotes neuronal death in spinal cord injury (32). It also has been shown that the heightened temperatures induced by i.c.v. injection of PGE2 play a significant role in escalating the neural damage caused by global ischemia (33) and that hypothermia rescues hippocampal CA1 neurons from forebrain ischemia (34). In our experimental model, the rectal temperature was maintained at 37°C during the operation; however, there may have been some differences in local brain temperature during and/or after the ischemia between genotypes. Therefore, further studies will be needed to identify the mechanisms by which mPGES-1 affects neurotoxicity.
In summary, we have shown that mPGES-1 plays a critical role in the infarction, edema, apoptotic neuronal death, behavioral dysfunctions, and PGE2 production observed after ischemia. Our results demonstrate that mPGES-1 is coinduced with COX-2 mainly in the neurons and microglia in the cortex after transient ischemia. Although augmented PGE2 synthesis has been demonstrated in the lesion sites of rodent transient ischemia models, the role of PGE2 in neuronal cell death has been controversial. We here demonstrate that postischemic PGE2 production is involved in the pathogenic events occurring in cerebral ischemia and thus that inhibition of postischemic PGE2 production may be a valuable therapeutic strategy. Considering that COX inhibitors may nonselectively suppress the production of many types of prostanoids that are essential for normal physiological function of the brain (8), a mPGES-1 inhibitor may be an injury-selective and fewer-side-effect inhibitor. Thus, our results suggest that mPGES-1 is a promising, previously undescribed target for treatment of human stroke.
Materials and Methods
Animals.
Male (270–300 g) Sprague–Dawley rats were purchased from the Shizuoka Laboratory Animal Cooperative (Shizuoka, Japan). mPGES-1 KO and WT mice (C57BL/6J × 129/SvJ background) back-crossed to C57BL/6J mice for >8 generations to avoid artifactual differences caused by genetic background were used (21). Our preliminary data showed no significant gender differences in infarct volume, degree of edema, neurological score, and motor activity 24 h after ischemia (see Table 2, which is published as supporting information on the PNAS web site). Therefore, both male and female mice were studied at weights of 24–30 g, and data from both sexes were pooled. In all studies, the animal care and experimental procedures complied with the guidelines of the Japanese Pharmacological Society.
Ischemia Model.
Transient occlusion of the right MCA for 2 h was carried out under halothane anesthesia and achieved by inserting a nylon monofilament up to the MCA. Body temperature was maintained ≈37°C. Details are provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. In sham-operated animals, an incision was made over the MCA, but the artery was not occluded. For i.c.v. injection, 1-μl solutions of either PGE2 (1.25 or 2.5 ng/μl) or vehicle (0.25% dimithylsulfoxide in artificial cerebrospinal fluid) were infused stereotaxically into the bilateral cerebroventricle just before the MCAO (total PGE2 of 0, 2.5, or 5 ng). Details are provided in Supporting Materials and Methods.
Staining of Brain Slice.
Animals were anesthetized 24 h after reperfusion. Frozen coronal sections (20 μm) were immunostained as described (24) according to the protocol of the avidin-biotin complex kit (Vector Laboratories). Details are provided in Supporting Materials and Methods. The number of caspase-3-positive cells was counted in each of three predesignated areas of 0.29 mm2 in the peri-infarct and ischemic core regions (Fig. 6G). For fluoroimmunostaining, the Cy3- or FITC-conjugated secondary antibodies (Jackson ImmunoResearch) were used and examined by using a confocal laser scanning system (LSM510; Zeiss). TUNEL staining was performed according to protocol of the In Situ Cell Death Detection Kit (Roche Diagnostics). The number of TUNEL-positive cells was counted in each of three predesignated areas of 25,600 μm2 in the peri-infarct region (Fig. 6A).
Western and Northern Blotting.
The cerebral hemispheres were dissected into the cerebral cortex, striatum, and hippocampus and lysed by homogenization. Western or Northern blotting was performed as described (24) by using mPGES-1, mPGES-2, cPGES, COX-1, COX-2, and caspase-3 antibodies or cDNA probes for mPGES-1, COX-2, and GAPDH, respectively. Details are provided in Supporting Materials and Methods.
PGE2 Assay.
The cerebral hemispheres were dissected as described above and then quickly frozen in liquid nitrogen and weighed to determine the wet weight. Prostanoids were extracted by homogenization of the tissues in 70% methanol solution containing 10 μM indomethacin and centrifugation at 15,000 × g for 20 min at 4°C. The supernatant was evaporated and dissolved with the assay buffer. The PGE2 concentration was determined according to protocol of the enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI).
Quantification of Infarct Volume.
The quantification of infarct volume was performed by 2,3,5-triphenyltetrazolium chloride (TTC) staining and with scion image software (Scion, Frederick, MD). Details are provided in Supporting Materials and Methods. Infarct volume of whole brain, cortex, or striatum = [infarct volume of whole brain, cortex, or striatum/volume of whole brain] × 100. Edema percent = [volume of postischemic hemisphere − volume of contralateral hemisphere]/volume of contralateral hemisphere × 100.
Behavioral Experiment.
The animals were scored for neurological deficits 24 h after transient ischemia as follows: 0, no deficit; 1, flexion of the torso and contralateral forelimb when lifted by the tail; 2, contralateral forelimb weakness upon application of pressure to the side of the body; 3, circling to the affected side; and 4, no spontaneous locomotor activity. To measure the spontaneous motor activities, the animals were placed in a transparent box (25 × 40 × 20 cm) in which the floor was marked with both longitudinal and transverse lines at 5-cm intervals, and the number of times they crossed the lines over a period of 1 min was measured.
Measurement of Physiological Parameters.
Cerebral blood flow was measured under 1% halothane anesthesia by the probe of laser-Doppler flowmetry (ALF21; Advance Co. Ltd., Tokyo, Japan) that was attached to the skull at 3 mm lateral and 1 mm caudal to the bregma. In a separate cohort of animals, mean arterial pressure was monitored, and arterial blood pH, pCO2, and pO2 were measured before, during, and 30 min after ischemia (Rapid Lab 248, Bayer, Wuppertal, Germany). For studies of vascular anatomy, four unoperated mice of each genotype were deeply anesthetized and perfused via the left ventricle with 0.5 ml of 50% carbon black containing 10% gelatin. The photograph of the ventral aspect of the brain was taken by a digital microscope (VHX; Keyence, Osaka, Japan).
Statistical Analysis.
Results are expressed as the mean ± SEM. Statistical significance was evaluated with one-way ANOVA followed by Tukey’s method. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported in part by Japan Society for the Promotion of Science Grant-in-Aid for the Scientific Research 16390069 (to Y.S.) and Grant-in-Aid for the Creative Scientific Research 13NP0401 (to Y.S.) and by Japanese Ministry of Education, Culture, Sports, Science, and Technology Grant-in-Aid for Young Scientists 18790063 (to Y.I.-M.).
Abbreviations
- PGE2
prostaglandin E2
- PGES
PGE2 synthase
- mPGES
microsomal PGES
- cPGES
cytosolic PGES
- MCA
middle cerebral artery
- MCAO
MCA occlusion
- COX
cyclooxygenase
- KO
knockout
- i.c.v.
intracerebroventricular.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Lyden P. D., Grotta J. C., Levine S. R., Marler J. R., Frankel M. R., Brott T. G. Neurology. 1997;49:14–20. doi: 10.1212/wnl.49.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Lyden P. D. Prog. Cardiovasc. Dis. 1999;42:175–183. doi: 10.1016/s0033-0620(99)70001-0. [DOI] [PubMed] [Google Scholar]
- 3.Koistinaho J., Hokfelt T. NeuroReport. 1997;8:i–viii. [PubMed] [Google Scholar]
- 4.Dirnagl U., Iadecola C., Moskowitz M. A. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 5.Barone F. C., Feuerstein G. Z. J. Cereb. Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Kempski O., Shohami E., von Lubitz D., Hallenbeck J. M., Feuerstein G. Stroke. 1987;18:111–119. doi: 10.1161/01.str.18.1.111. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C., Niwa K., Nogawa S., Zhao X., Nagayama M., Araki E., Morham S., Ross M. E. Proc. Natl. Acad. Sci. USA. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufmann W. E., Andreasson K. I., Isakson P. C., Worley P. F. Prostaglandins. 1997;54:601–624. doi: 10.1016/s0090-6980(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen S., Fusco F. R., Yrjanheikki J., Keinanen R., Hirvonen T., Roivainen R., Narhi M., Hokfelt T., Koistinaho J. Proc. Natl. Acad. Sci. USA. 1997;94:6500–6505. doi: 10.1073/pnas.94.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogawa S., Zhang F., Ross M. E., Iadecola C. J. Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iadecola C., Forster C., Nogawa S., Clark H. B., Ross M. E. Acta Neuropathol. 1999;98:9–14. doi: 10.1007/s004010051045. [DOI] [PubMed] [Google Scholar]
- 12.Yokota C., Kaji T., Kuge Y., Inoue H., Tamaki N., Minematsu K. Neurosci. Lett. 2004;357:219–222. doi: 10.1016/j.neulet.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 13.Tomimoto H., Akiguchi I., Wakita H., Lin J. X., Budka H. Acta Neuropathol. 2000;99:26–30. doi: 10.1007/pl00007402. [DOI] [PubMed] [Google Scholar]
- 14.Rose J. W., Hill K. E., Watt H. E., Carlson N. G. J. Neuroimmunol. 2004;149:40–49. doi: 10.1016/j.jneuroim.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki T., Kitagawa K., Yamagata K., Takemiya T., Tanaka S., Omura-Matsuoka E., Sugiura S., Matsumoto M., Hori M. J. Cereb. Blood Flow Metab. 2004;24:107–113. doi: 10.1097/01.WCB.0000100065.36077.4A. [DOI] [PubMed] [Google Scholar]
- 16.McCullough L., Wu L., Haughey N., Liang X., Hand T., Wang Q., Breyer R. M., Andreasson K. J. Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cazevieille C., Muller A., Meynier F., Dutrait N., Bonne C. Neurochem. Int. 1994;24:395–398. doi: 10.1016/0197-0186(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 18.Takadera T., Shiraishi Y., Ohyashiki T. Neurochem. Int. 2004;45:713–719. doi: 10.1016/j.neuint.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsson P. J., Thoren S., Morgenstern R., Samuelsson B. Proc. Natl. Acad. Sci. USA. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami M., Nakatani Y., Tanioka T., Kudo I. Prostaglandins Other Lipid Mediat. 2002;68–69:383–399. doi: 10.1016/s0090-6980(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 21.Uematsu S., Matsumoto M., Takeda K., Akira S. J. Immunol. 2002;168:5811–5816. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]
- 22.Engblom D., Saha S., Engstrom L., Westman M., Audoly L. P., Jakobsson P. J., Blomqvist A. Nat. Neurosci. 2003;6:1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
- 23.Kamei D., Yamakawa K., Takegoshi Y., Mikami-Nakanishi M., Nakatani Y., Oh-Ishi S., Yasui H., Azuma Y., Hirasawa N., Ohuchi K., et al. J. Biol. Chem. 2004;279:33684–33695. doi: 10.1074/jbc.M400199200. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda-Matsuo Y., Ikegaya Y., Matsuki N., Uematsu S., Akira S., Sasaki Y. J. Neurochem. 2005;94:1546–1558. doi: 10.1111/j.1471-4159.2005.03302.x. [DOI] [PubMed] [Google Scholar]
- 25.Kojima F., Naraba H., Sasaki Y., Okamoto R., Koshino T., Kawai S. J. Rheumatol. 2002;29:1836–1842. [PubMed] [Google Scholar]
- 26.Dore S., Otsuka T., Mito T., Sugo N., Hand T., Wu L., Hurn P. D., Traystman R. J., Andreasson K. Ann. Neurol. 2003;54:155–162. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- 27.Kochanek P. M., Hallenbeck J. M. Stroke. 1992;23:1367–1379. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- 28.Schilling M., Besselmann M., Leonhard C., Mueller M., Ringelstein E. B., Kiefer R. Exp. Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 29.Yamagata K., Matsumura K., Inoue W., Shiraki T., Suzuki K., Yasuda S., Sugiura H., Cao C., Watanabe Y., Kobayashi S. J. Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozaki-Okayama Y., Matsumura K., Ibuki T., Ueda M., Yamazaki Y., Tanaka Y., Kobayashi S. Crit. Care Med. 2004;32:795–800. doi: 10.1097/01.ccm.0000114576.60077.fc. [DOI] [PubMed] [Google Scholar]
- 31.Guay J., Bateman K., Gordon R., Mancini J. J. Biol. Chem. 2004;279:24866–24872. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]
- 32.Liu D., Li L., Augustus L. J. Neurochem. 2001;77:1036–1047. doi: 10.1046/j.1471-4159.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- 33.Thornhill J., Asselin J. Brain Res. 1999;825:36–45. doi: 10.1016/s0006-8993(99)01210-x. [DOI] [PubMed] [Google Scholar]
- 34.Colbourne F., Grooms S. Y., Zukin R. S., Buchan A. M., Bennett M. V. Proc. Natl. Acad. Sci. USA. 2003;100:2906–2910. doi: 10.1073/pnas.2628027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.